Study on Preparation and Humidity-Control Capabilities of Vermiculite/Poly(sodium Acrylate-acrylamide) Humidity Controlling Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Vermiculite/Poly(sodium Acrylate-acrylamide) Material
2.3. Characterization
3. Results
3.1. Morphology Analysis
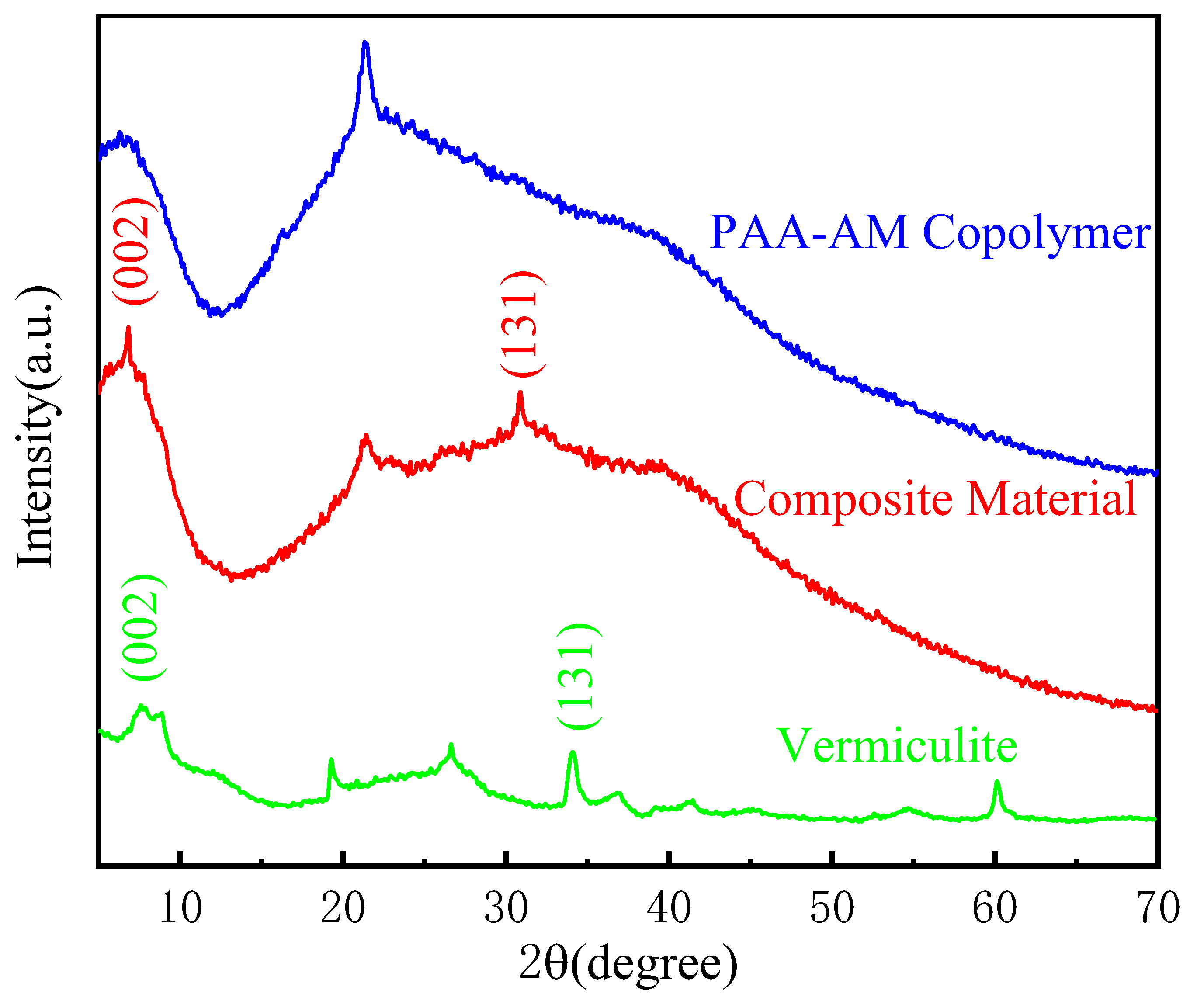
3.2. Structural Analysis
3.3. Infrared Analysis
3.4. BET Analysis
3.5. Humidity Controlling Performance of Composite Material
3.5.1. Single Factor Experiment
3.5.2. Orthogonal Experiment
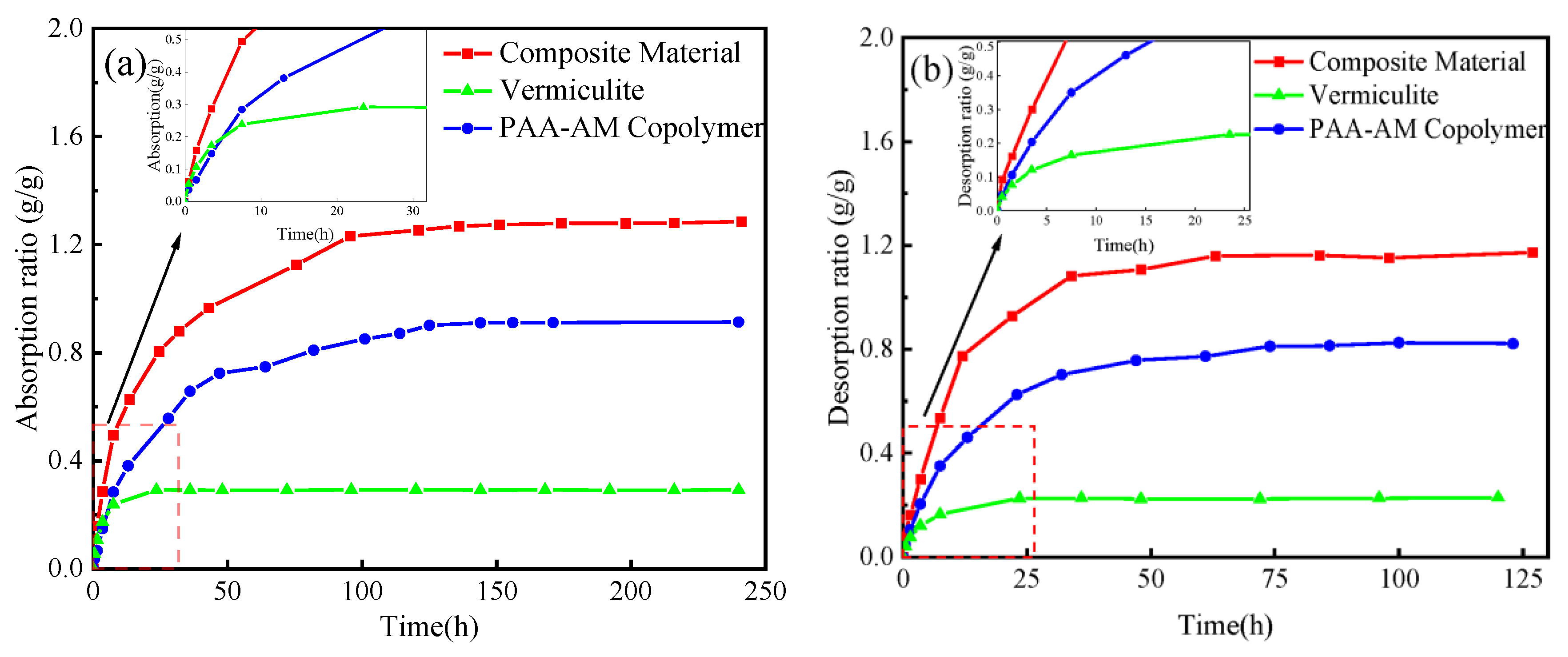
