Characterization of Interfacial Corrosion Behavior of Hybrid Laminate EN AW-6082 ∪ CFRP
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
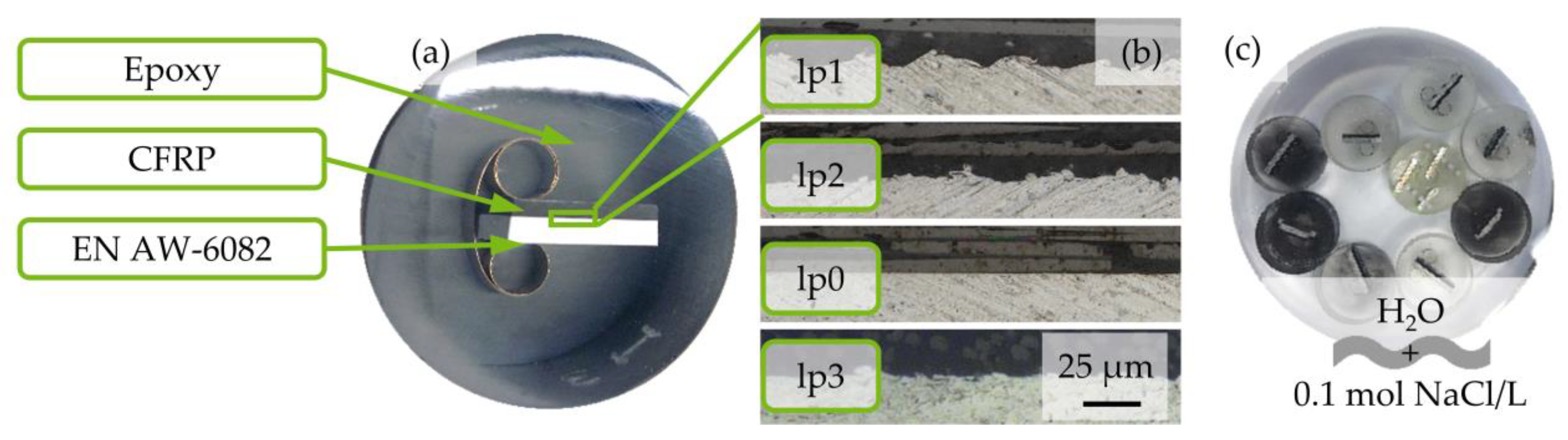
2.2. Specimen Preparation
2.3. Experimental Setup
2.4. Testing Method
3. Results
3.1. Specimen Dimensions
3.2. Weight Measurement
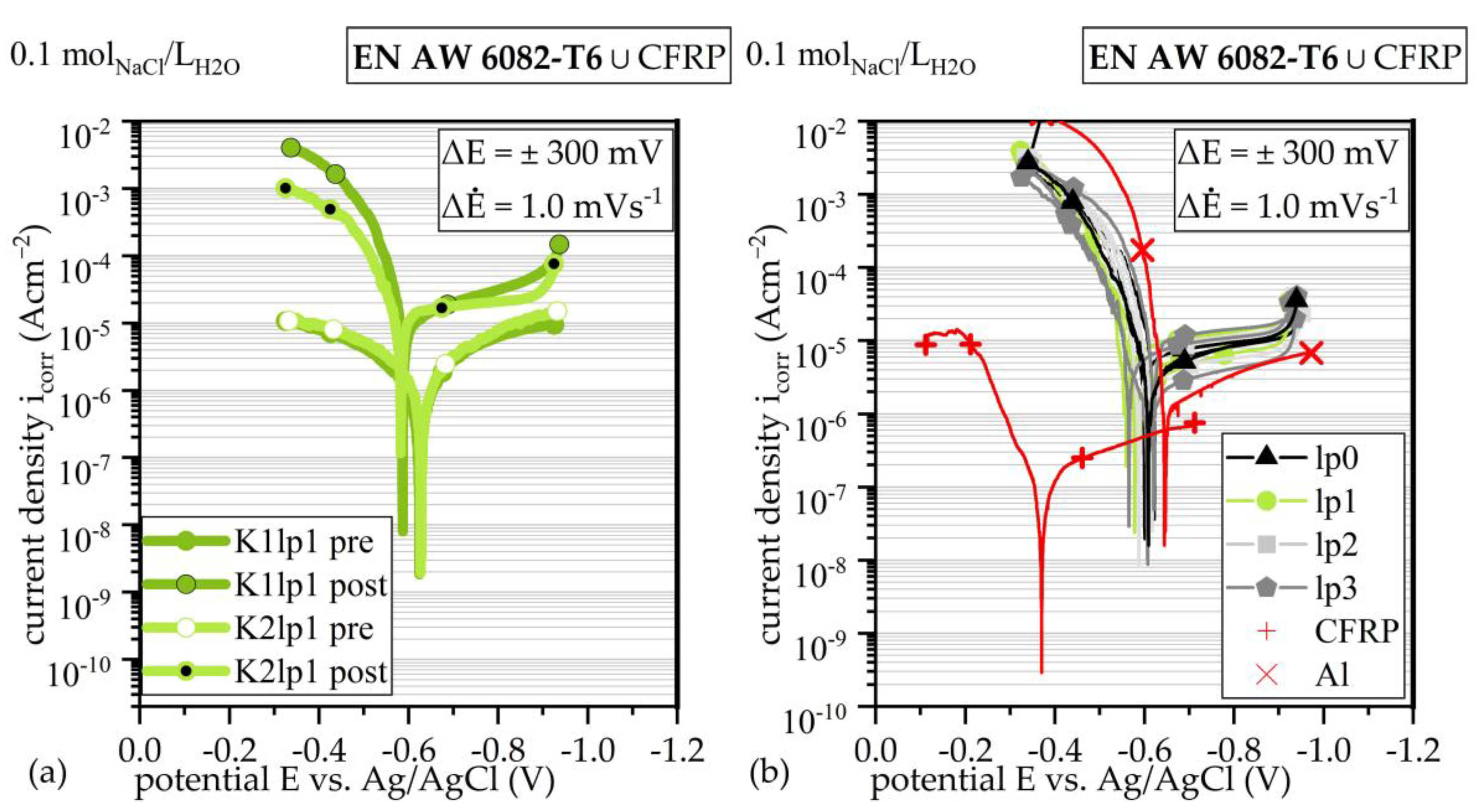
