Red Emitting Solid-State CDs/PVP with Hydrophobicity for Latent Fingerprint Detection
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Carbon Dots
2.3. Preparation of CDs/PVP
2.4. Visualization of Latent Fingerprints
2.5. Characterization
3. Results and Discussion
3.1. Effects of Molar Ratio and Concentration on CDs Synthesis
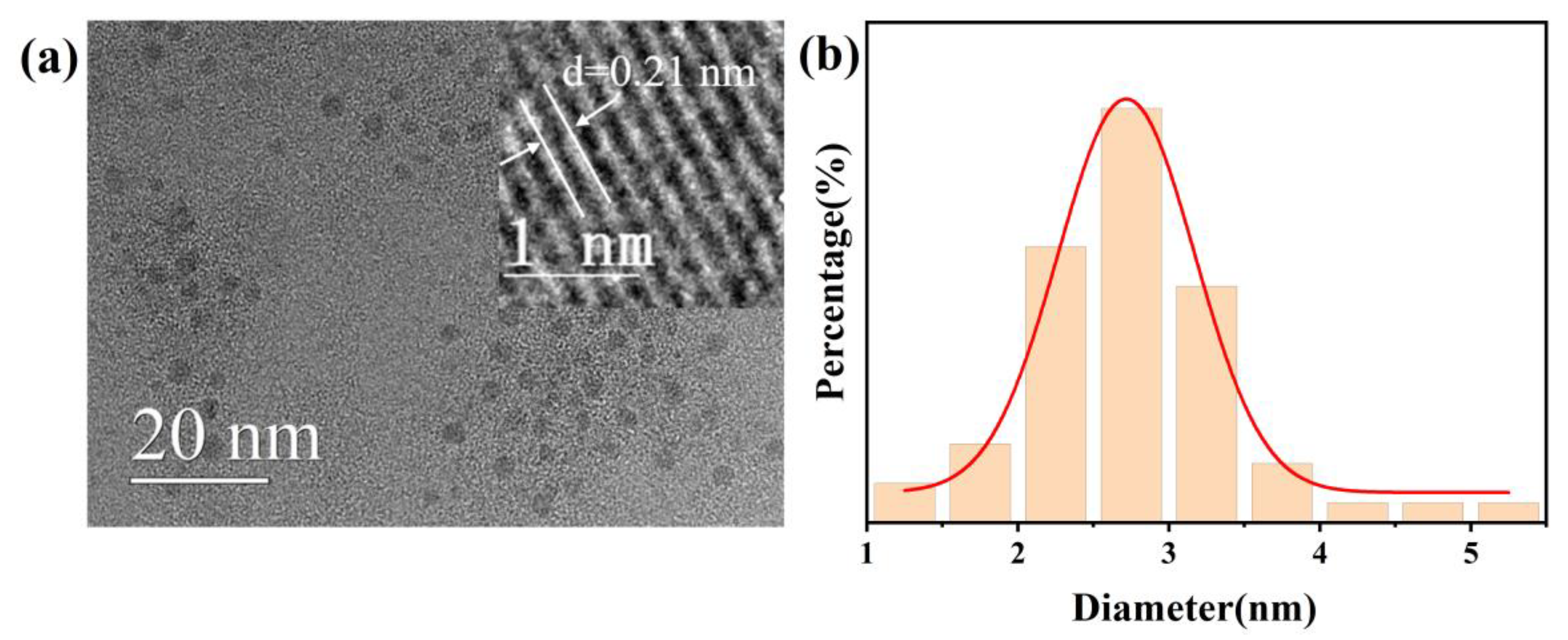
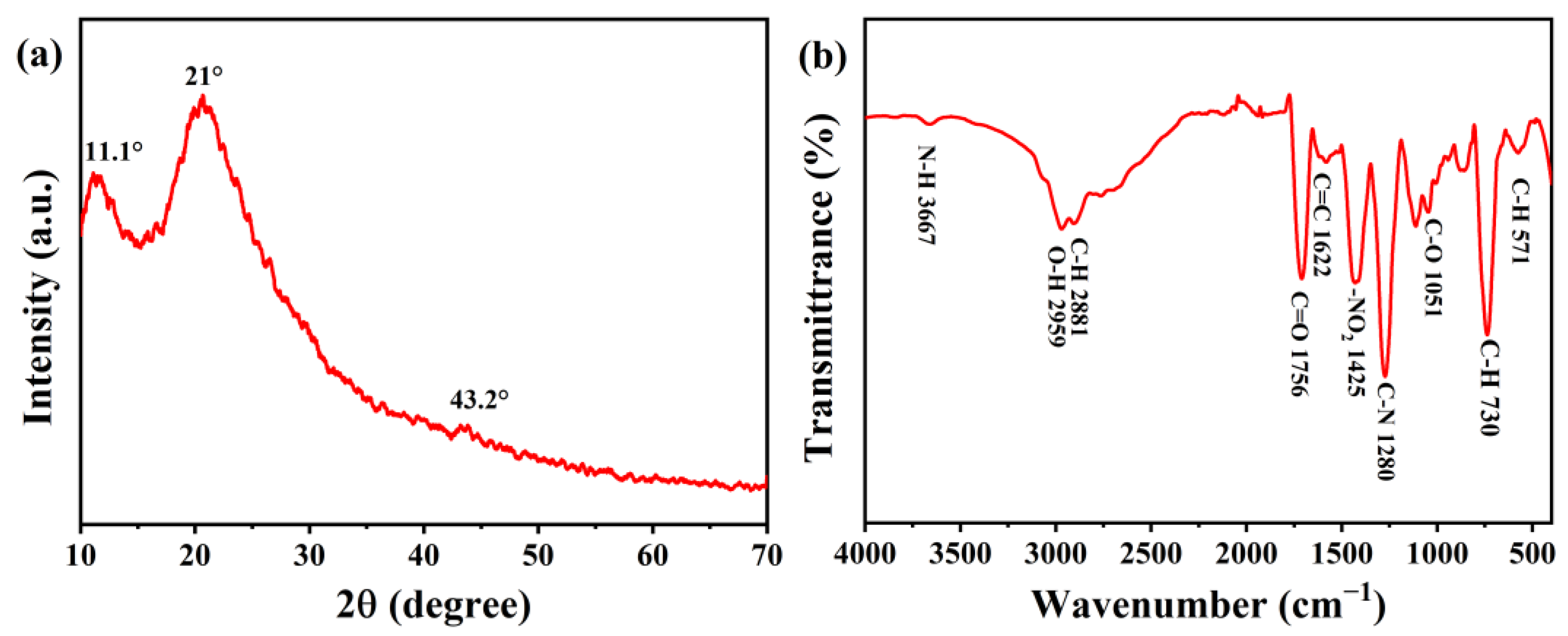
3.2. Morphology and Microstructure Analysis
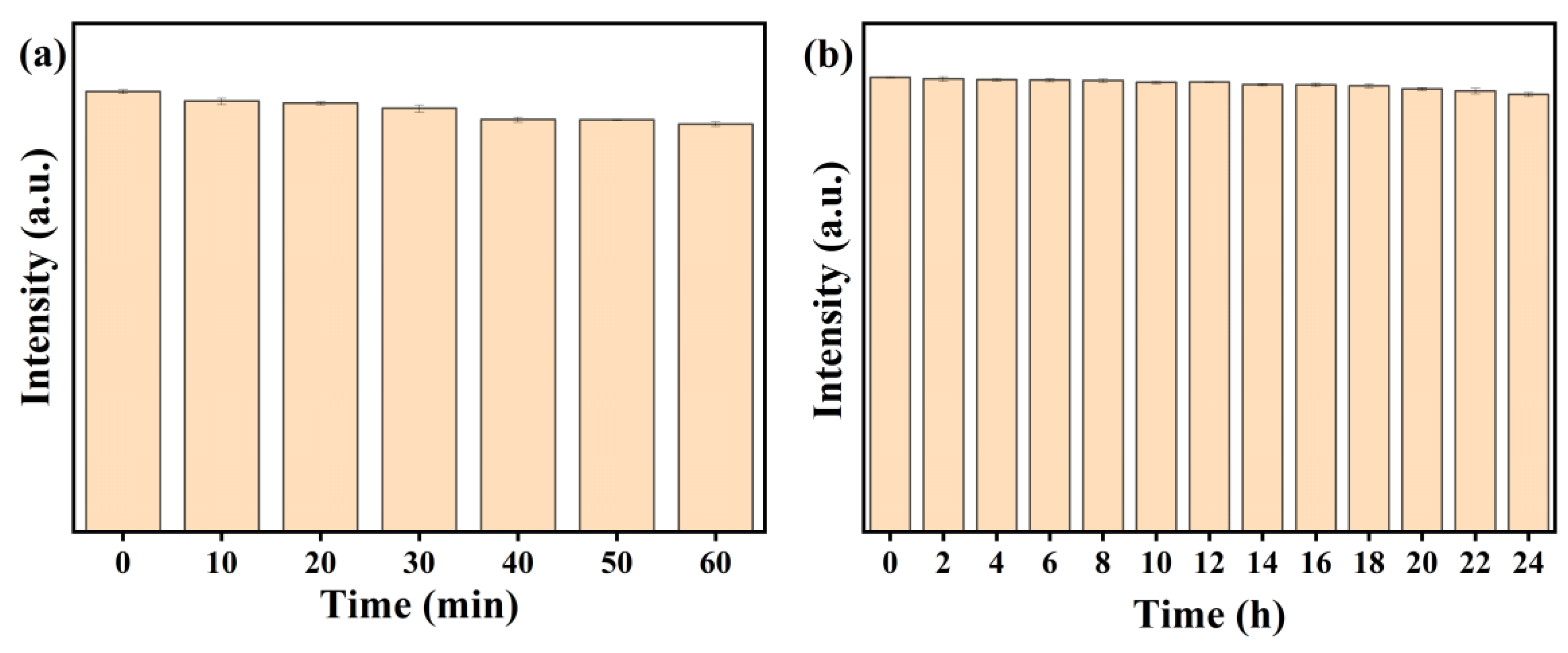
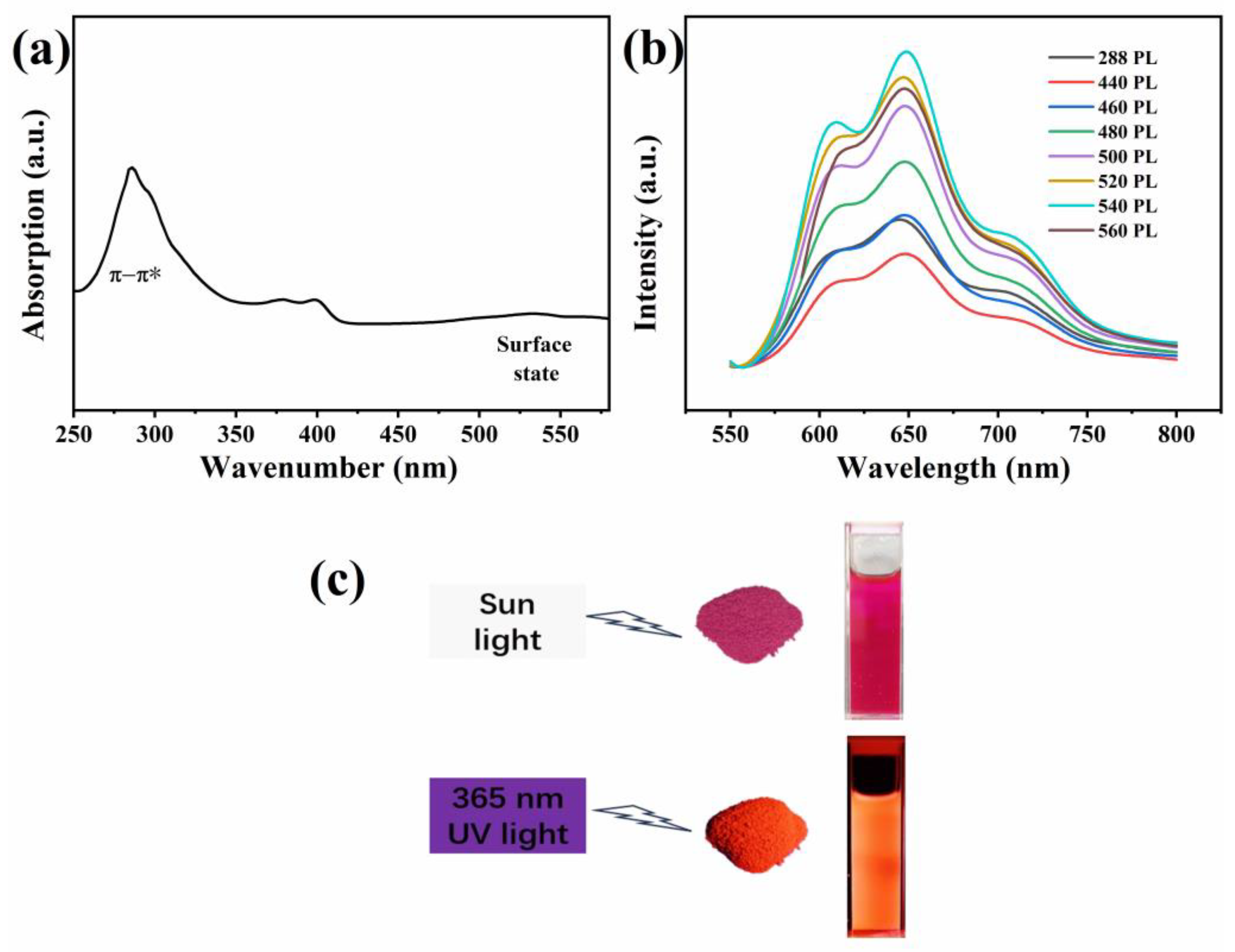
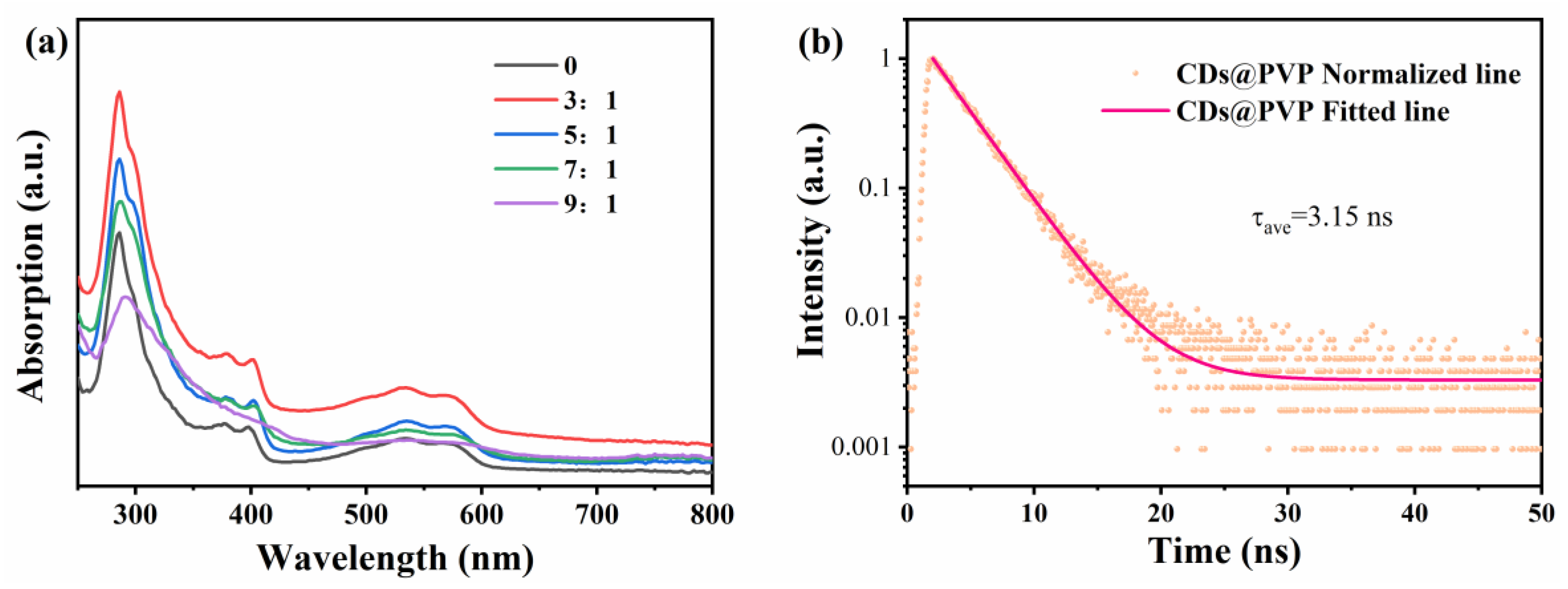
3.3. Optical Property Studies
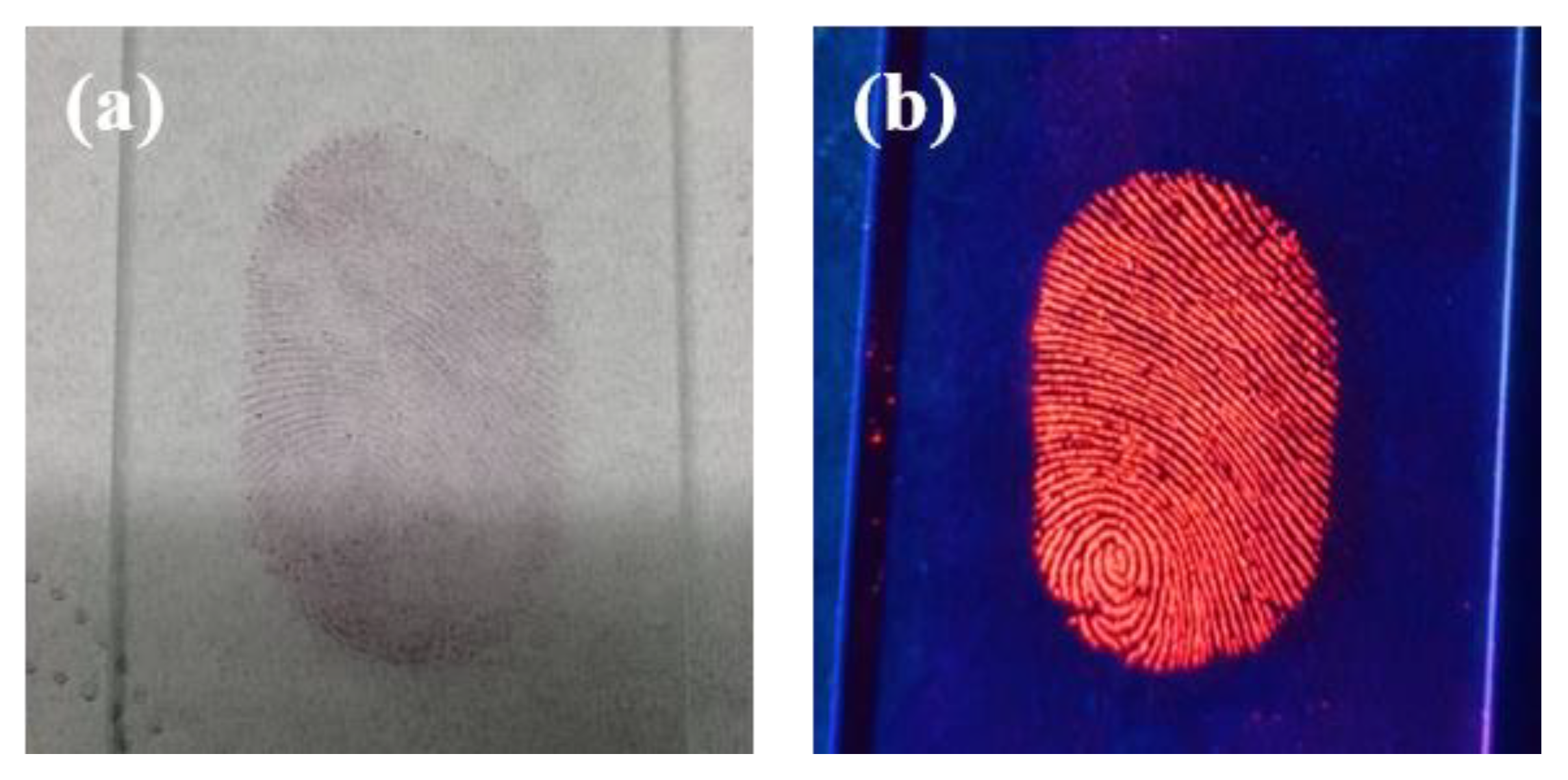
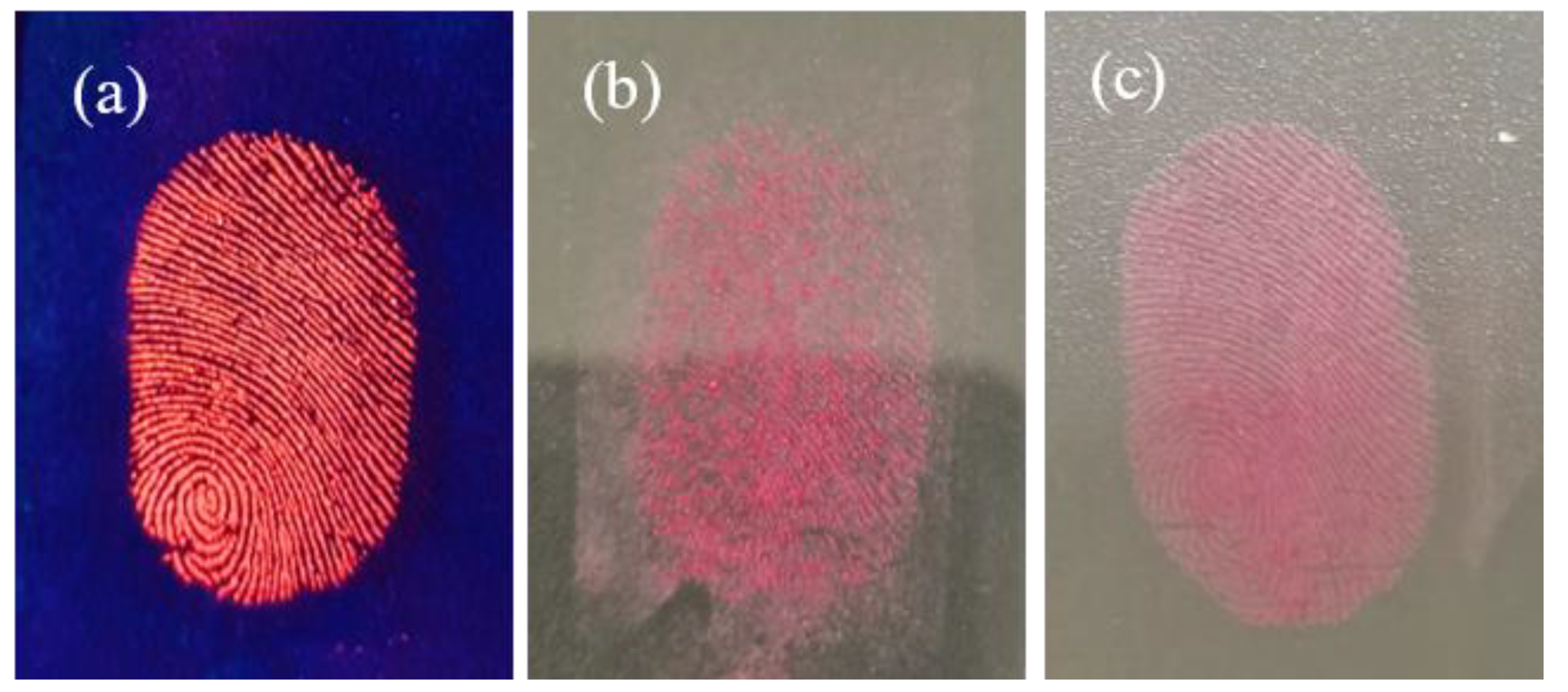
3.4. Application in Latent Fingerprint Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, B.; Lu, S. The light of carbon dots: From mechanism to applications. Matter 2022, 5, 110–149. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, C.; Fan, J.; Luo, J.; Liu, L.; Huang, J.; Liu, R.; Zhang, X. Red-emitting carbon dots as luminescent agent in wide-range water detection in organic solvents and polarity-selective zebrafish imaging. J. Alloys Compd. 2022, 920, 165963. [Google Scholar] [CrossRef]
