Effect of Ni Doping on the Thermoelectric Properties of YbCo2Zn20
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Synthesis
2.2. Sample Characterization
3. Results and Discussion
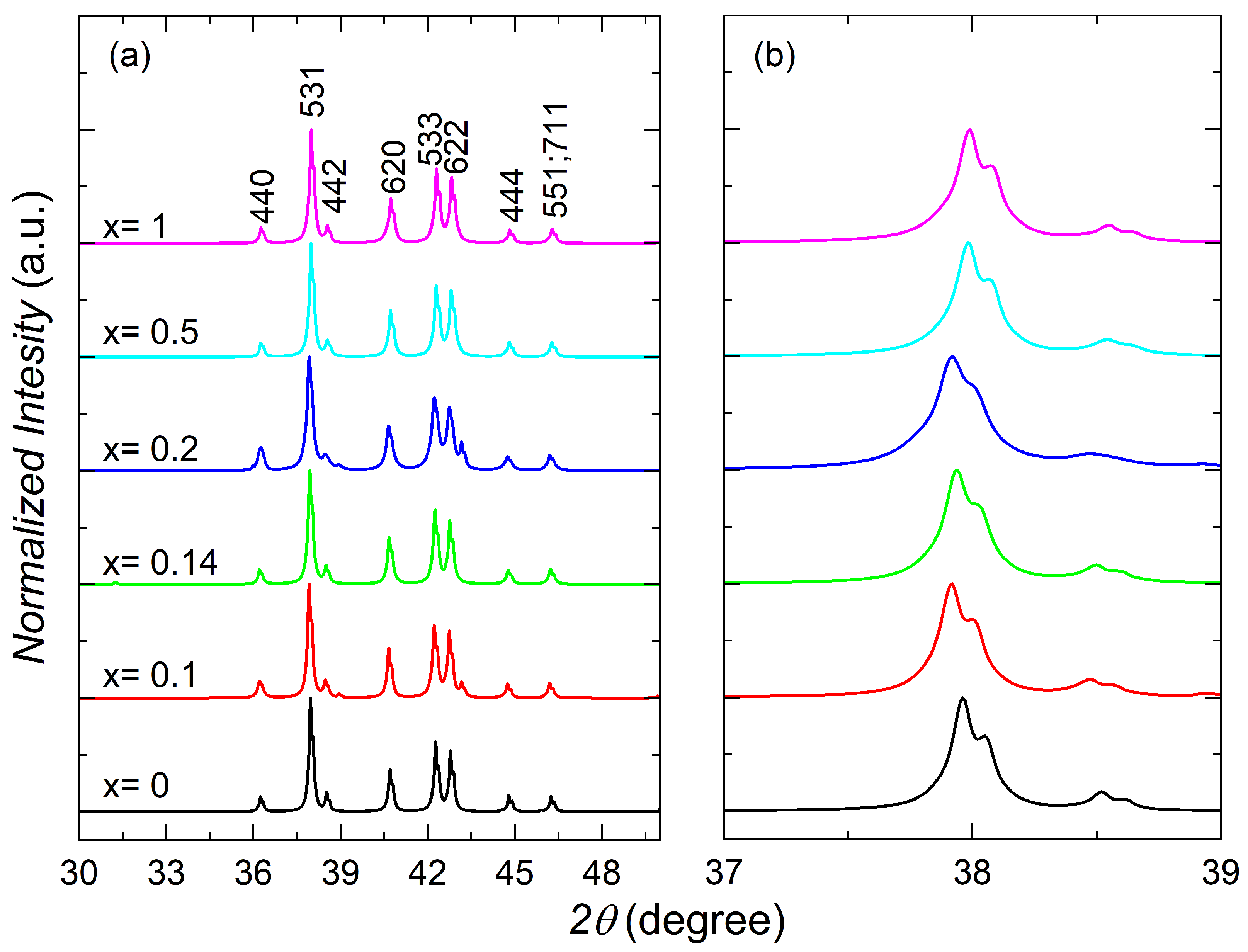
3.1. Structural Characterization
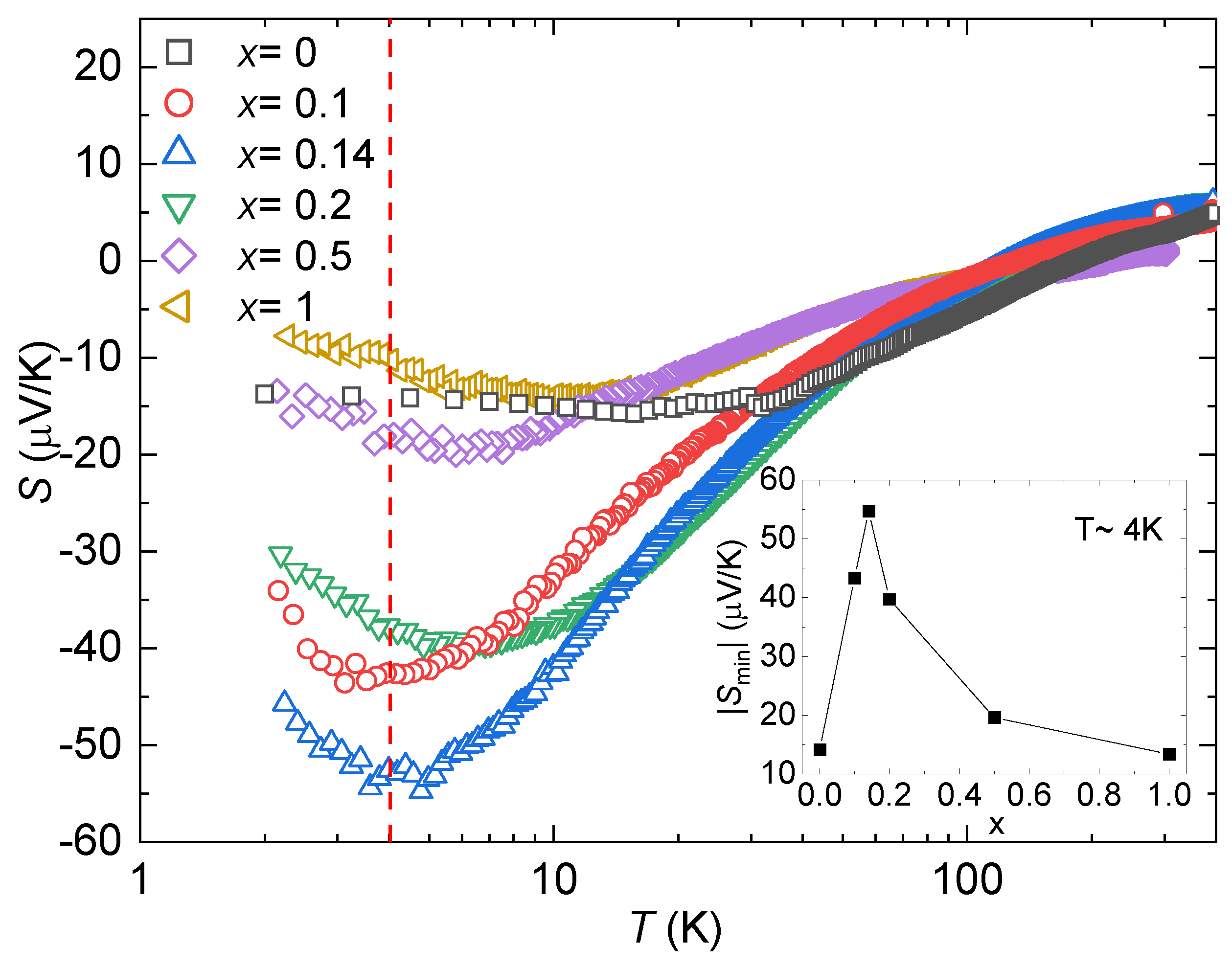
3.2. Electrical Transport and Thermoelectric Properties
3.3. Magnetic and Heat Capacity Properties
3.4. Hall Effect
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Galeano-Cabral, J.R.; Porto-Hernandez, L.; Vargas, J.; Ordonez, J. Exergetic Optimization of an Integrated Municipal Solid Waste Incinerator and Wastewater Treatment Plant. Int. J. Energy Clean Environ. 2022, 23, 95–108. [Google Scholar] [CrossRef]
- Porto-Hernandez, L.; Vargas, J.; Munoz, M.; Galeano-Cabral, J.; Ordonez, J.; Balmant, W.; Mariano, A. Fundamental optimization of steam Rankine cycle power plants. Energy Convers. Manag. 2023, 289, 117148. [Google Scholar] [CrossRef]
- Hsu, C.T.; Huang, G.Y.; Chu, H.S.; Yu, B.; Yao, D.J. Experiments and simulations on low-temperature waste heat harvesting system by thermoelectric power generators. Appl. Energy 2011, 88, 1291–1297. [Google Scholar] [CrossRef]
- Kumar, S.; Heister, S.D.; Xu, X.; Salvador, J.R. Optimization of thermoelectric components for automobile waste heat recovery systems. J. Electron. Mater. 2015, 44, 3627–3636. [Google Scholar] [CrossRef]
- Saqr, K.M.; Mansour, M.K.; Musa, M. Thermal design of automobile exhaust based thermoelectric generators: Objectives and challenges. Int. J. Automot. Technol. 2008, 9, 155–160. [Google Scholar] [CrossRef]
- Chein, R.; Huang, G. Thermoelectric cooler application in electronic cooling. Appl. Therm. Eng. 2004, 24, 2207–2217. [Google Scholar] [CrossRef]
- Simons, R.; Chu, R. Application of thermoelectric cooling to electronic equipment: A review and analysis. In Proceedings of the Sixteenth Annual IEEE Semiconductor Thermal Measurement and Management Symposium (Cat. No. 00CH37068), San Jose, CA, USA, 23 March 2000; pp. 1–9. [Google Scholar]
- Wang, P.; Bar-Cohen, A. On-chip hot spot cooling using silicon thermoelectric microcoolers. J. Appl. Phys. 2007, 102, 034503. [Google Scholar] [CrossRef]
- Hasan, M.N.; Nafea, M.; Nayan, N.; Mohamed Ali, M.S. Thermoelectric generator: Materials and applications in wearable health monitoring sensors and internet of things devices. Adv. Mater. Technol. 2022, 7, 2101203. [Google Scholar] [CrossRef]