3.5.3. Control Experiment
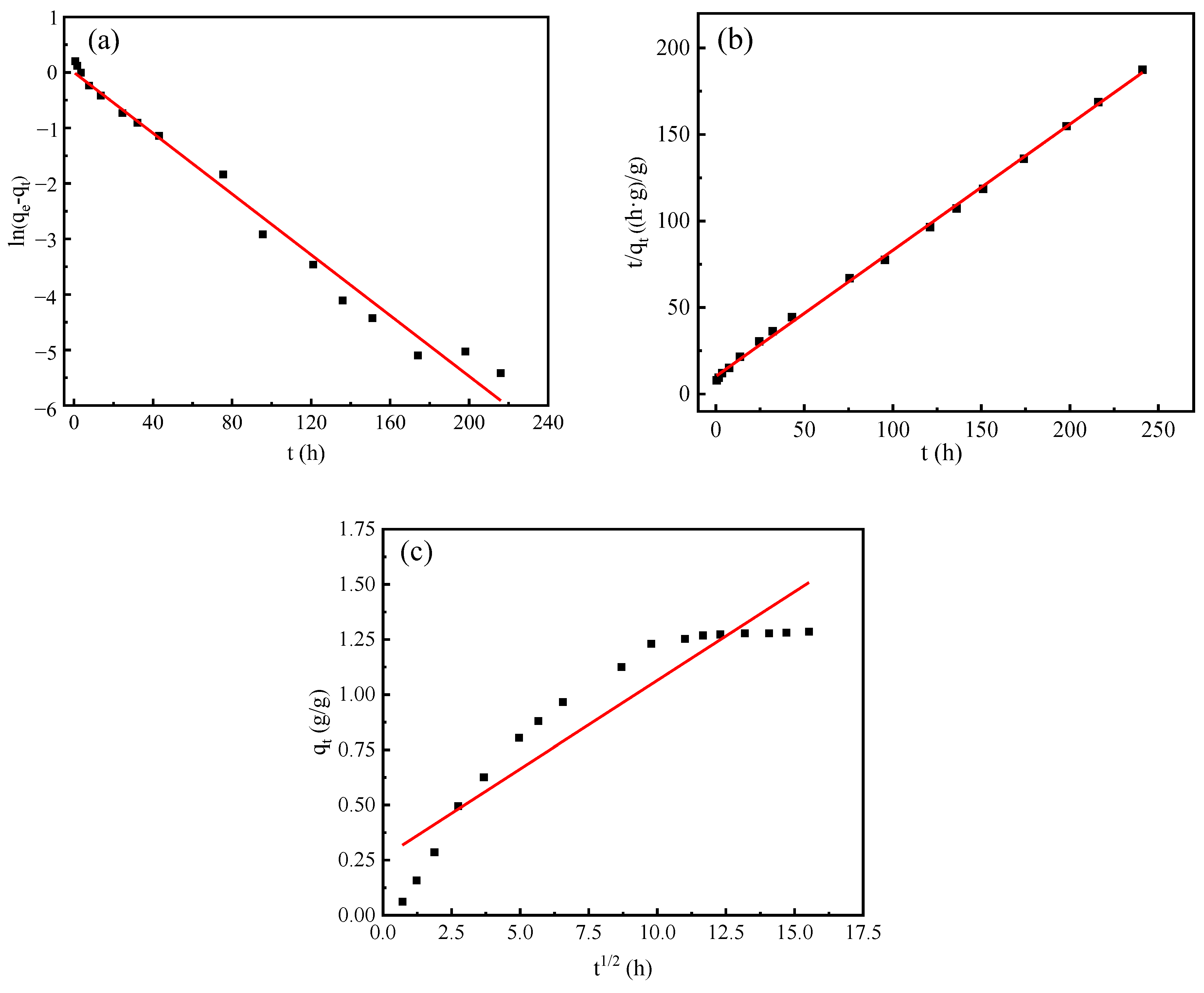
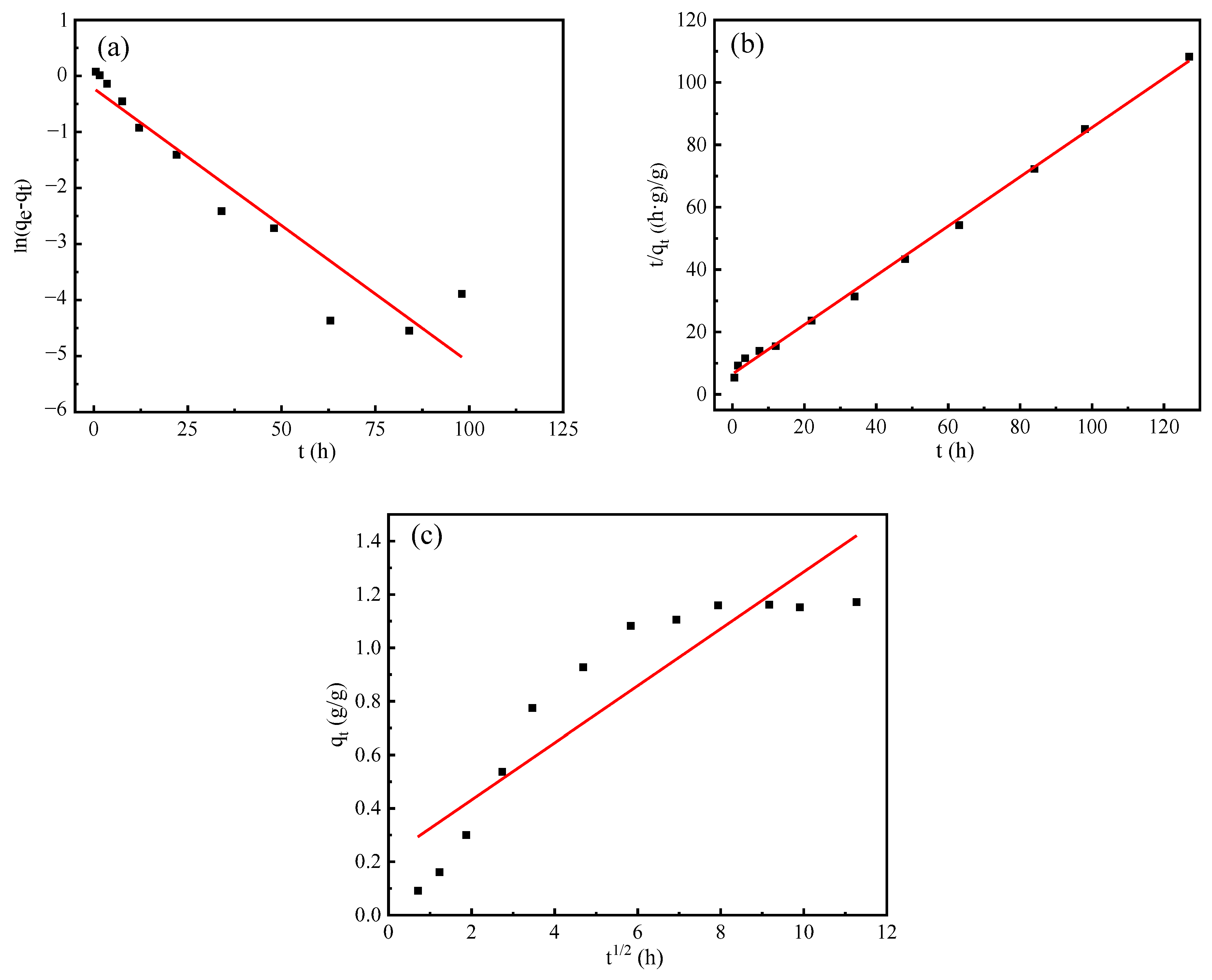
4. Adsorption and Desorption Kinetics
5. Conclusions
- The composite material consists of spherical particles with a rough surface, where the modified vermiculite effectively combines with the PAA-AM copolymer. The copolymerization of acrylic acid (AA) and acrylamide (AM) results in the infiltration of AA and AM into the middle layer of the modified vermiculite, expanding the interlayer spacing of the vermiculite. The results of BET analysis showed that the addition of vermiculite increased the specific surface area and pore volume of the composites and optimized the pore structure.
- Results from orthogonal and single-factor experiments indicate that the humidity control ability of the composite material is influenced by various factors. Notably, the vermiculite content and monomer ratio have a substantial impact on humidity control performance, while the neutralization degree plays a relatively minor role. The optimal preparation conditions include a vermiculite content of 4%, a neutralization degree of 90%, and a mass ratio of AA to AM of 4:1. Under these conditions, the composite material exhibits moisture absorption and dehumidification rates of 1.285 g/g and 1.172 g/g, respectively. Comparative tests reveal that the composite material’s moisture absorption and release rates surpass those of vermiculite and PAA-AM copolymer, enabling faster humidity adjustment. The incorporation of vermiculite introduces a mesoporous structure to the composite material, enhancing its surface roughness but diminishing its humidity control capacity.