- Bécue, A. Emerging fields in fingermark (meta)detection—A critical review. Anal. Methods 2016, 8, 7983–8003. [Google Scholar] [CrossRef]
- Yang, R.; Lian, J. Studies on the development of latent fingerprints by the method of solid-medium ninhydrin. Forensic Sci. Int. 2014, 242, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.J.; Downham, R.; Sears, V.G. Effect of substrate surface topography on forensic development of latent fingerprints with iron oxide powder suspension. Surf. Interface Anal. 2010, 42, 438–442. [Google Scholar] [CrossRef]
- Frick, A.A.; Busetti, F.; Cross, A.; Lewis, S.W. Aqueous Nile blue: A simple, versatile and safe reagent for the detection of latent fingermarks. Chem. Commun. 2014, 50, 3341–3343. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Hou, W.Y.; Kang, J.; Zhai, X.Y.; Chen, H.L.; Hao, Y.W.; Wan, G.Y. The facile preparation of solid-state fluorescent carbon dots with a high fluorescence quantum yield and their application in rapid latent fingerprint detection. Dalton Trans. 2021, 50, 12188–12196. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Shen, F.; Zhang, X.; Jing, P.; Li, D.; Yang, X.; Zhou, D.; Xu, X.; Qu, S. Synthesis of green emissive carbon dots@montmorillonite composites and their application for fabrication of light-emitting diodes and latent fingerprints markers. J. Colloid Interface Sci. 2019, 554, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, I.; Algarra, M.; Alcoholado, C.; Cifuentes, M.; Lázaro-Martínez, J.M.; Rodríguez-Castellón, E.; Mutavdžić, D.; Radotić, K.; Bandosz, T.J. Fingerprint imaging using N-doped carbon dots. Carbon 2019, 144, 791–797. [Google Scholar] [CrossRef]
- Chen, J.; Wei, J.S.; Zhang, P.; Niu, X.Q.; Zhao, W.; Zhu, Z.Y.; Ding, H.; Xiong, H.M. Red-Emissive Carbon Dots for Fingerprints Detection by Spray Method: Coffee Ring Effect and Unquenched Fluorescence in Drying Process. ACS Appl. Mater. Interfaces 2017, 9, 18429–18433. [Google Scholar] [CrossRef]
- Donato, K.Z.; Tan, H.L.; Marangoni, V.S.; Martins, M.V.; Ng, P.R.; Costa, M.C.; Jain, P.; Lee, S.J.; Koon, G.K.; Donato, R.K.; et al. Graphene oxide classification and standardization. Sci. Rep. 2023, 13, 6064. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, R.; Kumawat, M.K.; Thakur, M.; Srivastava, R. Multi-fluorescent cationic carbon dots for solid-state fingerprinting. J. Lumin. 2019, 208, 428–436. [Google Scholar] [CrossRef]