- Parás-Hernández, F.; Fabián-Mijangos, A.; Cardona-Castro, M.; Alvarez-Quintana, J. Enhanced performance nanostructured thermoelectric converter for self-powering health sensors. Nano Energy 2020, 74, 104854. [Google Scholar] [CrossRef]
- Rowe, D. Applications of nuclear-powered thermoelectric generators in space. Appl. Energy 1991, 40, 241–271. [Google Scholar] [CrossRef]
- Dai, Z.; Wang, C.; Zhang, D.; Tian, W.; Qiu, S.; Su, G. Thermoelectric characteristics analysis of thermionic space nuclear power reactor. Int. J. Energy Res. 2020, 44, 855–868. [Google Scholar] [CrossRef]
- Ordonez, J.C.; Ordonez, C. Thermoelectric insulation for cold temperature vaccine storage. In Proceedings of the 2021 IEEE Conference on Technologies for Sustainability (SusTech), Virtual, 22–24 April 2021; pp. 1–5. [Google Scholar]
- Ohara, B.; Sitar, R.; Soares, J.; Novisoff, P.; Nunez-Perez, A.; Lee, H. Optimization strategies for a portable thermoelectric vaccine refrigeration system in developing communities. J. Electron. Mater. 2015, 44, 1614–1626. [Google Scholar] [CrossRef]
- Mahan, G.; Sofo, J. The best thermoelectric. Proc. Natl. Acad. Sci. USA 1996, 93, 7436–7439. [Google Scholar] [CrossRef] [PubMed]
- Nolas, G.S.; Sharp, J.; Goldsmid, J. Thermoelectrics: Basic Principles and New Materials Developments; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2001; Volume 45. [Google Scholar]
- Morelli, D.T. Thermoelectric materials. In Springer Handbook of Electronic and Photonic Materials; Kasap, S., Capper, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; p. 1. [Google Scholar]
- Chaikin, P. An introduction to thermopower for those who might want to use it to study organic conductors and superconductors. In Organic Superconductivity; Springer: Berlin/Heidelberg, Germany, 1990; pp. 101–115. [Google Scholar]
- Tritt, T. Thermoelectric materials: Principles, structure, properties, and applications. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2002; pp. 1–11. [Google Scholar]
- Bauer, E. Kondo Systems and Heavy Fermions: Transport Phenomena. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 4372–4381. [Google Scholar]
- Kittel, C.; McEuen, P.; McEuen, P. Introduction to Solid State Physics; Wiley: New York, NY, USA, 1996; Volume 8. [Google Scholar]