- The humidity control process of the composite material is governed by pseudo second-order kinetics, which encompasses the complete adsorption process. This is attributed to the synergistic effect of the PAA-AM copolymer and vermiculite.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Izquierdo, M.; Moreno-Rodríguez, A.; González-Gil, A.; García-Hernando, N. Air conditioning in the region of Madrid, Spain: An approach to electricity consumption, economics and CO2 emissions. Energy 2011, 36, 1630–1639. [Google Scholar]
- Sultan, M.; Miyazaki, T.; Saha, B.B.; Koyama, S. An overview of solid desiccant dehumidification and air conditioning systems. Renew. Sustain. Energy Rev. 2015, 46, 16–29. [Google Scholar]
- Ichiro, S.; Akihiko, M.; Masao, T. Humidity changes in the house and wall materials. Res. Rep. Jpn. Archit. Soc. 1949, 3, 21–25. [Google Scholar]
- Tomson, A.E.; Sokolova, T.V.; Sosnovskaya, N.E.; Pekhtereva, V.S.; Goncharova, I.A.; Arashkova, A.A.; Kozhich, D.T. Prospects for the use of modified peat for indoor humidity control. Solid Fuel Chem. 2017, 51, 321–325. [Google Scholar]
- Pelliccia, G.; Baldinelli, G.; Bianconi, F.; Filippucci, M.; Fioravanti, M.; Goli, G.; Rotili, A.; Togni, M. Characterisation of wood hygromorphic panels for relative humidity passive control. J. Build. Eng. 2020, 32, 101829. [Google Scholar]
- Saatchi, D.; Oh, S.; Oh, I. Biomimetic and biophilic design of multifunctional symbiotic lichen-schwarz metamaterial. Adv. Funct. Mater. 2023, 33, 31. [Google Scholar]
- Zhou, L.; Hao, M.; Min, T.; Bian, X.; Du, H.; Sun, X.; Zhu, Z.; Wen, Y. Kaolin incorporated with thyme essential oil for humidity-controlled antimicrobial food packaging. Food Packag. Shelf Life 2023, 38, 101106. [Google Scholar]
- Lefers, R.M.; Bettahalli, N.M.S.; Fedoroff, N.V.; Ghaffour, N.; Davies, P.A.; Nunes, S.P.; Leiknes, T. Hollow fibre membrane-based liquid desiccant humidity control for controlled environment agriculture. Biosyst. Eng. 2019, 183, 47–57. [Google Scholar]
- Moriya, Y.; Ishii, N. Humidity Control System with Adsorption Materials. JSME Int. J. Ser. B Fluids Therm. Eng. 1996, 39, 653–659. [Google Scholar]
- Tomita, Y.; Takahashi, R.; Sato, S.; Sodesawa, T.; Otsuda, M. Humidity control ability of silica with bimodal pore structures prepared from water glass. J. Ceram. Soc. Jpn. 2004, 112, 491–495. [Google Scholar]
- Young, J.F. Humidity control in the laboratory using salt solutions—A review. J. Appl. Chem. 1967, 17, 241–245. [Google Scholar]
- Vakalova, T.V.; Revva, I.B. Use of zeolite rocks for ceramic bricks based on brick clays and clay loams with high drying sensitivity. Constr. Build. Mater. 2020, 225, 119324. [Google Scholar]
- Tomura, S.; Maeda, M.; Inukai, K.; Fumijilo, O.; Suzuki, M.; Shibasaki, Y.; Suzuki, S. Water vapor adsorption property of various clays and related materials for applications to humidity self-control materials. Clay Sci. 2011, 10, 195–203. [Google Scholar]