- Deng, Z.; Liu, C.; Jin, Y.; Pu, J.; Wang, B.; Chen, J. High quantum yield blue- and orange-emitting carbon dots: One-step microwave synthesis and applications as fluorescent films and in fingerprint and cellular imaging. Analyst 2019, 144, 4569–4574. [Google Scholar] [CrossRef] [PubMed]
- Che, H.; Che, G.; Zhou, P.; Liu, C.; Dong, H.; Li, C.; Song, N.; Li, C. Nitrogen doped carbon ribbons modified g-C3N4 for markedly enhanced photocatalytic H2-production in visible to near-infrared region. Chem. Eng. J. 2020, 382, 122870. [Google Scholar] [CrossRef]
- Qu, D.; Miao, X.; Wang, X.; Nie, C.; Li, Y.; Luo, L.; Sun, Z. Se & N co-doped carbon dots for high-performance fluorescence imaging agent of angiography. J. Mater. Chem. B 2017, 5, 4988–4992. [Google Scholar] [PubMed]
- Yu, H.; Li, H.; Chen, C.; Zhou, S.; Wang, P. Water-sensitive phase-transition of a carbon dot–calcium carbonate composite for moisture detection in organic solvents. Anal. Methods 2019, 11, 2634–2638. [Google Scholar] [CrossRef]
