Fabrication of Cu2Sn1-xGexS3 Thin-Film Solar Cells via Sulfurization of Cu2GeS3/Cu2SnS3 Stacked Precursors
Abstract
:1. Introduction
2. Materials and Methods
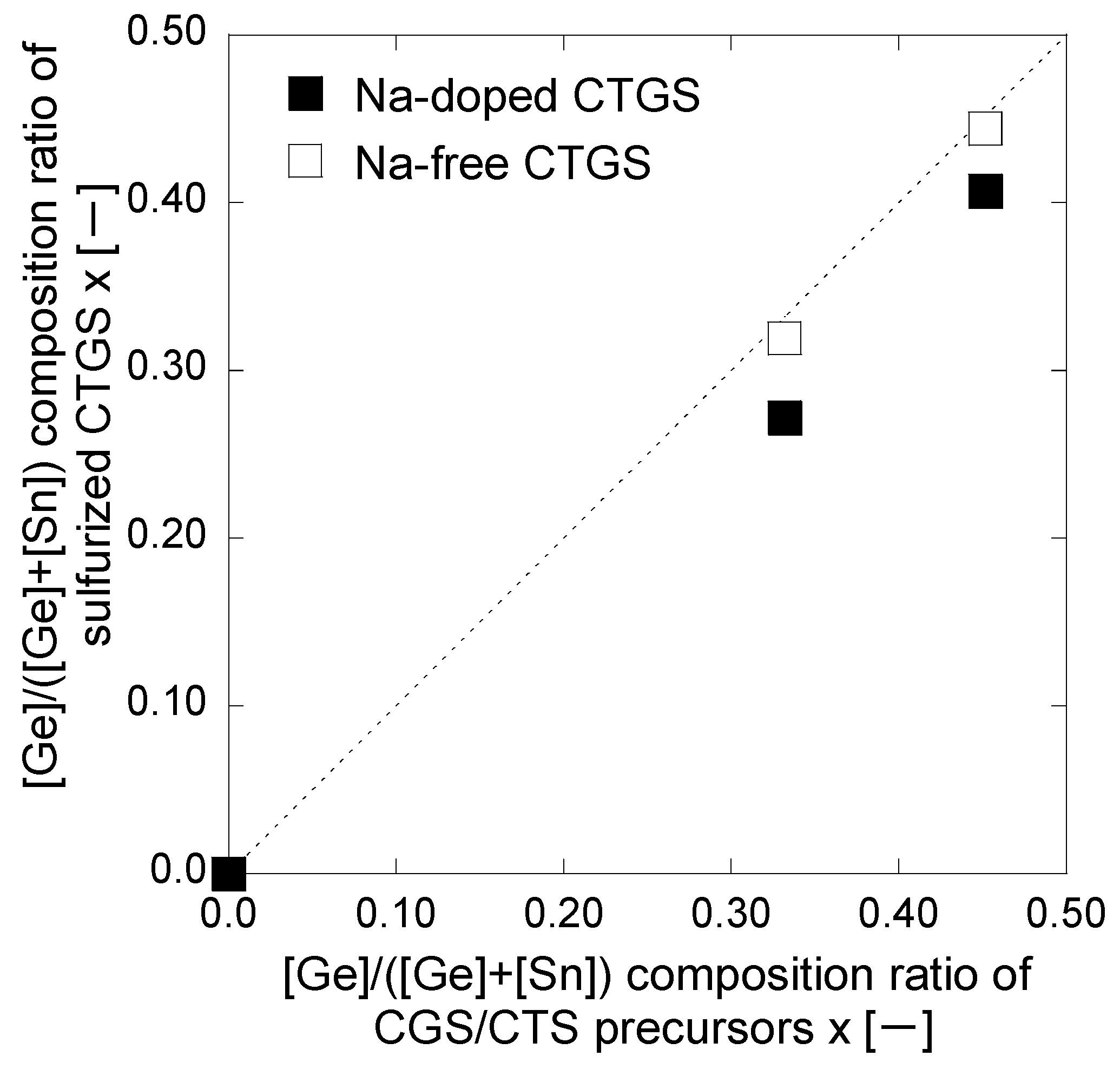
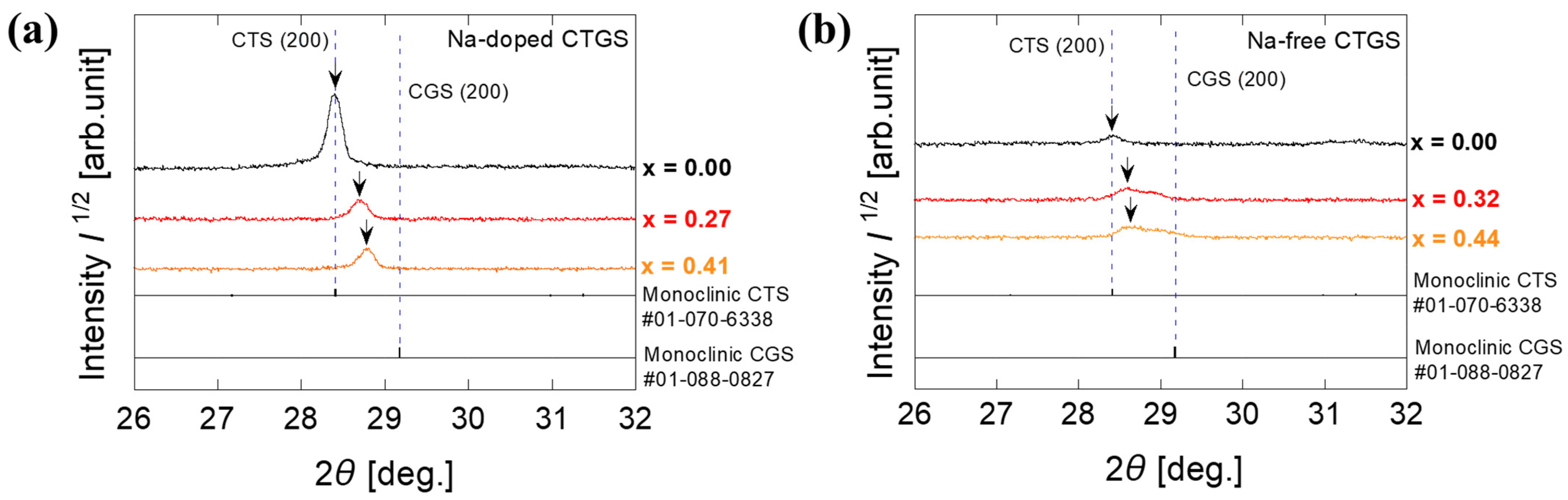
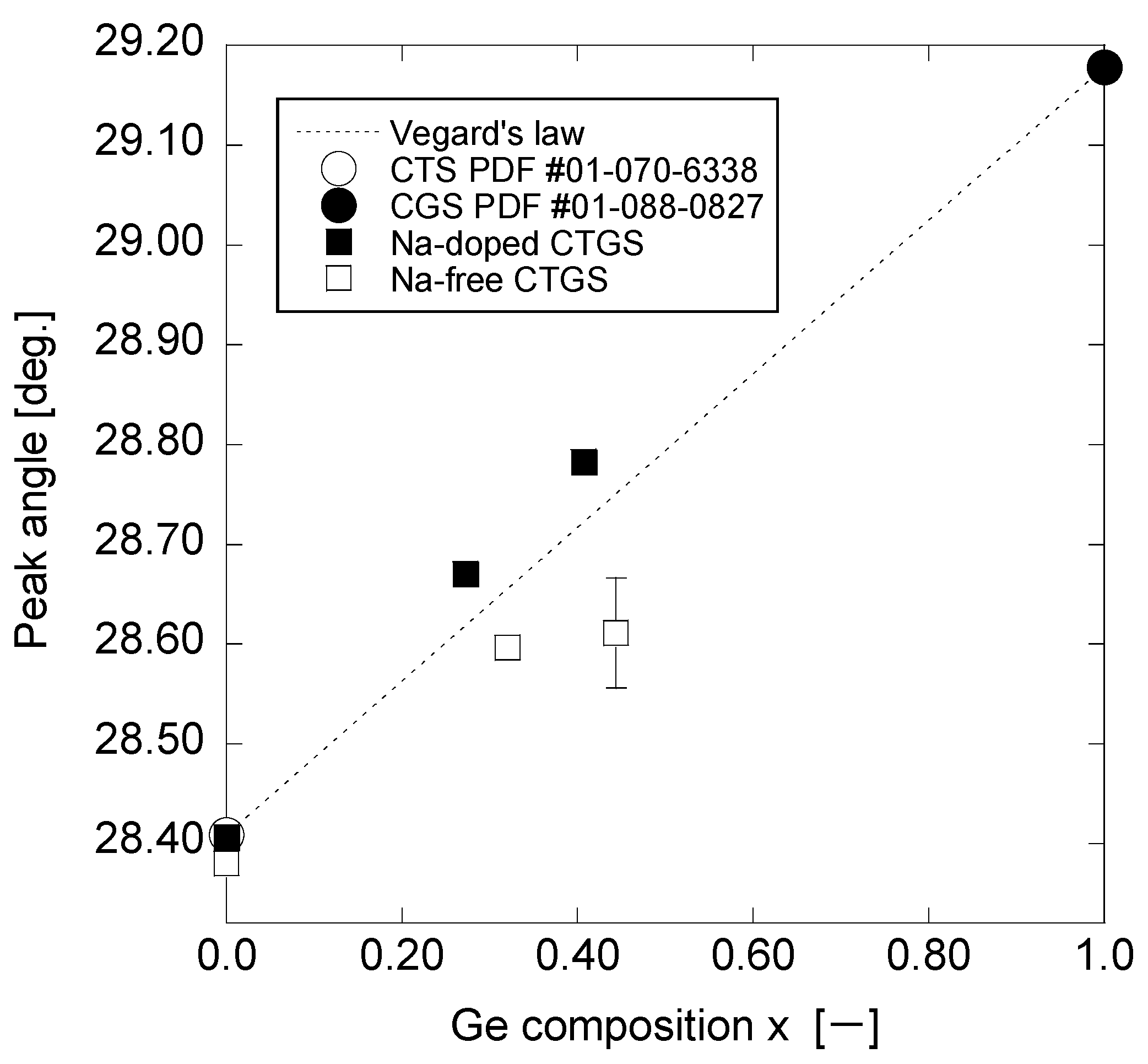
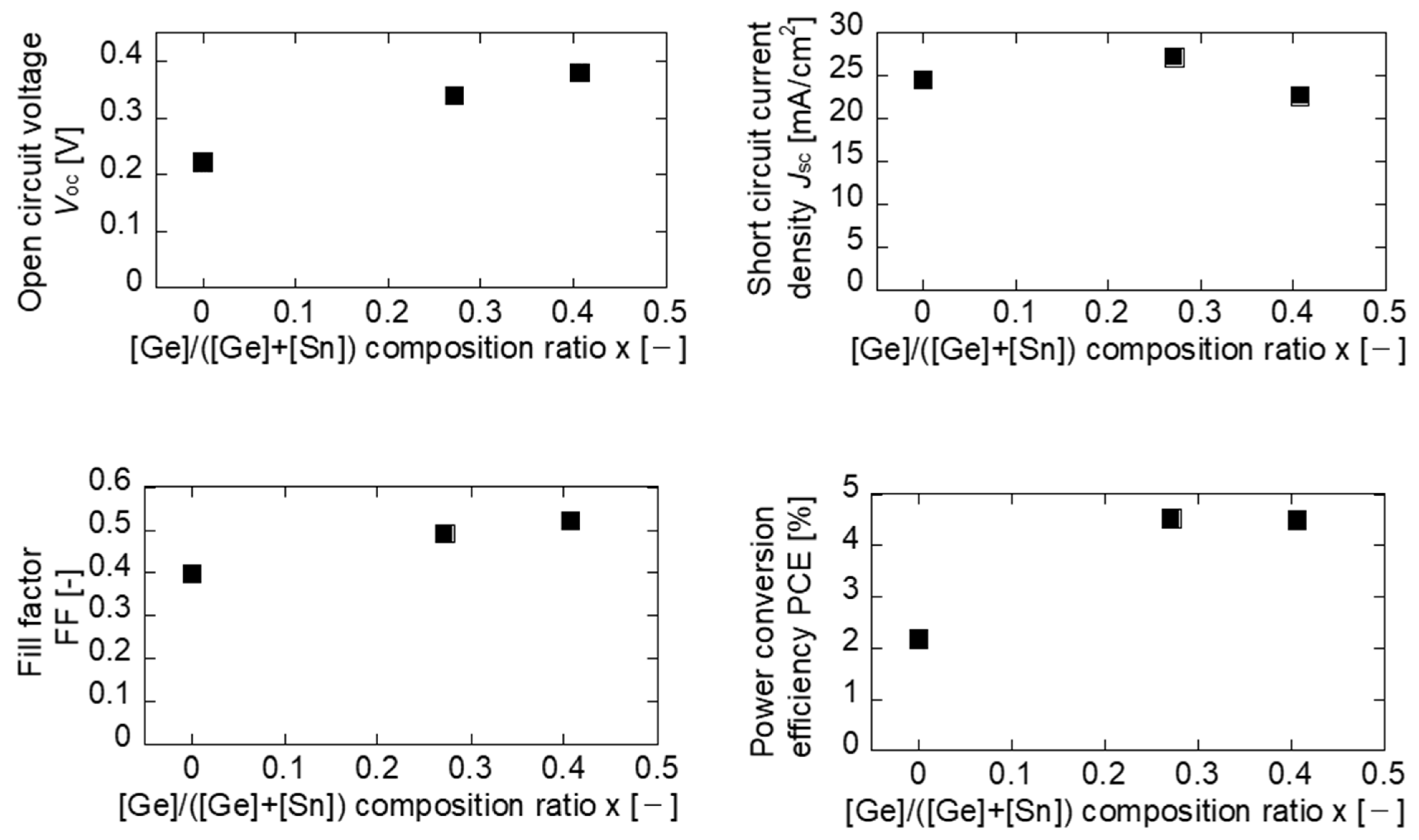
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aihara, N.; Araki, H.; Takeuchi, A.; Jimbo, K.; Katagiri, H. Fabrication of Cu2SnS3 thin films by sulfurization of evaporated Cu-Sn precursors for solar cells. Phys. Status Solidi C 2013, 10, 1086–1092. [Google Scholar] [CrossRef]
- De Chalbaud, L.M.; de Delgado, G.D.; Delgado, J.M. Synthesis and single-crystal structural study of Cu2GeS3. Mater. Res. Bull. 1997, 32, 1371–1376. [Google Scholar] [CrossRef]
- Chen, Q.; Maeda, T.; Wada, T. Optical properties and electronic structures of Cu2SnS3, Cu2GeS3, and their solid solution Cu2(Ge,Sn)S3. Jpn. J. Appl. Phys. 2018, 57, 08RC20. [Google Scholar] [CrossRef]
- Araki, H.; Yamano, M.; Nishida, G.; Takeuchi, A.; Aihara, N.; Tanaka, K. Synthesis and characterization of Cu2Sn1-xGexS3. Phys. Status Solidi C 2017, 10, 1600199. [Google Scholar] [CrossRef]
- Fujita, R.; Saito, N.; Kosugi, K.; Tanaka, K. Preparation of Cu2Sn1-xGexS3 bulk single crystals by chemical vapor transport with iodine. J. Cryst. Growth 2018, 498, 258–262. [Google Scholar] [CrossRef]