3.3. Linear Sweep Voltammetry
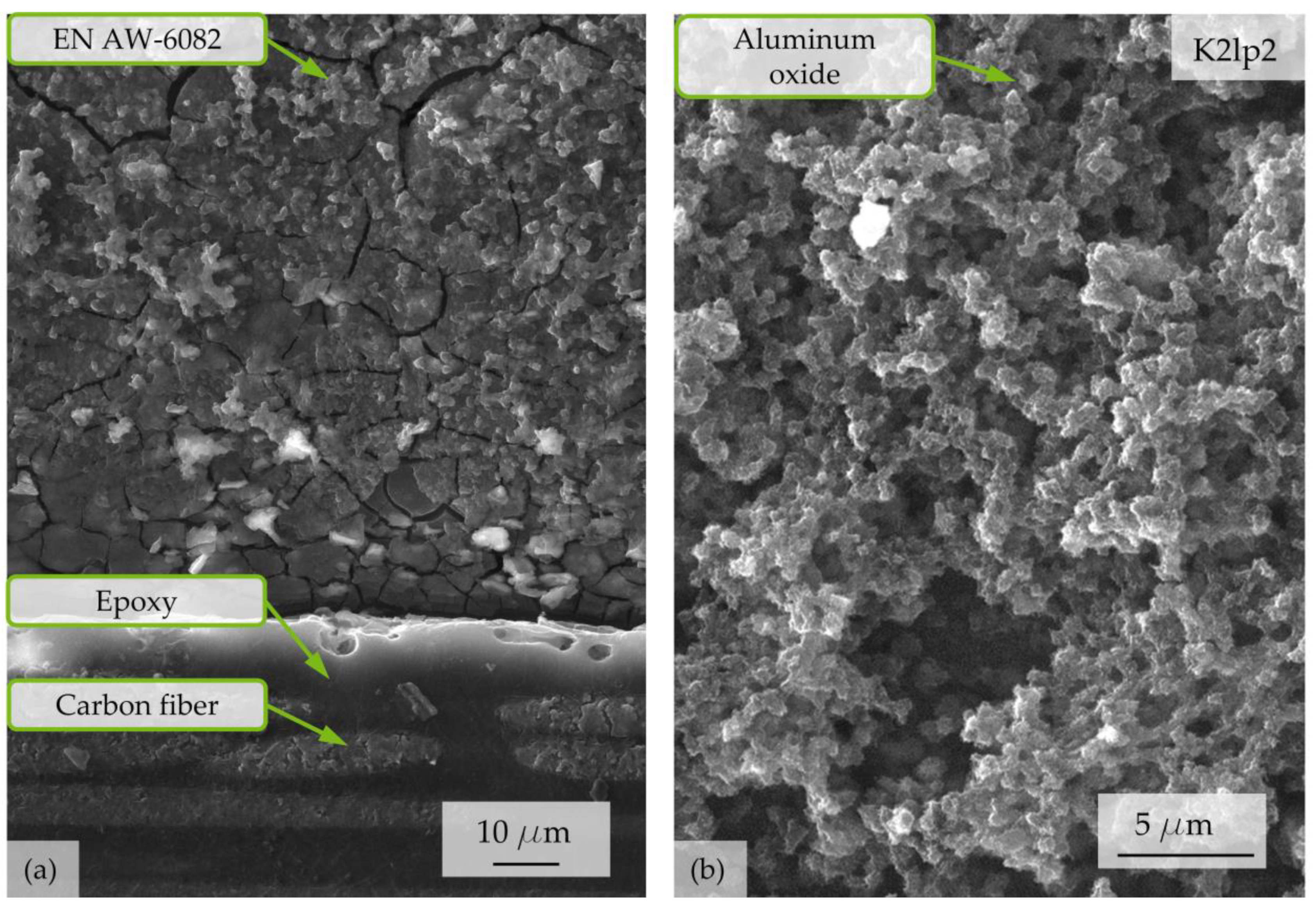
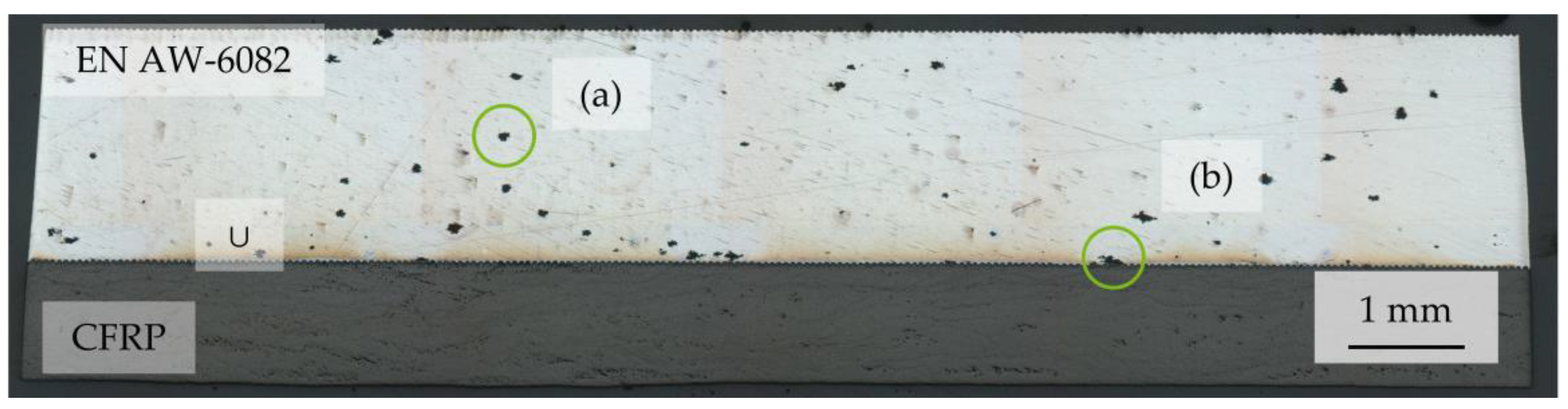
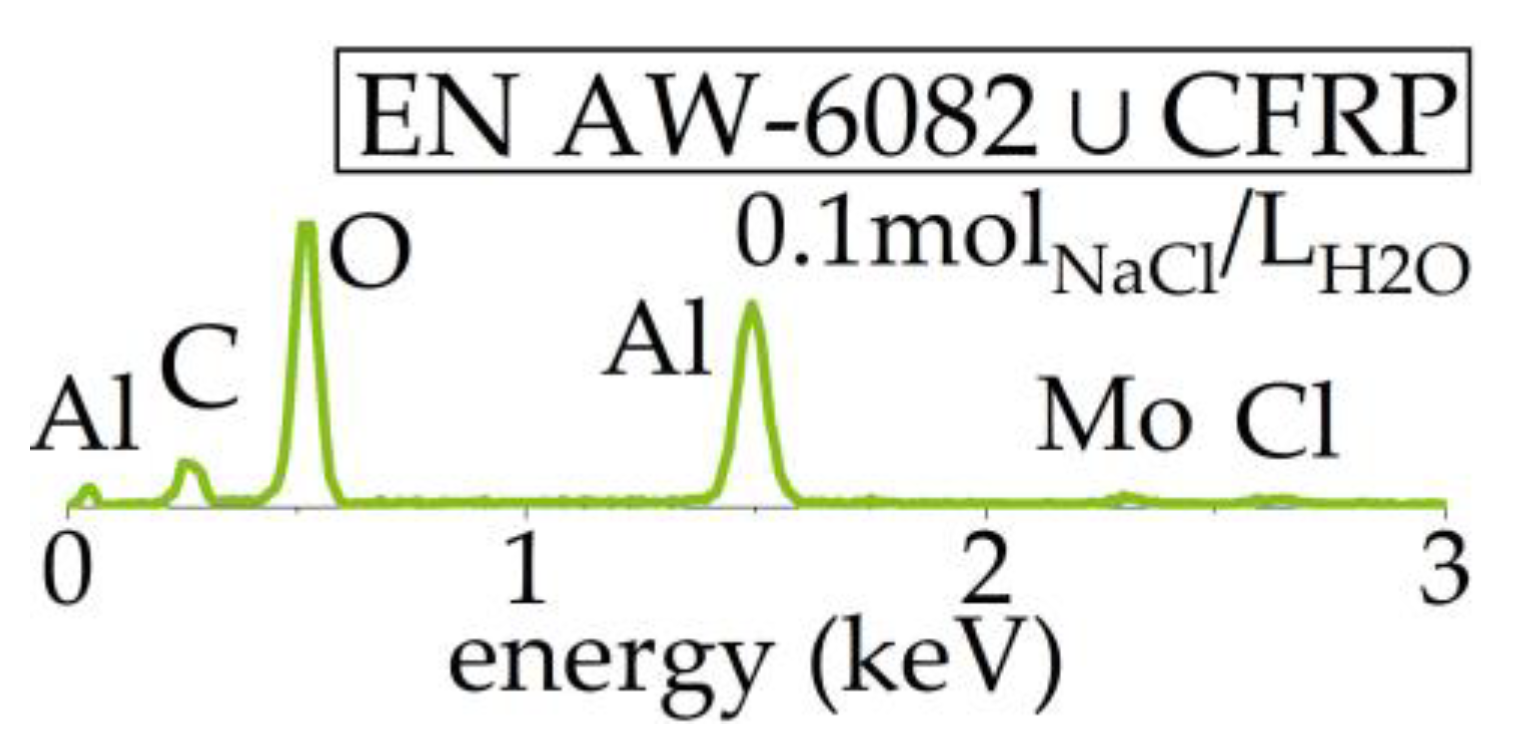
3.4. Microscopic Analyses
4. Discussion
5. Conclusions
- Galvanic coupling and passivation of the Al component, limited conductivity of the carbon fibers, and the random distribution of exposed fibers at specimen cut leads to high statistical scatter of the lsv measurement as well as uncertainties in the determination of the true surface and therefore limits the applicability of lsv for the hybrid material to qualitative comparisons.
- A continuous mass loss was detected during corrosion exposure tests and could be allocated to the direct contact region of the interface, proving the dominance of galvanic corrosion on the long-term corrosion evolution of EN AW-6082 ∪ CFRP, while the corrosion mechanism of pure EN AW-6082 under same condition was identified as pitting corrosion. The interface acts comparably to a sacrificial anode for the Al base material.