- Li, W.-K.; Feng, J.-T.; Ma, Z.-Q. Nitrogen, sulfur, boron and flavonoid moiety co-incorporated carbon dots for sensitive fluorescence detection of pesticides. Carbon 2020, 161, 685–693. [Google Scholar] [CrossRef]
- Cao, M.; Li, Y.; Zhao, Y.; Shen, C.; Zhang, H.; Huang, Y. A novel method for the preparation of solvent-free, microwave-assisted and nitrogen-doped carbon dots as fluorescent probes for chromium(vi) detection and bioimaging. RSC Adv. 2019, 9, 8230–8238. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, J.; Li, X. Leek-derived codoped carbon dots as efficient fluorescent probes for dichlorvos sensitive detection and cell multicolor imaging. Anal. Bioanal. Chem. 2019, 411, 7879–7887. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, D.W.; Ma, J.; Qin, A.; Tang, B.Z. A ratiometric fluorescent tag based on dual-emissive hydrophobic carbon dots for in-situ monitoring of seafood freshness. Anal. Chim. Acta 2023, 1250, 340931. [Google Scholar] [CrossRef]
- Guo, G.; Li, T.; Wang, Y.; Hu, H.; Xing, H.; Tang, S.; Gao, S.; Leng, X.; Chen, D. Aggregation-induced bimodal excitation of nitrogen-doped carbon dots for ratiometric sensing of new coccine and solid-state multicolor lighting. J. Colloid Interface Sci. 2023, 645, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, X.; Yang, K.; Wang, L.; Lee, C.-S. Oxygen/nitrogen-related surface states controlled carbon nanodots with tunable full-color luminescence: Mechanism and bio-imaging. Carbon 2020, 160, 298–306. [Google Scholar] [CrossRef]
- Lin, S.; Lin, C.; He, M.; Yuan, R.; Zhang, Y.; Zhou, Y.; Xiang, W.; Liang, X. Solvatochromism of bright carbon dots with tunable long-wavelength emission from green to red and their application as solid-state materials for warm WLEDs. RSC Adv. 2017, 7, 41552–41560. [Google Scholar] [CrossRef]
- Li, P.; Xue, S.; Sun, L.; Zong, X.; An, L.; Qu, D.; Wang, X.; Sun, Z. Formation and fluorescent mechanism of red emissive carbon dots from o-phenylenediamine and catechol system. Light Sci. Appl. 2022, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Soni, N.; Singh, S.; Sharma, S.; Batra, G.; Kaushik, K.; Rao, C.; Verma, N.C.; Mondal, B.; Yadav, A.; Nandi, C.K. Absorption and emission of light in red emissive carbon nanodots. Chem. Sci. 