- Fort, J.; Dolezelova, M.; Koci, V.; Cerny, R. Functional properties of SAP-based humidity control plasters. Polymers 2021, 13, 2279. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.; da Silva, F.E.; Franco, C.V.; Nuernberg, R.B.; Gomes, T.; Miranda, R.; da Silva Paula, M.M. Humidity and pH sensor based on sulfonated poly-{styrene–acrylic acid} polymer.: Synthesis and characterization. Mater. Sci. Eng. 2009, 29, 599–601. [Google Scholar]
- Chen, G.; Bai, X.; Zhang, X.; Li, Q. Research on characteristics of biomass-based humidity-controlling materials. Heat. Vent. Air Cond. 2007, 37, 14–17. [Google Scholar]
- Zhou, X.; Jin, H.; Gu, A.; Li, X.; Sun, L.; Mao, P.; Yang, Y.; Ding, S.; Chen, J.; Yun, S. Eco-friendly hierarchical porous palygorskite/wood fiber aerogels with smart indoor humidity control. J. Clean. Prod. 2022, 335, 130367. [Google Scholar]
- Maria, I.; Petrisor, S.; Corneliu, C.; Gabriela, S.; Igor, C.; Valeria, H. Porous polymer/inorganic composite matrices as efficient desiccants for air dehumidification. Appl. Surf. Sci. 2019, 487, 1189–1197. [Google Scholar]
- Vu, D.H.; Wang, K.S.; Bac, B.H.; Nam, B.X. Humidity control materials prepared from diatomite and volcanic ash. Constr. Build. Mater. 2013, 38, 1066–1072. [Google Scholar]
- Fort, J.; Kocí, J.; Pokorny, J.; Podolka, L.; Kraus, M.; Cerny, R. Characterization of responsive plasters for passive moisture and temperature control. Appl. Sci. 2020, 10, 9116. [Google Scholar] [CrossRef]
- Zheng, X.; Yuan, L. Recent progress on composite humidity control materials. Chem. Ind. Eng. Prog. 2020, 39, 1378–1388. [Google Scholar]
- Zhao, H.; Zhang, T.; Dai, J.; Jiang, K.; Fei, T. Preparation of hydrophilic organic groups modified mesoporous silica materials and their humidity sensitive properties. Sens. Actuators B Chem. 2017, 240, 681–688. [Google Scholar]
- Fraine, Y.; Seladji, C.; Ait-Mokhtar, A. Effect of microencapsulation phase change material and diatomite composite filling on hygrothermal performance of sintered hollow bricks. Build. Environ. 2019, 154, 145–154. [Google Scholar]
- Fleischer, M.; Mandarino, J.A. Glossary of Mineral Species; The Mineralogical Record Inc.: Tucson, AZ, USA, 1991; pp. 23–26. [Google Scholar]
- Potter, M.J. Vermiculite. In US Geological Survery Minerals Yearbook; US Geological Survey: Reston, VA, USA, 2002; pp. 14–15. [Google Scholar]
- Malamis, S.; Katsou, E. A review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite: Examination of process parameters, kinetics and isotherms. J. Hazard. Mater. 2013, 253, 428–461. [Google Scholar]
- Padilla-Ortega, E.; Leyva-Ramos, R.; Mendoza-Barron, J. Role of electrostatic interactions in the adsorption of cadmium (II) from aqueous solution onto vermiculite. Appl. Clay Sci. 2014, 88–89, 10–17. [Google Scholar]