- Corrosion products were identified as aluminum oxides.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bader, B.; Türck, E.; Vietor, T. Multi material design. A current overview of the used potential in automotive industries. In Technologies for Economical and Functional Lightweight Design. Zukunftstechnologien für den Multifunktionalen Leichtbau; Springer: Berlin/Heidelberg, Germany, 2019; pp. 3–13. [Google Scholar] [CrossRef]
- Min, J.; Li, Y.; Li, J.; Carlson, B.E.; Lin, J. Friction stir blind riveting of carbon fiber reinforced polymer composite and aluminum alloy sheets. Int. J. Adv. Manuf. Technol. 2015, 76, 1403–1410. [Google Scholar] [CrossRef]
- Vermeeren, C.A.J.R. An historic overview of the development of fibre metal laminates. Appl. Compos. Mater. 2003, 10, 189–205. [Google Scholar] [CrossRef]
- Malekinejad, H.; Carbas, R.J.C.; Akhavan-Safar, A.; Marques, E.A.S.; Castro Sousa, F.; da Silva, L.F.M. Enhancing fatigue life and strength of adhesively bonded composite joints: A comprehensive review. Materials 2023, 16, 6468. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Basak, A.K.; Dong, Y.; Sarker, P.K.; Uddin, M.S.; Littlefair, G.; Dixit, A.R.; Chattopadhyaya, S. Jointing of carbon fibre reinforced polymer (CFRP) composites and aluminium alloys—A review. Compos. Part A Appl. Sci. Manuf. 2017, 101, 1–29. [Google Scholar] [CrossRef]
- Li, S.; Khan, H.A.; Hihara, L.H.; Cong, H.; Li, J. Corrosion behavior of friction stir blind riveted AL/CFRP and Mg/CFRP joints exposed to a marine environment. Corros. Sci. 2018, 132, 303–309. [Google Scholar] [CrossRef]
- Park, S.Y.; Choi, W.J.; Choi, H.S.; Kwon, H.; Kim, S.H. Recent trends in surface treatment technologies for airframe adhesive bonding processing: A review (1995–2008). J. Adhes. 2010, 86, 192–221. [Google Scholar] [CrossRef]
- Kwon, D.S.; Yoon, S.H.; Hwang, H.Y. Effects of residual oils on the adhesion characteristics of metal-CFRP adhesive joints. Compos. Struct. 2019, 207, 240–254. [Google Scholar] [CrossRef]
- Ireland, R.; Arronche, L.; Saponara, V.L. Electrochemical investigation of galvanic corrosion between aluminum 7075 and glass fibre/epoxy composites modified with carbon nanotubes. Compos. Part B Eng. 2012, 43, 183–194. [Google Scholar] [CrossRef]
- Xhanari, K.; Finšgar, M. The corrosion inhibition of AA6082 aluminium alloy by certain azoles in chloride solution: Electrochemistry and surface analysis. Coatings 2019, 9, 380. [Google Scholar] [CrossRef]
- Lumley, R. Fundamentals of Aluminium Metallurgy; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780081020647. [Google Scholar]
- Rechner, R.; Jansen, I.; Beyer, E. Influence on the strength and aging resistance of aluminium joints by laser pre-treatment and surface modification. Int. J. Adhes. 2010, 30, 595–601. [Google Scholar] [CrossRef]
- Heckert, A.; Zaeh, M.F. Laser surface pre-treatment of aluminium for hybrid joints with glass fibre reinforced thermoplastics. Phys. Procedia 2014, 56, 1171–1181. [Google Scholar] [CrossRef]
- Steinert, P.; Dittes, A.; Schimmelpfennig, R.; Scharf, I.; Lampke, T.; Schubert, A. Design of high strength polymer metal interfaces by laser microstructured surfaces. IOP Conf. Ser. Mater. Sci. Eng. 2018, 373, 012015. [Google Scholar] [CrossRef]