- Nowotny, H.; Gratz, E. Boltzmann Equation and Scattering Mechanisms. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.T.; Zhou, X.M.; Zhen, Y.; Chen, N.Y.; Xu, H.; Zhu, J.Z. The prediction and synthesis of some new intermetallic compounds between transition metals and rare earth metals. J. Less Common Met. 1989, 147, 167–173. [Google Scholar] [CrossRef]
- Brooks, M.; Nordström, L.; Johansson, B. Rare-earth transition-metal intermetallics. Phys. B Condens. Matter 1991, 172, 95–100. [Google Scholar] [CrossRef]
- Gignoux, D.; Schmitt, D. Rare earth intermetallics. J. Magn. Magn. Mater. 1991, 100, 99–125. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, R.; Ahmad, I. Physical properties and possible applications of gold-based rare earth intermetallics (R-Au): A review. J. Magn. Magn. Mater. 2019, 490, 165477. [Google Scholar] [CrossRef]
- Zada, Z.; Khan, J.; Khan, A.A.; Reshak, A.H.; Ali, D.; Rehman, F.U.; Urrahman, I.; Saqib, M.; Irfan, M.; Ramli, M.M. Structural, Thermoelectric, Electronic, and Magnetic Properties of Pristine Intermetallic Rare-Earth-Based XMn2Si2 (X= Dy, Er) Compounds. ECS J. Solid State Sci. Technol. 2023, 12, 043012. [Google Scholar] [CrossRef]
- Pawar, H.; Aynyas, M.; Sanyal, S.P. Thermoelectric properties of rare-earth based RENi2 (RE= Dy, Ho and Er) Laves phase compounds. J. Magn. Magn. Mater. 2018, 468, 123–131. [Google Scholar] [CrossRef]
- Nasch, T.; Jeitschko, W.; Rodewald, U.C. Ternary rare earth transition metal zinc compounds RT2Zn20 with T= Fe, Ru, Co, Rh, and Ni. Z. Naturforsch. B 1997, 52, 1023–1030. [Google Scholar] [CrossRef]
- Mun, E.; Jia, S.; Bud’ko, S.L.; Canfield, P.C. Thermoelectric power of the YbT2Zn20 (T= Fe, Ru, Os, Ir, Rh, and Co) heavy fermions. Phys. Rev. B 2012, 86, 115110. [Google Scholar] [CrossRef]
- Wei, K.; Neu, J.N.; Lai, Y.; Chen, K.W.; Hobbis, D.; Nolas, G.S.; Graf, D.E.; Siegrist, T.; Baumbach, R.E. Enhanced thermoelectric performance of heavy-fermion compounds YbTM2Zn20 (TM= Co, Rh, Ir) at low temperatures. Sci. Adv. 2019, 5, eaaw6183. [Google Scholar] [CrossRef] [PubMed]
- Galeano-Cabral, J.R.; Karr, E.; Schundelmier, B.; Oladehin, O.; Choi, E.S.; Siegrist, T.; Ordonez, J.; Shastri, S.; Petkov, V.; Baumbach, R.E.; et al. Enhanced thermoelectric properties of heavy-fermion compounds YbxCeySmzIr2Zn20 (x + y + z = 1). Phys. Rev. Mater. 2023, 7, 025406. [Google Scholar] [CrossRef]
