Synthesis of New Chiral β-Carbonyl Selenides with Antioxidant and Anticancer Activity Evaluation—Part I
Abstract
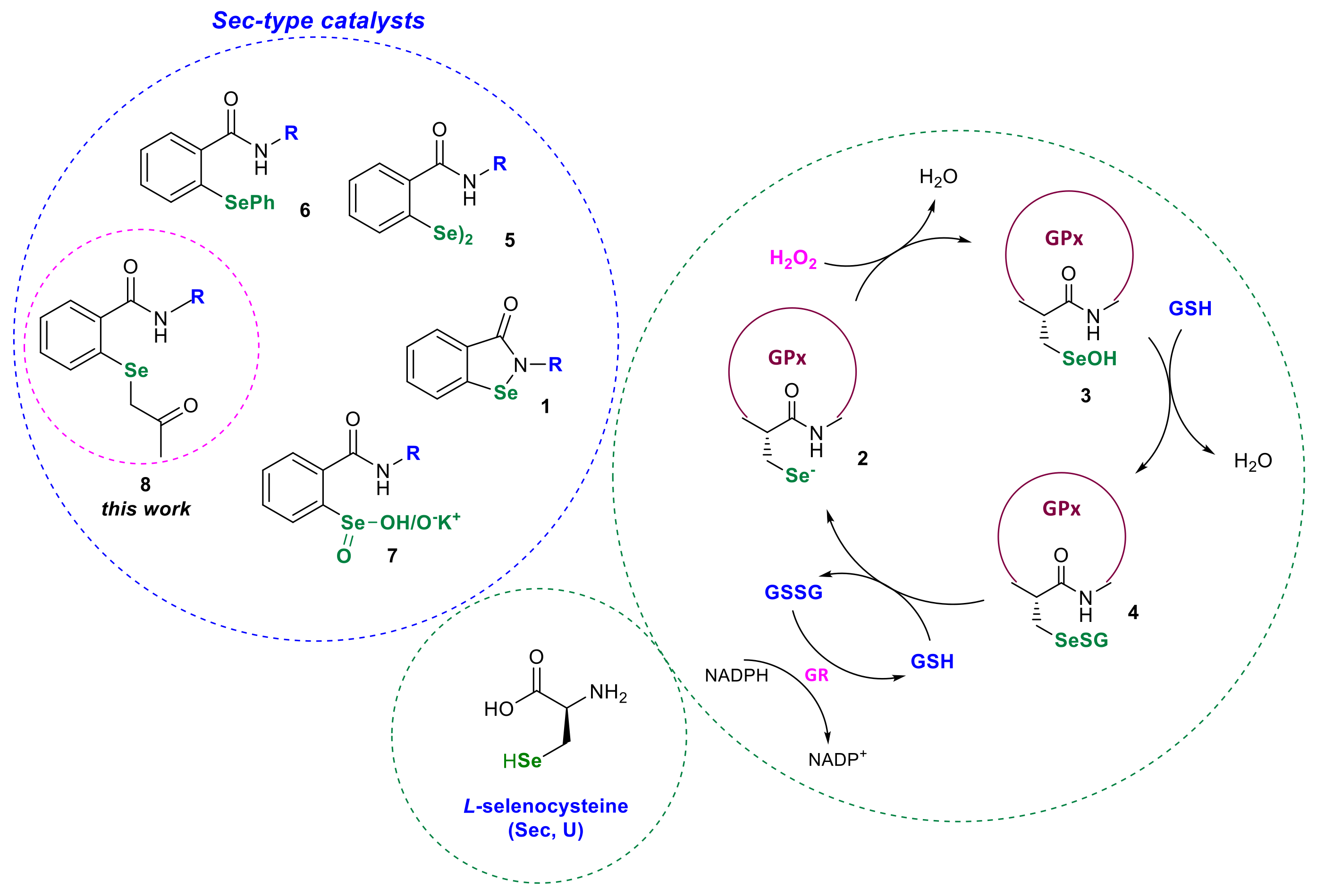
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. General Procedure and Analysis Data
3.3. Antioxidant Activity Evaluation
3.3.1. DTT Activity Assay
3.3.2. DPPH Radical Scavenging Assay
3.4. MTT Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, K.; Kumar, A.; Alok, S.; Kamal, K.; Singh, S.P. Stereochemistry and its role in drug design. IJPSR 2014, 5, 4644–4659. [Google Scholar]
- Nguyen, A.N.; He, H.; Pham-Huy, C. Chiral Drugs: An Overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar]
- McConathy, J.; Owens, M.J. Stereochemistry in Drug Action. Prim. Care Companion J. Clin. Psychiatry 2003, 5, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Orian, L.; Toppo, S. Organochalcogen peroxidase mimetics as potential drugs: A long story of a promise still unfulfilled. Free Radic. Biol. Med. 2014, 66, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Weekley, C.M.; Harris, H.H. Which form is that? The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem. Soc. Rev. 2013, 42, 8870–8894. [Google Scholar] [CrossRef] [PubMed]
- Chuai, H.; Zhang, S.Q.; Bai, H.; Li, J.; Wang, Y.; Sun, J.; Wen, E.; Zhang, J.; Xin, M. Small molecule selenium-containing compounds: Recent development and therapeutic applications. Eur. J. Med. Chem. 2021, 223, 113621. [Google Scholar] [CrossRef]
- Singh, F.V.; Wirth, T. Selenium reagents as catalysts. Catal. Sci. Technol. 2019, 9, 1073–1091. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Chen, Z.; Pan, X.; Zhang, Z.; Zhu, J.; Zhua, X. Organoselenium chemistry-based polymer synthesis. Org. Chem. Front. 2020, 7, 2815–2841. [Google Scholar] [CrossRef]
- Pacuła, A.J.; Mangiavacchi, F.; Sancineto, L.; Lenardao, E.J.; Ścianowski, J.; Santi, C. An Update on “Selenium Containing Compounds from Poison to Drug Candidates: A Review on the GPx-like Activity”. Curr. Chem. Biol. 2015, 9, 97–112. [Google Scholar] [CrossRef]
- Singh, F.V.; Wirth, T. Synthesis of Organoselenium Compounds with Potential Biological Activities. In Organoselenium Compounds in Biology and Medicine: Synthesis, Biological and Therapeutic Treatments; Royal Society of Chemistry: London, UK, 2017; pp. 77–121. [Google Scholar]