- Bazhirov, T.S.; Protsenko, V.; Bazhirov, N.S.; Dauletiyarov, M.S.; Serikbayev, B.; Bazhirova, K.N. Physicochemical investigations of vermiculite-microporous component for heat-resistant materials. Ser. Chem. Technol. 2019, 437, 136–142. [Google Scholar]
- Dordio, A.V.; Miranda, S.; Prates Ramalho, J.P.; Palace Carvalho, A.J. Mechanisms of removal of three widespread pharmaceuticals by two clay materials. J. Hazard. Mater. 2017, 323, 575–583. [Google Scholar] [PubMed]
- Marosz, M.; Kowalczyk, A.; Chmielarz, L. Modified vermiculites as effective catalysts for dehydration of methanol and ethanol. Catal. Today 2020, 355, 466–475. [Google Scholar]
- Sari, A.; Biçer, A.; Hekimoglu, G. Effects of carbon nanotubes additive on thermal conductivity and thermal energy storage properties of a novel composite phase change material. J. Compos. Mater. 2019, 53, 2967–2980. [Google Scholar]
- Zhao, H.; Huang, T.; Liu, T.; Lei, M.; Zhang, M. Synthesis of MgCl2/vermiculite and its water vapor adsorption-desorption performance. Int. J. Energy Res. 2021, 45, 21375–21389. [Google Scholar]
- Boonsiriwit, A.; Yao, X.; Kathuria, A.; Lee, Y. Effect of moisture-controlled packaging treatment with acid-modified expanded vermiculite-calcium chloride on the quality of fresh mushrooms (Agaricus bisporus) during low-temperature storage. J. Sci. Food Agric. 2022, 102, 3029–3037. [Google Scholar] [PubMed]
- Kiatkamjornwong, S.; Chomsaksakul, W.; Sonsuk, M. Radiation modification of water absorption of cassava starch by acrylic acid/acrylamide. Radiat. Phys. Chem. 2000, 59, 413–427. [Google Scholar]
- Rubinger, C.P.L.; Martins, C.R.; De Paoli, M.-A.; Rubinger, R.M. Sulfonated polystyrene polymer humidity sensor: Synthesis and characterization. Sens. Actuators B Chem. 2007, 123, 42–49. [Google Scholar]
- Dong, F.; Wang, J.; Wang, Y.; Ren, S. Synthesis and humidity controlling properties of halloysite/poly(sodium acrylate-acrylamide) composite. J. Mater. Chem. 2012, 22, 11093–11100. [Google Scholar]
- Li, M. Study on preparation and humidity-control performance of organobentonite/sodium polyacrylate composite material. Sci. Eng. Compos. Mater. 2015, 22, 1–6. [Google Scholar]
- Yang, H.; Peng, Z.; Zhou, Y.; Zhao, F.; Zhang, J.; Cao, X.; Hu, Z. Preparation and performances of a novel intelligent humidity control composite material. Energy Build. 2011, 43, 386–392. [Google Scholar]
- Xu, P.; Yao, Q.; Yu, N.; Zhao, Y.; Zhao, F.; Wang, B.; Peng, Z.; Hu, Z. Narrow-dispersed Konjac glucomannan nanospheres with high moisture adsorption and desorption ability by inverse emulsion crosslinking. Mater. Lett. 2014, 137, 59–61. [Google Scholar]




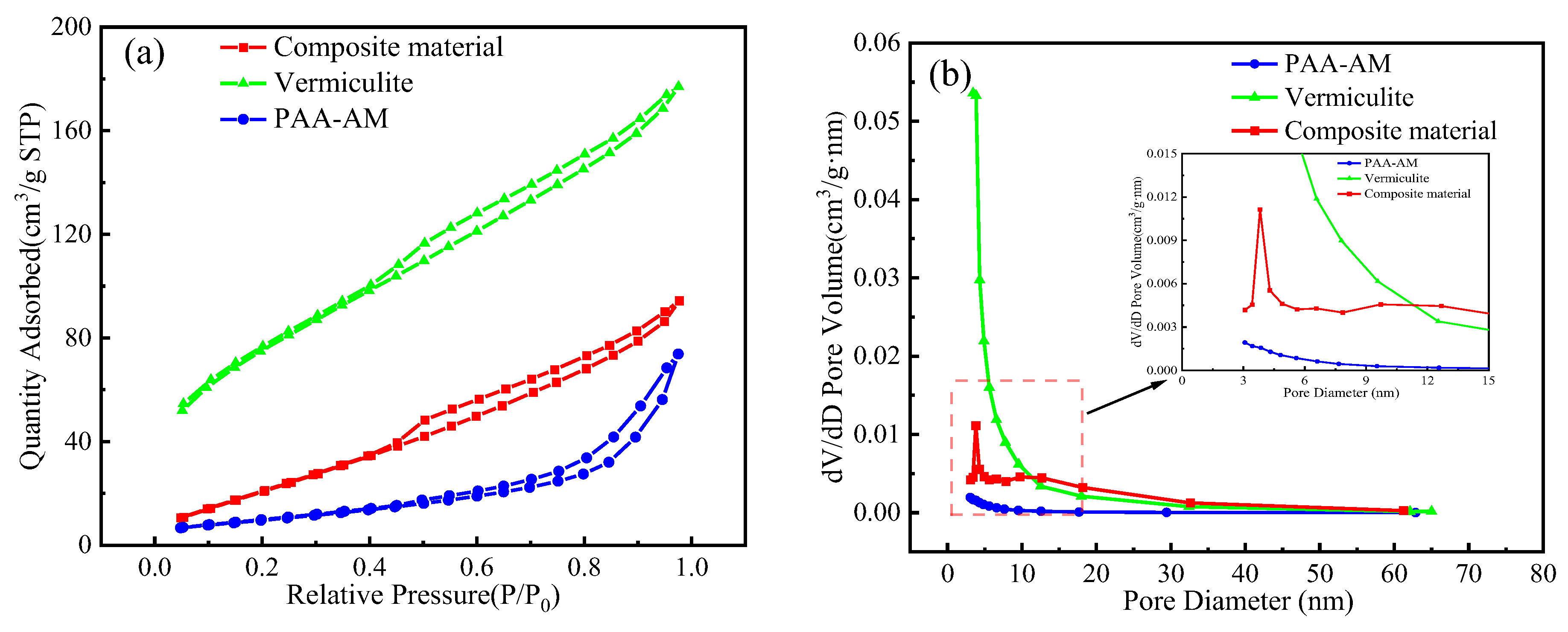


| Sample | SBET (m2/g) | Pore Diameter (nm) | Vtot (cm3/g) |
|---|---|---|---|
| Vermiculite | 36.65 | 3.473 | 0.175 |
| PAA-AM | 6.14 | 3.073 | 0.008 |
| Composite material | 278.34 | 3.815 | 0.114 |
| Sample No. | Factors and Levels | Humidity Controlling Properties | |||
|---|---|---|---|---|---|