- Lenoir, B.; Cassart, M.; Michenaud, J.P.; Scherrer, H.; Scherrer, S. Transport properties of Bi-rich Bi-Sb alloys. J. Phys. Chem. Solids 1996, 57, 89–99. [Google Scholar] [CrossRef]
- Doroshenko, A.; Rogacheva, E.; Drozdova, A.; Martynova, K.; Men’shov, Y.V. Thermoelectric properties of polycrystalline Bi1−xSbx solid solutions in the concentration range x= 0–0.25. J. Thermoelectr. 2016, 4, 23–36. [Google Scholar]
- Saleemi, M.; Tafti, M.Y.; Jacquot, A.; Jägle, M.; Johnsson, M.; Toprak, M.S. Chemical synthesis of iron antimonide (FeSb2) and its thermoelectric properties. Inorg. Chem. 2016, 55, 1831–1836. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.Y.; Hogan, T.P.; Rocci-Lane, M.; Brazis, P.; Ireland, J.R.; Kannewurf, C.R.; Bastea, M.; Uher, C.; Kanatzidis, M.G. A new thermoelectric material: CsBi4Te6. J. Am. Chem. Soc. 2004, 126, 6414–6428. [Google Scholar] [CrossRef]
- Rowe, D.; Kuznetsov, V.; Kuznetsova, L.; Min, G. Electrical and thermal transport properties of intermediate-valence YbAl3. J. Phys. D Appl. Phys. 2002, 35, 2183. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- US Geological Survey (USGS). Mineral Commodity Summaries 2020; US Geological Survey: Reston, VA, USA, 2020. [Google Scholar]
- Van den Brink, S.; Kleijn, R.; Sprecher, B.; Tukker, A. Identifying supply risks by mapping the cobalt supply chain. Resour. Conserv. Recycl. 2020, 156, 104743. [Google Scholar] [CrossRef]
- Al-Omari, I.; Skomski, R.; Thomas, R.; Leslie-Pelecky, D.; Sellmyer, D.J. High-temperature magnetic properties of mechanically alloyed SmCo5 and YCo5 magnets. IEEE Trans. Magn. 2001, 37, 2534–2536. [Google Scholar] [CrossRef]
- Torikachvili, M.; Jia, S.; Mun, E.; Hannahs, S.; Black, R.; Neils, W.; Martien, D.; Bud’Ko, S.; Canfield, P. Six closely related YbT2Zn20 (T= Fe, Co, Ru, Rh, Os, Ir) heavy fermion compounds with large local moment degeneracy. Proc. Natl. Acad. Sci. USA 2007, 104, 9960–9963. [Google Scholar] [CrossRef] [PubMed]
- Canfield, P.C.; Kong, T.; Kaluarachchi, U.S.; Jo, N.H. Use of frit-disc crucibles for routine and exploratory solution growth of single crystalline samples. Philos. Mag. Lett. 2016, 96, 84–92. [Google Scholar] [CrossRef]