- Aihara, N.; Tanaka, K. Photoluminescence characterization of Cu2Sn1-xGexS3 bulk single crystals. AIP Adv. 2018, 8, 095323. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, S.; Xiang, Y. Solvothermal synthesis of Cu2Zn(Sn1-xGex)S4 and Cu2(Sn1-xGex)S3 nanoparticles with tunable band gap energies. J. Alloys Comp. 2015, 640, 75–81. [Google Scholar] [CrossRef]
- Ghorpade, U.V.; Suryawanshi, M.P.; Shin, S.W.; Kim, I.; Ahn, S.K.; Yun, J.H.; Jeong, C.; Kolekar, S.S.; Kim, J.H. Colloidal wurtzite Cu2SnS3 (CTS) nanocrystals and their applications in solar cells. Chem. Mater. 2016, 28, 3308–3317. [Google Scholar] [CrossRef]
- Liu, H.; Xiang, L.; Gao, P.; Wang, D.; Yang, J.; Chen, X.; Li, S.; Shi, Y.; Gao, F.; Zhang, Y. Improvement strategies for stability and efficiency of perovskite solar cells. Nanomaterials 2022, 12, 3295. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.-H.; Jiang, Y.; Hu, J.-S. Instability of solution-processed perovskite films: Origin and mitigation strategies. Mater. Futures 2023, 2, 012102. [Google Scholar] [CrossRef]
- Chantana, J.; Tai, K.; Hayashi, H.; Nishimura, T.; Kawano, Y.; Minemoto, T. Investigation of carrier recombination of Na-doped Cu2SnS3 solar cell for its improved conversion efficiency of 5.1%. Sol. Energy Mater Sol. Cells 2020, 206, 110261. [Google Scholar] [CrossRef]
- Kanai, A.; Sugiyama, M. Na induction effects for J–V properties of Cu2SnS3 (CTS) solar cells and fabrication of a CTS solar cell over-5.2% efficiency. Sol. Energy Mater Sol. Cells 2021, 231, 111315. [Google Scholar] [CrossRef]