- Zhang, Z.; Shan, J.; Tan, X.; Zhang, J. Improvement of the laser joining of CFRP and aluminum via laser pre-treatment. Int. J. Adv. Manuf. Technol. 2017, 90, 3465–3472. [Google Scholar] [CrossRef]
- Schanz, J.; Meinhard, D.; Dostal, I.; Riegel, H.; De Silva, A.K.M.; Harrison, D.K.; Knoblauch, V. Comprehensive study on the influence of different pretreatment methods and structural adhesives on the shear strength of hybrid CFRP/aluminum joints. J. Adhes. 2022, 98, 1772–1800. [Google Scholar] [CrossRef]
- Wu, S.; Delp, A.; Freund, J.; Walther, F.; Haubrich, J.; Löbbecke, M.; Tröster, T. Adhesion properties of the hybrid system made of laser-structured aluminium EN AW 6082 and CFRP by co-bonding-pressing. J. Adhes. 2023, 100, 1–29. [Google Scholar] [CrossRef]
- Delp, A.; Freund, J.; Wu, S.; Scholz, R.; Löbbecke, M.; Haubrich, J.; Tröster, T.; Walther, F. Influence of laser-generated surface micro-structuring on the intrinsically bonded hybrid system CFRP-EN AW 6082-T6 on its corrosion properties. Compos Struct. 2022, 285, 115238. [Google Scholar] [CrossRef]
- Narsimhachary, D.; Rai, P.K.; Shariff, S.M.; Padmanabham, G.; Mondal, K.; Basu, A. Corrosion behavior of laser-brazed surface made by joining of AA6082 and galvanized steel. J. Mater. Eng. Perform. 2019, 28, 2115–2127. [Google Scholar] [CrossRef]
- Song, G.-L.; Zhang, C.; Chen, X.; Zheng, D. Galvanic activity of carbon fiber reinforced polymers and electrochemical behavior of carbon fiber. Corros. Commun. 2021, 1, 26–39. [Google Scholar] [CrossRef]
- Li, Z.; Peng, M.; Wei, H.; Zhang, W.; Lv, Q.; Zhang, F.; Shan, Q. First-principles study on surface corrosion of 6082 aluminum alloy in H+ and Cl medium. J. Mol. Struct. 2023, 1294, 136570. [Google Scholar] [CrossRef]
- Freund, J.; Lützenkirchen, I.; Löbbecke, M.; Delp, A.; Walther, F.; Wu, S.; Tröster, T.; Haubrich, J. Transferability of the structure-property relationships from laser-pretreated metal-polymer joints to aluminum-CFRP hybrid joints. J. Compos. Sci. 2023, 7, 427. [Google Scholar] [CrossRef]
- McCafferty, E. Introduction to Corrosion Science; Springer: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- ISO 17475:2005+Cor.1:2006; German version EN ISO 17475:2008: Corrosion of Metals and Alloys—Electrochemical Test Methods—Guidelines for Conducting Potentiostatic and Potentiodynamic Polarization Measurements. DIN e.V.: Beuth Verlag, Berlin, 2006.
- Zappalorto, M.; Panozzo, F.; Carraro, P.A.; Quaresimin, M. Electrical response of laminate with a delamination: Modelling and experiments. Compos. Sci. Technol. 2017, 143, 31–45. [Google Scholar] [CrossRef]
- Zhang, D.-Q.; Li, J.; Joo, H.G.; Lee, K.Y. Corrosion properties of Nd:YAG laser-GMA hybrid welded AA6061 Al alloy and its microstructure. Corros. Sci. 2009, 51, 1399–1404. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, K.; Zhu, S.; Xu, H.; Cao, D.; Zhao, L.; Zhang, R.; Yin, W. Review on the electrical resistance/conductivity of carbon fiber reinforced polymer. Appl. Sci. 2019, 9, 2390. [Google Scholar] [CrossRef]



| Laser Parameter | f (kHz) | P (W) | o (10−2) | N |
|---|---|---|---|---|
| lp0 | N/A | N/A | N/A | N/A |
| lp1 | 60 | 20 | 10 | 5 |
| lp2 | 40 | 20 | 50 | 1 |
| lp3 | 60 | 15 | 50 | 1 |