- Ścianowski, J.; Rafiński, Z. Organoselenium Chemistry: Between Synthesis and Biochemistry; Santi, C., Ed.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2014; pp. 8–60. [Google Scholar]
- Santi, C.; Scimmi, C.; Sancineto, L. Ebselen and Analogues: Pharmacological Properties and Synthetic Strategies for Their Preparation. Molecules 2021, 26, 4230. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Zhou, J.; Shao, Q. Glutathione Peroxidase GPX1 and Its Dichotomous Roles in Cancer. Cancers 2022, 14, 2560. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Worley, B.L.; Phaëton, R.; Hempel, N. Extracellular Glutathione Peroxidase GPx3 and Its Role in Cancer. Cancers 2020, 12, 2197. [Google Scholar] [CrossRef]
- Nirgude, S.; Choudhary, B. Insights into the role of GPX3, a highly efficient plasma antioxidant, in cancer. Biochem. Pharmacol. 2021, 184, 114365. [Google Scholar] [CrossRef]
- Ye, S.; Lin, R.; Guo, N.; Xing, J.; Liu, K.; Yang, W.; Guo, X. Bioinformatics analysis on the expression of GPX family in gastric cancer and its correlation with the prognosis of gastric cancer. Heliyon 2022, 8, e12214. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, D.; Mugesh, G. Introduction of a catalytic triad increases the glutathione peroxidase-like activity of diaryl diselenides. Org. Biomol. Chem. 2015, 13, 9072–9082. [Google Scholar] [CrossRef] [PubMed]
- Pacuła, A.J.; Kaczor, K.B.; Antosiewicz, J.; Janecka, A.; Długosz, A.; Janecki, T.; Wojtczak, A.; Ścianowski, J. New Chiral Ebselen Analogues with Antioxidant and Cytotoxic Potential. Molecules 2017, 22, 492. [Google Scholar] [CrossRef]
- Laskowska, A.; Pacuła-Miszewska, A.J.; Obieziurska-Fabisiak, M.; Drogosz-Stachowicz, J.; Janecka, A.; Wojtczak, A.; Scianowski, J. Attachment of Chiral Functional Groups to Modify the Activity of New GPx Mimetics. Materials 2021, 15, 2068. [Google Scholar] [CrossRef]
- Laskowska, A.; Pacuła-Miszewska, A.J.; Obieziurska-Fabisiak, M.; Jastrzębska, A.; Gach-Janczak, K.; Janecka, A.; Ścianowski, J. Facile synthesis of chiral phenylselenides as novel antioxidants and cytotoxic agents. RSC Adv. 2023, 13, 14698–14702. [Google Scholar] [CrossRef]
