Porous Silicone Rubber Composite Supported 1,4-Diphenylethynyl Benzene for Hydrogen Absorption with Pd/C Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
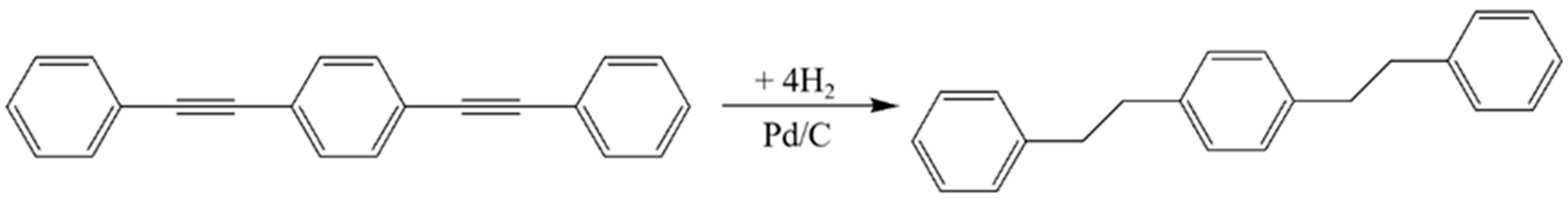
2.2. Preparation of DEB-Pd/C Getter
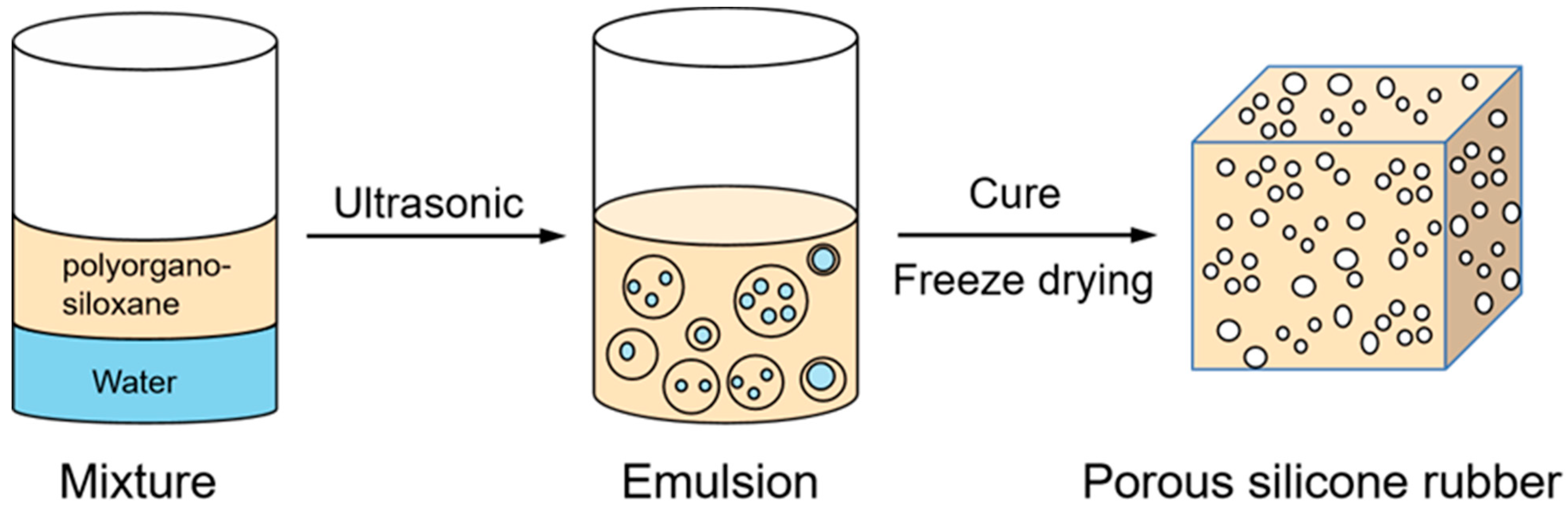
2.3. Preparation of Polysiloxane Emulsion
2.4. Preparation of Porous Silicone Rubber
2.5. Gel Fraction