- Htay, M.T.; Mandokoro, T.; Seki, H.; Sakaizawa, T.; Momose, N.; Taishi, T.; Hashimoto, Y.; Ito, K. Influence of Ge composition in the Cu2Sn1-xGexS3 thin-film photovoltaic absorber prepared by sulfurization of laminated metallic precursor. Sol. Energy Mater. Sol. Cells 2015, 140, 312–319. [Google Scholar] [CrossRef]
- Hayashi, H.; Chantana, J.; Kawano, Y.; Nishimura, T.; Minemoto, T. Influence of Ge/(Ge+Sn) composition ratio in Cu2Sn1-xGexS3 thin-film solar cells on their physical properties and photovoltaic performances. Sol. Energy Mater Sol. Cells 2020, 208, 110382. [Google Scholar] [CrossRef]
- Umehara, M.; Takeda, Y.; Motohiro, T.; Sakai, T.; Awano, H.; Maekawa, R. Cu2Sn1-xGexS3(x = 0.17) Thin-film solar cells with high conversion efficiency of 6.0%. Appl. Phys. Express 2013, 6, 045501. [Google Scholar] [CrossRef]
- Umehara, M.; Tajima, S.; Aoki, Y.; Takeda, Y.; Motohiro, T. Cu2Sn1−xGexS3 solar cells fabricated with a graded bandgap structure. Appl. Phys. Express 2016, 9, 072301. [Google Scholar] [CrossRef]
- He, M.; Kim, J.; Suryawanshi, M.P.; Lokhande, A.C.; Gang, M.; Ghorpade, U.V.; Lee, D.S.; Kim, J.H. Influence of sulfurization temperature on photovoltaic properties of Ge alloyed Cu2SnS3 (CTGS) thin film solar cells. Sol. Energy Mater. Sol. Cells 2018, 174, 94–101. [Google Scholar] [CrossRef]
- Malaquias, J.C.; Wu, M.; Lin, J.; Robert, E.V.C.; Sniekers, J.; Binnemans, K.; Dale, P.J.; Fransaer, J. Electrodeposition of germanium-containing precursors for Cu2(Sn,Ge)S3 thin film solar cells. Electrochim. Acta 2017, 251, 651–659. [Google Scholar] [CrossRef]