- Florida State University. FSU: Biological Science Imaging Resource. Available online: https://bsir.bio.fsu.edu/fei-nova-400-nanosem (accessed on 12 April 2024).
- Swatek, P.; Daszkiewicz, M.; Kaczorowski, D. Crystal structure of the new compound UOs2Zn20. J. Alloys Compd. 2014, 586, 754–756. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Fujino, T.; Ito, K.; Nakamura, M.; Yoshizawa, M.; Saiga, Y.; Kosaka, M.; Uwatoko, Y. Elastic constants of the single crystalline Yb based heavy-fermion compound YbCo2Zn20. Phys. Rev. B 2009, 80, 184418. [Google Scholar] [CrossRef]
- Burnett, V.; Yazici, D.; White, B.; Dilley, N.; Friedman, A.; Brandom, B.; Maple, M. Structure and physical properties of RT2Cd20 (R= rare earth, T= Ni, Pd) compounds with the CeCr2Al20-type structure. J. Solid State Chem. 2014, 215, 114–121. [Google Scholar] [CrossRef]
- Niemann, S.; Jeitschko, W. Ternary aluminides AT2Al20 (A= rare earth elements and uranium; T= Ti, Nb, Ta, Mo, and W) with CeCr2Al20-type structure. J. Solid State Chem. 1995, 114, 337–341. [Google Scholar] [CrossRef]
- Slater, J.C. Atomic radii in crystals. J. Chem. Phys. 1964, 41, 3199–3204. [Google Scholar] [CrossRef]
- Jang, S.; Denlinger, J.; Allen, J.; Zapf, V.; Maple, M.; Kim, J.N.; Jang, B.G.; Shim, J.H. Evolution of the Kondo lattice electronic structure above the transport coherence temperature. Proc. Natl. Acad. Sci. USA 2020, 117, 23467–23476. [Google Scholar] [CrossRef] [PubMed]
- Lavagna, M.; Lacroix, C.; Cyrot, M. Resistivity of the Kondo lattice. J. Phys. F Met. Phys. 1982, 12, 745. [Google Scholar] [CrossRef]
- Yang, Y.f.; Fisk, Z.; Lee, H.O.; Thompson, J.; Pines, D. Scaling the Kondo lattice. Nature 2008, 454, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Mugiraneza, S.; Hallas, A.M. Tutorial: A beginner’s guide to interpreting magnetic susceptibility data with the Curie-Weiss law. Commun. Phys. 2022, 5, 95. [Google Scholar] [CrossRef]
- Batyev, E.G. Pauli paramagnetism and Landau diamagnetism. Physics-Uspekhi 2009, 52, 1245. [Google Scholar] [CrossRef]
- Van Den Handel, J. Paramagnetism. In Advances in Electronics and Electron Physics; Elsevier: Amsterdam, The Netherlands, 1954; Volume 6, pp. 463–518. [Google Scholar]
- Kittel, C. Introduction to Solid State Physics, 8th ed.; John Wiley & Sons: Hoboken, NJ. USA, 2004. [Google Scholar]
- Dalal, M. A Textbook of Inorganic Chemistry; Dalal Institute: Rohtak, India, 2017; Volume 1. [Google Scholar]