2021, 12, 3615–3626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hu, J.; Liu, S.; Hao, X.; Li, L.; Zou, G.; Hou, H.; Ji, X. Channel regulation of TFC membrane with hydrophobic carbon dots in forward osmosis. Chin. Chem. Lett. 2021, 32, 2882–2886. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, D.-W.; Ma, J.; Cheng, J.; Wang, Z.; Tang, B.Z. A volatile basic nitrogens-responsive tag based on aggregation-induced emission luminogen for real-time monitoring and in situ visualization of salmon freshness. Anal. Chim. Acta 2022, 1221, 340122. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Li, Y.; Qiang, M.; Lou, W.; Dai, B.; Lin, H.; Han, Z.; Hong, R.; Zhang, D. Color Tunable Composite Phosphor Ceramics Based on SrAlSiN3:Eu2+/Lu3Al5O12:Ce3+ for High-Power and High-Color-Rendering-Index White LEDs/LDs Lighting. Materials 2023, 16, 6007. [Google Scholar] [CrossRef]
- Zhao, D.; Ma, W.; Xiao, X. The Recognition of Sweat Latent Fingerprints with Green-Emitting Carbon Dots. Nanomaterials 2018, 8, 612. [Google Scholar] [CrossRef]


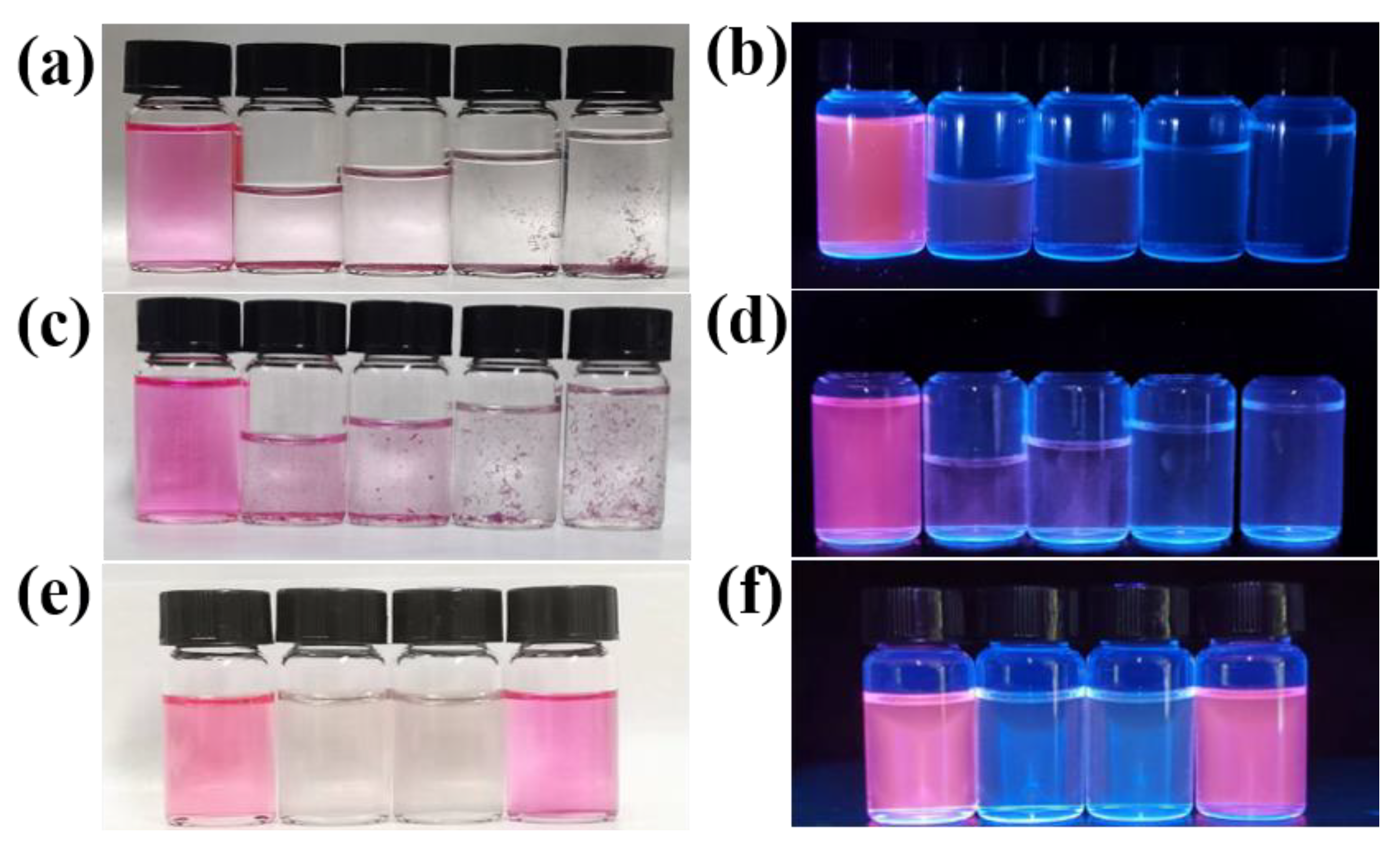