- Obieziurska, M.; Pacuła, A.J.; Laskowska, A.; Długosz-Pokorska, A.; Janecka, A.; Ścianowski, J. Seleninic Acid Potassium Salts as Water-Soluble Biocatalysts with Enhanced Bioavailability. Materials 2020, 13, 661. [Google Scholar] [CrossRef]
- Zhang, Z.; Ren, S.; Wan, S.; Li, W.; Jiang, T. Synthesis of D-glucosamine-modified benzo[d][1,2]selenazol-3-(2h)-one derivatives. Syn. Comm. 2010, 40, 3438–3446. [Google Scholar]
- Feng, S.; Qi, K.; Guo, Y.; Wang, J.; Gu, G.; Liu, P.; Ma, J.; Qu, L.; Zhang, S. A novel synthesis of 2-((2-oxopropyl)selanyl) benzamide derivatives by cascade selenenylation-acylation reaction and in vitro cytotoxicity evaluation. Tetrahedron Lett. 2020, 61, 152561. [Google Scholar] [CrossRef]
- Feng, S.; Qi, K.; Ma, J.; Guo, Y.; Gao, J.; Liu, P.; Wang, J.; Gu, G.; Dong, L.; Wang, J.; et al. Synthesis of novel unsymmetrical alkyl-aryl-selenides: β-carbonyl-selenides derivatives and anticancer evaluation. Chem. Pap. 2022, 76, 5471–5485. [Google Scholar] [CrossRef]
- Kumakura, F.; Mishra, B.; Priyadarsini, K.I.; Iwaoka, M. A Water-Soluble Cyclic Selenide with Enhanced Glutathione Peroxidase-Like Catalytic Activities. Eur. J. Org. Chem. 2010, 3, 440–444. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Gulcin, I.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety—Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; El-Dabea, T.; Khalaf, M.M.; Abu-Dief, A.M. Recent Overview of Potent Antioxidant Activity of Coordination Compounds. Antioxidants 2023, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Deutchoua, A.D.D.D.; Ngueumaleu, Y.; Liendji, R.W.; Hanga, S.S.P.; Nguelo, B.B.; Dedzo, G.K.; Ngameni, E. Unusual reactivity of 2,2-diphenyl-1-picrylhydrazyl (DPPH) with Fe3+ controlled by competing reactions. RSC Adv. 2024, 14, 1354. [Google Scholar] [CrossRef]
- Janković, N.; Tadí, J.; Milović, E.; Marković, Z.; Jeremić, S.; Petronijević, J.; Joksimović, N.; Borović, T.T.; Bukhari, S.N.A. Investigation of the radical scavenging potential of vanillin-based pyrido-dipyrimidines: Experimental and in silico approach. RSC Adv. 2023, 13, 15236. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant Property of Coffee Components: Assessment of Methods that Define Mechanisms of Action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Fang, L.J.; Shao, X.T.; Wang, S.; Lu, G.H.; Xu, T.; Zhou, J.Y. Sesquiterpene lactone parthenolide markedly enhances sensitivity of human A549 cells to low-dose oxaliplatin via inhibition of NF-kappaB activation and induction of apoptosis. Planta Med. 2010, 76, 258–264. [Google Scholar] [CrossRef]