- Araki, H.; Chino, K.; Kimura, K.; Aihara, N.; Jimbo, K.; Katagiri, H. Fabrication of Cu2GeS3-based thin film solar cells by sulfurization of Cu/Ge stacked precursors. Jpn. J. Appl. Phys. 2014, 53, 05FW10. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, L.; Jiang, G.; Liu, W.; Zhu, C. High open-circuit voltage of ternary Cu2GeS3 thin film solar cells from combustion synthesized Cu-Ge alloy. Sol. Energy Mater. Sol. Cells 2017, 160, 319–327. [Google Scholar] [CrossRef]
- Kanai, A.; Araki, H.; Takeuchi, A.; Katagiri, H. Annealing temperature dependence of photovoltaic properties of solar cells containing Cu2SnS3 thin films produced by co-evaporation. Phys. Status Solidi B 2015, 252, 1239–1243. [Google Scholar] [CrossRef]
- Kanai, A.; Toyonaga, K.; Chino, K.; Katagiri, H.; Hideaki, A. Fabrication of Cu2SnS3 thin-film solar cells with power conversion efficiency of over 4%. Jpn. J. Appl. Phys. 2015, 54, 08KC06. [Google Scholar] [CrossRef]
- Sasagawa, S.; Nishida, G.; Takeuchi, A.; Katagiri, H.; Hideaki, A. Effect of sodium addition on Cu2SnS3 thin-film solar cells fabricated on alkali-free glass substrates. Jpn. J. Appl. Phys. 2018, 57, 08RC11. [Google Scholar] [CrossRef]
- Sasagawa, S.; Yago, A.; Kanai, A.; Araki, H. Cu2(Sn1-xGex)S3 solar cells prepared via co-evaporation and annealing in germanium sulfide and sulfur vapor. Phys. Status Solidi C 2017, 14, 1600193. [Google Scholar] [CrossRef]