| Parameter Set | Fiber Direction (°) | Number | lsv | Total Exposure Time (h) |
|---|---|---|---|---|
| lp1 | 90 | K1lp1 | yes | 168 |
| lp1 | 0 | K2lp1 | yes | 168 |
| lp1 | 0 | K3lp1 | no | 744 |
| lp1 | 90 | K4lp1 | no | 744 |
| lp2 | 0 | K1lp2 | no | 168 |
| lp2 | 90 | K2lp2 | no | 168 |
| lp2 | 90 | K3lp2 | no | 744 |
| lp2 | 0 | K4lp2 | no | 744 |
| EN AW-6082 | N/A | ref | no | 744 |
| lp0 | 0 | lp0 | yes | N/A |
| lp1 | 0 | lp1 | yes | N/A |
| lp2 | 0 | lp2 | yes | N/A |
| lp3 | 0 | lp3 | yes | N/A |
| EN AW-6082 | N/A | Al | yes | N/A |
| CFRP | N/A | CFRP | yes | N/A |
| Number | Diameter (mm) | Height (mm) | Area CFRP (mm2) | Area Al (mm2) | Volume (mm3) |
|---|---|---|---|---|---|
| K1lp1 | 29.8 | 17.3 | 11.26 | 18.02 | 12,054 |
| K2lp1 | 30.0 | 11.3 | 19.25 | 30.18 | 7988 |
| K3lp1 | 29.8 | 12.5 | 19.70 | 31.11 | 8703 |
| K4lp1 | 29.8 | 17.4 | 18.23 | 11.84 | 12,091 |
| K1lp2 | 30.1 | 12.2 | 19.17 | 31.53 | 8660 |
| K2lp2 | 29.8 | 17.1 | 10.47 | 18.64 | 11,935 |
| K3lp2 | 30.0 | 17.1 | 10.94 | 18.58 | 12,080 |
| K4lp2 | 29.9 | 12.7 | 17.82 | 29.63 | 8927 |
| ref | 30.0 | 11.4 | 00.00 | 00.00 | 8062 |
| Weight (g) | 0 h (D0) | 24 h (D1) | 48 h (D2) | 72 h (D3) | 96 h (D4) | 168 h (W1) | 336 h (W2) | 504 h (W3) | 744 h (W4) |
|---|---|---|---|---|---|---|---|---|---|
| K1lp1 | 14.6750 | 14.6802 | 14.6844 | 14.6855 | 14.6880 | 14.6934 | n.a. | n.a. | n.a. |
| K2lp1 | 10.5495 | 10.5519 | 10.5555 | 10.5567 | 10.5591 | 10.5637 | n.a. | n.a. | n.a. |
| K3lp1 | 10.8513 | 10.8587 | 10.862 | 10.8629 | 10.8651 | 10.8706 | 10.8759 | 10.8792 | 10.8794 |
| K4lp1 | 14.6666 | 14.6734 | 14.6788 | 14.6791 | 14.6817 | 14.6886 | 14.6959 | 14.7008 | 14.7012 |
| K1lp2 | 10.5432 | 10.5462 | 10.5493 | 10.5505 | 10.5526 | 10.5568 | n.a. | n.a. | n.a. |
| K2lp2 | 10.6009 | 10.6085 | 10.6130 | 10.6136 | 10.6166 | 10.6217 | n.a. | n.a. | n.a. |
| K3lp2 | 13.6981 | 13.7044 | 13.709 | 13.7094 | 13.7129 | 13.7174 | 13.7248 | 13.7296 | 13.7301 |
| K4lp2 | 11.1066 | 11.1121 | 11.1150 | 11.1162 | 11.1190 | 11.1258 | 11.1324 | 11.1376 | 11.1379 |
| ref | 10.1690 | 10.1740 | 10.1783 | 10.1797 | 10.1828 | 10.1893 | 10.1976 | 10.2035 | 10.2055 |
| Batch | Initial κ (mS/cm) | Final κ (mS/cm) | Remaining Water Volume (mL) | Resulting c (mol/L) |
|---|---|---|---|---|
| s1 | 10.50 | 11.24 | 1791 | 0.1117 |
| s2 | 10.58 | 10.89 | 1817 | 0.1101 |
| s3 | 10.29 | 11.76 | 1803 | 0.1109 |
| s4 | 10.35 | 11.12 | 1763 | 0.1134 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delp, A.; Wu, S.; Freund, J.; Scholz, R.; Löbbecke, M.; Tröster, T.; Haubrich, J.; Walther, F. Characterization of Interfacial Corrosion Behavior of Hybrid Laminate EN AW-6082 ∪ CFRP. Materials 2024, 17, 1907. https://doi.org/10.3390/ma17081907
Delp A, Wu S, Freund J, Scholz R, Löbbecke M, Tröster T, Haubrich J, Walther F. Characterization of Interfacial Corrosion Behavior of Hybrid Laminate EN AW-6082 ∪ CFRP. Materials. 2024; 17(8):1907. https://doi.org/10.3390/ma17081907
Chicago/Turabian StyleDelp, Alexander, Shuang Wu, Jonathan Freund, Ronja Scholz, Miriam Löbbecke, Thomas Tröster, Jan Haubrich, and Frank Walther. 2024. "Characterization of Interfacial Corrosion Behavior of Hybrid Laminate EN AW-6082 ∪ CFRP" Materials 17, no. 8: 1907. https://doi.org/10.3390/ma17081907