- Marchetti, P.; Galla, D.A.; Russo, F.P.; Ricevuto, E.; Flati, V.; Porzio, G.; Ficorella, C.; Cifone, M.G. Apoptosis induced by oxaliplatin in human colon cancer HCT15 cell line. Anticancer Res. 2004, 24, 219–226. [Google Scholar]
- Oliveira, M.D.S.; Barbosa, M.I.; de Souza, T.B.; Moreira, D.R.; Martins, F.T.; Villarreal, W.; Machado, R.P.; Doriguetto, A.C.; Soares, M.B.P.; Bezerra, D.P. A novel platinum complex containing a piplartine derivative exhibits enhanced cytotoxicity, causes oxidative stress and triggers apoptotic cell death by ERK/p38 pathway in human acute promyelocytic leukemia HL-60 cells. Redox Biol. 2019, 20, 182–194. [Google Scholar] [CrossRef]
- Brummelhaus de Menezes, B.; Mironuk Frescura, L.; Duarte, R.; Villetti, M.A.; Barcellos da Rosa, M. A critical examination of the DPPH method: Mistakes and inconsistencies in stoichiometry and IC50 determination by UV-Vis spectroscopy. Anal. Chim. Acta 2021, 1157, 338398–338405. [Google Scholar] [CrossRef]




 | ||||
|---|---|---|---|---|
| Entry | Solvent (Solubilization of A) | NaHCO3 | Conditions and Order of Addition | Yield (%) |
| 1 | DCM | - | 1. A + acetone, rt, 1 h 2. C, rt, 20 min | 30 |
| 2 | DCM | 1 eq. | 1. A + B, rt, 1 h 2. C, rt, 3 h | 41 |
| 3 | - | 1 eq. (0.75 M NaHCO3/H2O) | 1. A + C, rt, 20 min 2. B, 0 °C to rt, 2 h | 37 |
| 4 | Acetone | 1 eq. (0.75 M NaHCO3/H2O) | 1. B (acetone + H2O solution of NaHCO3, rt, 25 min) + A, 0 °C to rt, 1 h 2. C, rt, 2 h | 0 |
| 5 | THF | 1 eq. | 1. B (acetone + NaHCO3, rt, 30 min) + A, rt, 1 h 2. C, rt, 3 h | 65 |
| 6 | Acetone | 1 eq. | 1. B (acetone + NaHCO3, rt, 30 min) + A, rt, 1 h 2. C, rt, 3 h | 87 |
 | ||||
|---|---|---|---|---|
| Remaining DTTred (%) | ||||
| Catalyst (0.1 equiv.) | 5 min | 15 min | 30 min | 60 min |
| 11/12 | 79 ± 5.4 | 61 ± 4.8 | 44 ± 2.8 | 13 ± 3.7 |
| 13/14 | 72 ± 7.4 | 61 ± 6.1 | 48 ± 3.7 | 21 ± 1.7 |
| 15/16 | 86 ± 0.1 | 78 ± 0.2 | 69 ± 0.1 | 56 ± 0.5 |
| 17/18 | 91 ± 2.2 | 90 ± 0.7 | 82 ± 1.4 | 73 ± 0.5 |
| 19/20 | 93 ± 0.1 | 90 ± 0.6 | 83 ± 0.8 | 70 ± 0.2 |