- Kanai, A.; Araki, H.; Ohashi, R.; Sugiyama, M. Sulfurization of Cu2(Sn,Ge)S3 thin films deposited by co-evaporation. Jpn. J. Appl. Phys. 2020, 59, SCCD01. [Google Scholar] [CrossRef]
- Green, M.A.; Dunlop, E.D.; Yoshita, M.; Kopidakis, N.; Bothe, K.; Siefer, G.; Hao, X. Solar cell efficiency tables (Version 63). Prog. Photovolt. 2024, 32, 3–13. [Google Scholar] [CrossRef]
- Scarpulla, M.A.; McCandless, B.; Phillips, A.B.; Yan, Y.; Heben, M.J.; Wolden, C.; Xiong, G.; Metzger, W.K.; Mao, D.; Krasikov, D.; et al. CdTe-based thin film photovoltaics: Recent advances, current challenges and future prospects. Sol. Energy Mater. Sol. Cells 2023, 255, 112289. [Google Scholar] [CrossRef]
- Huang, C.-H.; Chuang, W.-J.; Lin, C.-P.; Jan, Y.-L.; Shih, Y.-C. Deposition technologies of high-efficiency CIGS solar cells: Development of two-step and co-evaporation processes. Crystals 2018, 8, 296. [Google Scholar] [CrossRef]
- Hamamura, K.; Chantana, J.; Suzuki, K.; Minemoto, T. Influence of Cu/(Ge + Sn) composition ratio on photovoltaic performances of Cu2Sn1-xGexS3 solar cell. Sol. Energy 2017, 149, 341–346. [Google Scholar] [CrossRef]
- Nikolaev, R.E.; Vasilyeva, I.G. A new way of phase identification, of AgGaGeS4∙nGeS2 crystals. J. Solid State Chem. 2013, 203, 340. [Google Scholar] [CrossRef]
- Piacente, V.; Foglia, S.; Scardala, P. Sublimation study of the tin sulphides SnS2, Sn2S3 and SnS. J. Alloys Comp. 1991, 177, 17–30. [Google Scholar] [CrossRef]