| Vermiculite Content (%) | Neutralization Degree (%) | Mass Ratio of AA to AM | Absorption Ratio (g/g) | Desorption Ratio (g/g) | |
| 1 | 1 | 70 | 1:1 | 0.798 | 0.679 |
| 2 | 1 | 80 | 4:1 | 1.047 | 0.959 |
| 3 | 1 | 90 | 7:1 | 1.098 | 0.940 |
| 4 | 1 | 100 | 10:1 | 1.068 | 0.915 |
| 5 | 4 | 70 | 4:1 | 1.197 | 1.062 |
| 6 | 4 | 80 | 1:1 | 0.848 | 0.724 |
| 7 | 4 | 90 | 10:1 | 1.115 | 0.988 |
| 8 | 4 | 100 | 7:1 | 1.228 | 1.080 |
| 9 | 7 | 70 | 7:1 | 0.863 | 0.768 |
| 10 | 7 | 80 | 10:1 | 0.92 | 0.855 |
| 11 | 7 | 90 | 1:1 | 0.789 | 0.708 |
| 12 | 7 | 100 | 4:1 | 0.912 | 0.821 |
| 13 | 10 | 70 | 10:1 | 0.887 | 0.793 |
| 14 | 10 | 80 | 7:1 | 0.879 | 0.769 |
| 15 | 10 | 90 | 4:1 | 0.985 | 0.841 |
| 16 | 10 | 100 | 1:1 | 0.729 | 0.630 |
| Absorption Ratio (g/g) | Desorption Ratio (g/g) | |||||
|---|---|---|---|---|---|---|
| Vermiculite Content (%) | Neutralization Degree (%) | Mass Ratio of AA to AM | Vermiculite Content (%) | Neutraliztion Degree (%) | Mass Ratio of AA to AM | |
| K1 | 1.003 | 0.936 | 0.791 | 0.873 | 0.826 | 0.685 |
| K2 | 1.097 | 0.923 | 1.035 | 0.964 | 0.827 | 0.921 |
| K3 | 0.871 | 0.997 | 1.017 | 0.788 | 0.869 | 0.889 |
| K4 | 0.870 | 0.984 | 0.997 | 0.758 | 0.861 | 0.888 |
| R | 0.227 | 0.075 | 0.244 | 0.206 | 0.043 | 0.236 |
| Optimal level | A2 | B3 | C2 | A2 | B3 | C2 |
| Pseudo First-Order | Pseudo Second-Order | Intra-Particle Diffusion | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k1 | qe1 | R2 | k2 | qe2 | R2 | kid | Intercept | R2 | ||
| composite materials | Absorption process | 0.0630 | 0.9993 | 0.9847 | 0.0517 | 1.3720 | 0.9990 | 0.0803 | 0.2614 | 0.8754 |
| Desorption process | 0.1126 | 0.7988 | 0.9043 | 0.0955 | 1.2650 | 0.9980 | 0.1067 | 0.2181 | 0.8266 | |
| Vermiculite | Absorption process | 0.0465 | 0.6491 | 0.3135 | 2.5761 | 0.2938 | 0.9999 | 0.0111 | 0.1611 | 0.5433 |
| Desorption process | 0.1064 | 0.0664 | 0.6175 | 1.7630 | 0.2335 | 0.9996 | 0.0166 | 0.0869 | 0.7190 | |
| PAA-AM | Absorption process | 0.0808 | 1.0180 | 0.9570 | 0.0519 | 1.0810 | 0.9983 | 0.0660 | 0.1220 | 0.8899 |
| Desorption process | 0.1227 | 0.7436 | 0.9837 | 0.1016 | 0.9104 | 0.9989 | 0.0782 | 0.0115 | 0.8840 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Z.; Wang, J.; Diao, Y.; Hu, W. Study on Preparation and Humidity-Control Capabilities of Vermiculite/Poly(sodium Acrylate-acrylamide) Humidity Controlling Composite. Materials 2024, 17, 1920. https://doi.org/10.3390/ma17081920
Xue Z, Wang J, Diao Y, Hu W. Study on Preparation and Humidity-Control Capabilities of Vermiculite/Poly(sodium Acrylate-acrylamide) Humidity Controlling Composite. Materials. 2024; 17(8):1920. https://doi.org/10.3390/ma17081920
Chicago/Turabian StyleXue, Zhichang, Jihui Wang, Yaqi Diao, and Wenbin Hu. 2024. "Study on Preparation and Humidity-Control Capabilities of Vermiculite/Poly(sodium Acrylate-acrylamide) Humidity Controlling Composite" Materials 17, no. 8: 1920. https://doi.org/10.3390/ma17081920