| 21/22 | 72 ± 2.5 | 52 ± 0.2 | 27 ± 2.7 | 6 ± 4.3 |
| 23/24 | 79 ± 0.3 | 56 ± 5.3 | 30 ± 3.3 | 10 ± 2.4 |
| Ebselen | 75 | 64 | 58 | 52 |
| Compound | IC50 (mM) | Antioxidant Capacity (mM TE·1 g−1) |
|---|---|---|
| 11/12 | 5.61 | 0.10 |
| 13/14 | 0.89 | 0.47 |
| 15/16 | 2.28 | 0.16 |
| 17/18 | 2.59 | 0.12 |
| 19/20 | 1.62 | 0.23 |
| 21/22 | 2.51 | 0.19 |
| 23/24 | 0.96 | 0.39 |
| Compound | IC50 [µM] ± SEM | |
|---|---|---|
| MCF-7 | HL-60 | |
| 11 | 46.1 ± 1.6 | 181 ± 11 |
| 12 | 55 ± 1.4 | 101 ± 14 |
| 13 | 39.2 ± 0.9 | 14.4 ± 0.5 |
| 14 | 35.7 ± 0.6 | 16.2 ± 1.1 |
| 15 | 115 ± 21 | 111 ± 3.5 |
| 16 | 188.3 ± 0.3 | 142 ± 1.7 |
| 17 | 235 ± 1 | 303 ± 3 |
| 18 | 237 ± 11 | 23.5 ± 1.4 |
| 19 | 87.4 ± 0.5 | 101 ± 0.6 |
| 20 | 72.4 ± 0.8 | 84.5 ± 0.6 |
| 21 | 39.2 ± 0.7 | 27.3 ± 0.5 |
| 22 | 296 ± 5 | 225 ± 2 |
| 23 | 37 ± 0.3 | 23.7 ± 1.1 |
| 24 Oxaliplatin | 38 ± 1.1 35 [36,37] | 23.8 ± 1.1 0.8 [38] |
| IC50 [µM] ± SEM | ||||
|---|---|---|---|---|
 |  | |||
| Se-derivative | MCF-7 | HL-60 | MCF-7 | HL-60 |
| β-Carbonylselenide | 39.2 ± 0.9 | 14.4 ± 0.5 | 35.7 ± 0.6 | 16.2 ± 1.1 |
| Phenylselenide | >150 | >150 | >150 | >150 |
| Benzisoselenazolone | 38.3 ± 1.3 | 26,0. ± 1.7 | 35.1 ± 0.5 | 33.3 ± 0.5 |
| Diselenide | 37.00 ± 4.25 | 8.67 ± 0.14 | >100 | 10.10 ± 0.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laskowska, A.; Pacuła-Miszewska, A.J.; Obieziurska-Fabisiak, M.; Jastrzębska, A.; Długosz-Pokorska, A.; Gach-Janczak, K.; Ścianowski, J. Synthesis of New Chiral β-Carbonyl Selenides with Antioxidant and Anticancer Activity Evaluation—Part I. Materials 2024, 17, 899. https://doi.org/10.3390/ma17040899
Laskowska A, Pacuła-Miszewska AJ, Obieziurska-Fabisiak M, Jastrzębska A, Długosz-Pokorska A, Gach-Janczak K, Ścianowski J. Synthesis of New Chiral β-Carbonyl Selenides with Antioxidant and Anticancer Activity Evaluation—Part I. Materials. 2024; 17(4):899. https://doi.org/10.3390/ma17040899
Chicago/Turabian StyleLaskowska, Anna, Agata J. Pacuła-Miszewska, Magdalena Obieziurska-Fabisiak, Aneta Jastrzębska, Angelika Długosz-Pokorska, Katarzyna Gach-Janczak, and Jacek Ścianowski. 2024. "Synthesis of New Chiral β-Carbonyl Selenides with Antioxidant and Anticancer Activity Evaluation—Part I" Materials 17, no. 4: 899. https://doi.org/10.3390/ma17040899