- Ye, Q.; Xu, D.; Cai, B.; Lu, J.; Yi, H.; Ma, C.; Zheng, Z.; Yao, J.; Ouyang, G.; Yang, G. High-performance hierarchical O-SnS/I-ZnIn2S4 photodetectors by leveraging the synergy of optical regulation and band tailoring. Mater. Horiz. 2022, 9, 2364–2375. [Google Scholar] [CrossRef] [PubMed]
- Pyeon, J.J.; Baek, I.-H.; Song, Y.G.; Kim, G.S.; Cho, A.-J.; Lee, G.-Y.; Han, J.H.; Chung, T.-M.; Hwang, C.S.; Kang, C.-Y.; et al. Highly sensitive flexible NO2 sensor composed of vertically aligned 2D SnS2 operating at room temperature. J. Mater. Chem. C 2020, 8, 11874–11881. [Google Scholar] [CrossRef]
- Suzuki, K.; Chantana, J.; Minemoto, T. Na role during sulfurization of NaF/Cu/SnS2 stacked precursor for formation of Cu2SnS3 thin film as absorber of solar cell. Appl. Surf. Sci. 2017, 414, 140–146. [Google Scholar] [CrossRef]




| Precursor | Method | [Ge]/([Ge] + [Sn]) | PCE [%] | Ref. |
|---|---|---|---|---|
| Soda lime glass (SLG)/Mo/Cu-SnS2/NaF | Sputtering and annealing with S and SnS | 0 | 5.1 | [11] |
| SLG/Mo/Cu/SnS2/NaF | Sputtering and annealing with S | 0 | 5.2 | [12] |
| SLG/Mo/Ge, Sn, and Cu laminated layers | Sputtering and annealing with S in a closed tube | 0–1.0 | ~2 | [13] |
| SLG/Mo/Ge/Cu–SnS2 | Sputtering and annealing with S and SnS2 | 0–0.58 | 5.6 | [14] |
| SLG/Mo/Cu–Sn | Co-sputtering and annealing with S and GeS2 | 0, 0.17 | 6.0 | [15] |
| SLG/Mo/Cu–Sn | Co-sputtering and annealing with S and GeS2 | Graded band gap structure | 6.7 | [16] |
| SLG/Mo/Sn/Ge/Cu | Sputtering and annealing with S | 0.061–0.110 | 2.14 | [17] |