- Mitric, M.; Antic, B.; Balanda, M.; Rodic, D.; Napijalo, M.L. An X-ray diffraction and magnetic susceptibility study of YbxY2−xO3. J. Phys. Condens. Matter 1997, 9, 4103. [Google Scholar] [CrossRef]
- Besara, T.; Ramirez, D.; Sun, J.; Whalen, J.; Tokumoto, T.; McGill, S.; Singh, D.; Siegrist, T. Ba2TeO: A new layered oxytelluride. J. Solid State Chem. 2015, 222, 60–65. [Google Scholar] [CrossRef]
- Pengra, D.; Stoltenberg, J.; Van Dyck, R.; Vilches, O. The Hall Effect (Updated 19 June 2015). University of Washington. Available online: https://courses.washington.edu/phys431/hall_effect/hall_effect.pdf (accessed on 17 March 2024).
- Goswami, A.; Kanetkar, S. Thermoelectrics in cryogenic cooling. In Proceedings of the 2020 IEEE 22nd Electronics Packaging Technology Conference (EPTC), Singapore, 2–4 December 2020; pp. 355–358. [Google Scholar]
- Sidorenko, N.; Dashevsky, Z. Cryogenic thermoelectric cooler for operating temperatures below 90 K. Semiconductors 2019, 53, 752–755. [Google Scholar] [CrossRef]







| x | [cm] | [V/K] | [] | [] | [K] | [] | [K] | |
|---|---|---|---|---|---|---|---|---|
| 0 | 33.09 | 14.15 | 0.0024 | 0.005 | 4.63 | 4.60 | 348 | 244 |
| 0.1 | 30.48 | 43.35 | 0.0392 | 0.002 | 4.39 | −5.74 | 358 | 237 |
| 0.14 | 36.93 | 54.77 | 0.0816 | 0.001 | 4.40 | 1.05 | 334 | 243 |
| 0.2 | 35.23 | 39.72 | 0.0353 | 0.003 | 4.57 | −2.59 | 245 | 246 |
| 0.5 | 49.29 | 19.62 | 0.0095 | 0.001 | 4.55 | −9.34 | 267 | 244 |
| 1 | 49.17 | 13.41 | 0.0034 | 0.001 | 4.69 | 2.34 | 275 | 243 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galeano-Cabral, J.R.; Schundelmier, B.; Oladehin, O.; Feng, K.; Ordonez, J.C.; Baumbach, R.E.; Wei, K. Effect of Ni Doping on the Thermoelectric Properties of YbCo2Zn20. Materials 2024, 17, 1906. https://doi.org/10.3390/ma17081906
Galeano-Cabral JR, Schundelmier B, Oladehin O, Feng K, Ordonez JC, Baumbach RE, Wei K. Effect of Ni Doping on the Thermoelectric Properties of YbCo2Zn20. Materials. 2024; 17(8):1906. https://doi.org/10.3390/ma17081906
Chicago/Turabian StyleGaleano-Cabral, Jorge R., Benny Schundelmier, Olatunde Oladehin, Keke Feng, Juan C. Ordonez, Ryan E. Baumbach, and Kaya Wei. 2024. "Effect of Ni Doping on the Thermoelectric Properties of YbCo2Zn20" Materials 17, no. 8: 1906. https://doi.org/10.3390/ma17081906