| Mole Ratio of Reagents (OPDA:Phthalic Acid) | Mass Ratio of Reagents (OPDA:Phthalic Acid) | Reaction Conditions | Optimal Emission Wavelength (nm) |
|---|---|---|---|
| 2:1 | 0.504:0.415 g | 200 °C, 6 h | 569 |
| 3:1 | 0.81:0.415 g | 200 °C, 6 h | 597 |
| 4:1 | 1.08:0.415 g | 200 °C, 6 h | 569 |
| 5:1 | 0.45:0.14 g | 200 °C, 6 h | 569 |
| 5:1 | 1.35:0.415 g | 200 °C, 6 h | 649 |
| 5:1 | 2.16:0.83 g | 200 °C, 6 h | 649 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Han, Z.; Ding, S.; Jing, Y.; Wei, Z.; Zhang, D.; Hong, R.; Tao, C. Red Emitting Solid-State CDs/PVP with Hydrophobicity for Latent Fingerprint Detection. Materials 2024, 17, 1917. https://doi.org/10.3390/ma17081917
Zhang Z, Han Z, Ding S, Jing Y, Wei Z, Zhang D, Hong R, Tao C. Red Emitting Solid-State CDs/PVP with Hydrophobicity for Latent Fingerprint Detection. Materials. 2024; 17(8):1917. https://doi.org/10.3390/ma17081917
Chicago/Turabian StyleZhang, Zhihong, Zhaoxia Han, Shuhui Ding, Yujing Jing, Zhenjie Wei, Dawei Zhang, Ruijin Hong, and Chunxian Tao. 2024. "Red Emitting Solid-State CDs/PVP with Hydrophobicity for Latent Fingerprint Detection" Materials 17, no. 8: 1917. https://doi.org/10.3390/ma17081917