| SLG/Mo/Cu(Sn,Ge) | Electrodeposition and annealing with S and GeS | 0.83 | 0.7 | [18] |
| SLG/Mo/Cu/Ge | Evaporation and annealing with S | 1.0 | 1.70 | [19] |
| SLG/Mo/Cu–Ge | Annealing of Cu-Ge alloy prepared by combustion method with S | 1.0 | 2.67 | [20] |
| tCGS [h] | [Cu]/([Ge] + [Sn]) | [Ge]/([Ge] + [Sn]) x | [S]/([Cu] + [Ge] + [Sn]) | |
|---|---|---|---|---|
| CGS/CTS precursor | 0.0 | 1.58 | 0.00 | 1.07 |
| 1.0 | 1.67 | 0.33 | 1.00 | |
| 1.5 | 1.68 | 0.45 | 0.98 | |
| Na-doped CTGS | 0.0 | 1.60 | 0.00 | 1.15 |
| 1.0 | 1.91 | 0.27 | 1.26 | |
| 1.5 | 1.94 | 0.41 | 1.16 | |
| Na-free CTGS | 0.0 | Peeled off | Peeled off | Peeled off |
| 1.0 | 1.80 | 0.32 | 1.07 | |
| 1.5 | 1.81 | 0.44 | 1.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tasaki, T.; Jimbo, K.; Motai, D.; Takahashi, M.; Araki, H. Fabrication of Cu2Sn1-xGexS3 Thin-Film Solar Cells via Sulfurization of Cu2GeS3/Cu2SnS3 Stacked Precursors. Materials 2024, 17, 1886. https://doi.org/10.3390/ma17081886
Tasaki T, Jimbo K, Motai D, Takahashi M, Araki H. Fabrication of Cu2Sn1-xGexS3 Thin-Film Solar Cells via Sulfurization of Cu2GeS3/Cu2SnS3 Stacked Precursors. Materials. 2024; 17(8):1886. https://doi.org/10.3390/ma17081886
Chicago/Turabian StyleTasaki, Takeshi, Kazuo Jimbo, Daiki Motai, Masaya Takahashi, and Hideaki Araki. 2024. "Fabrication of Cu2Sn1-xGexS3 Thin-Film Solar Cells via Sulfurization of Cu2GeS3/Cu2SnS3 Stacked Precursors" Materials 17, no. 8: 1886. https://doi.org/10.3390/ma17081886