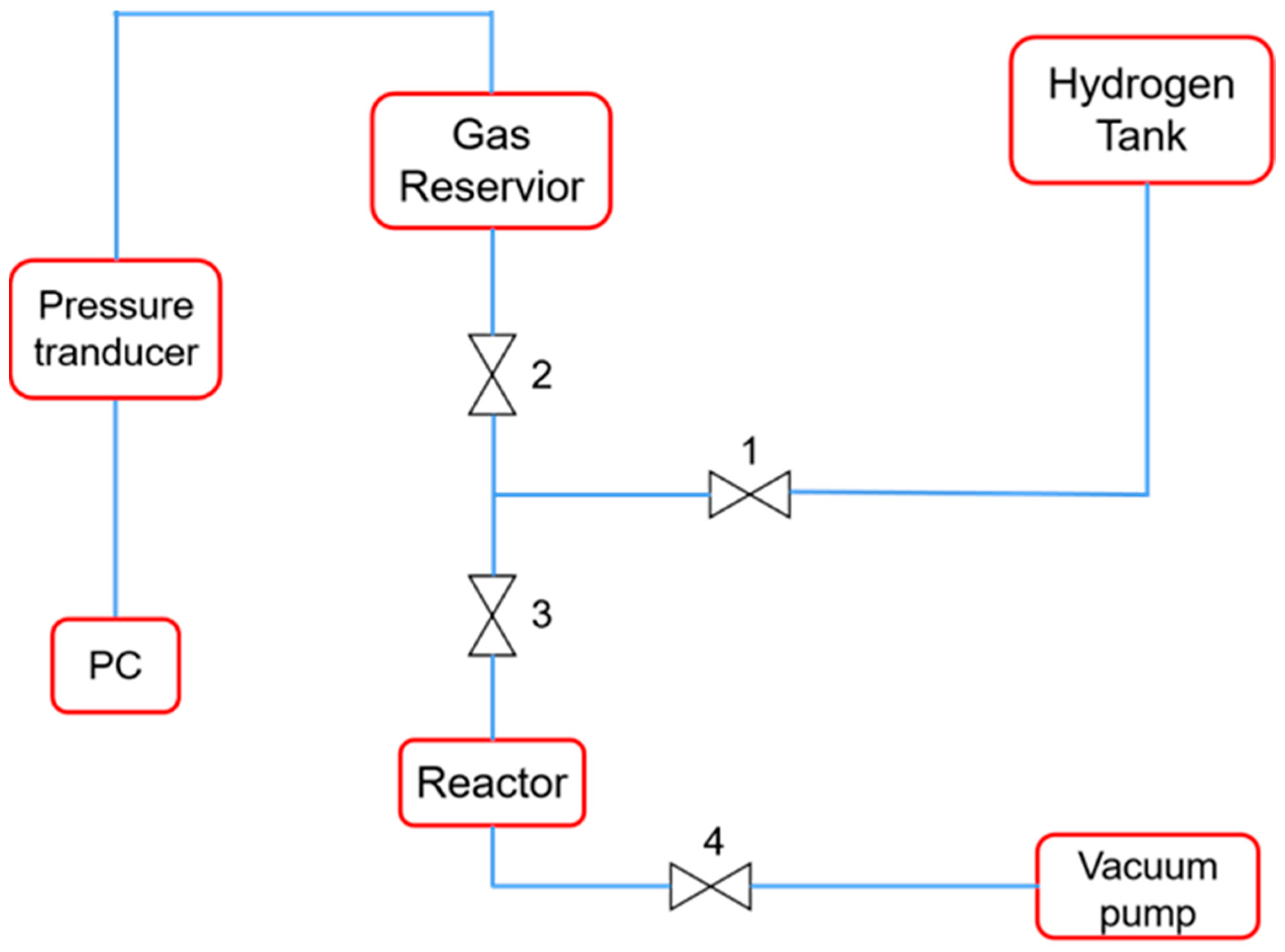
2.6. Hydrogen Absorption Test
2.7. Characterization
3. Results and Discussion
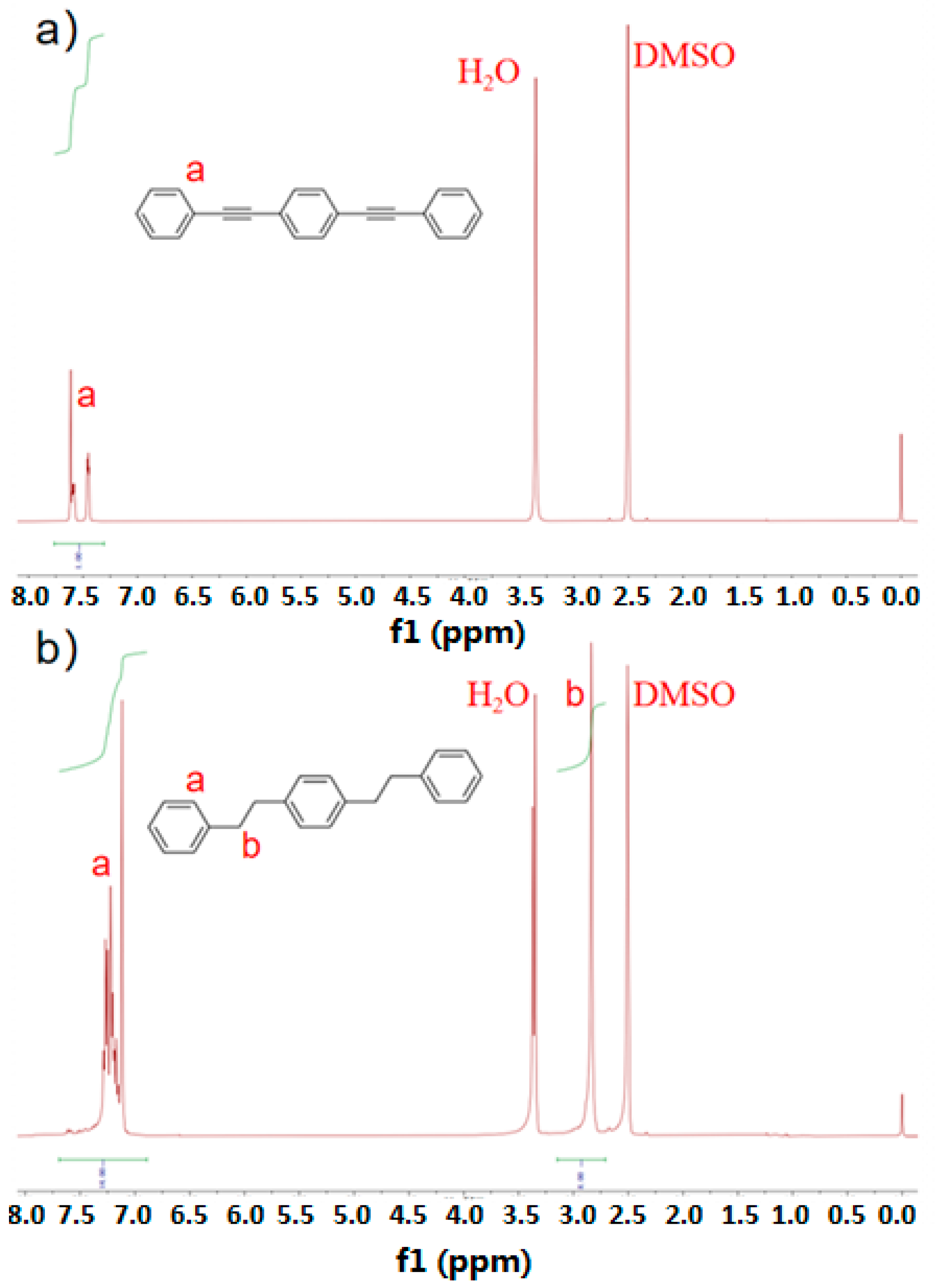
3.1. Characterization of DEB before and after Hydrogenation
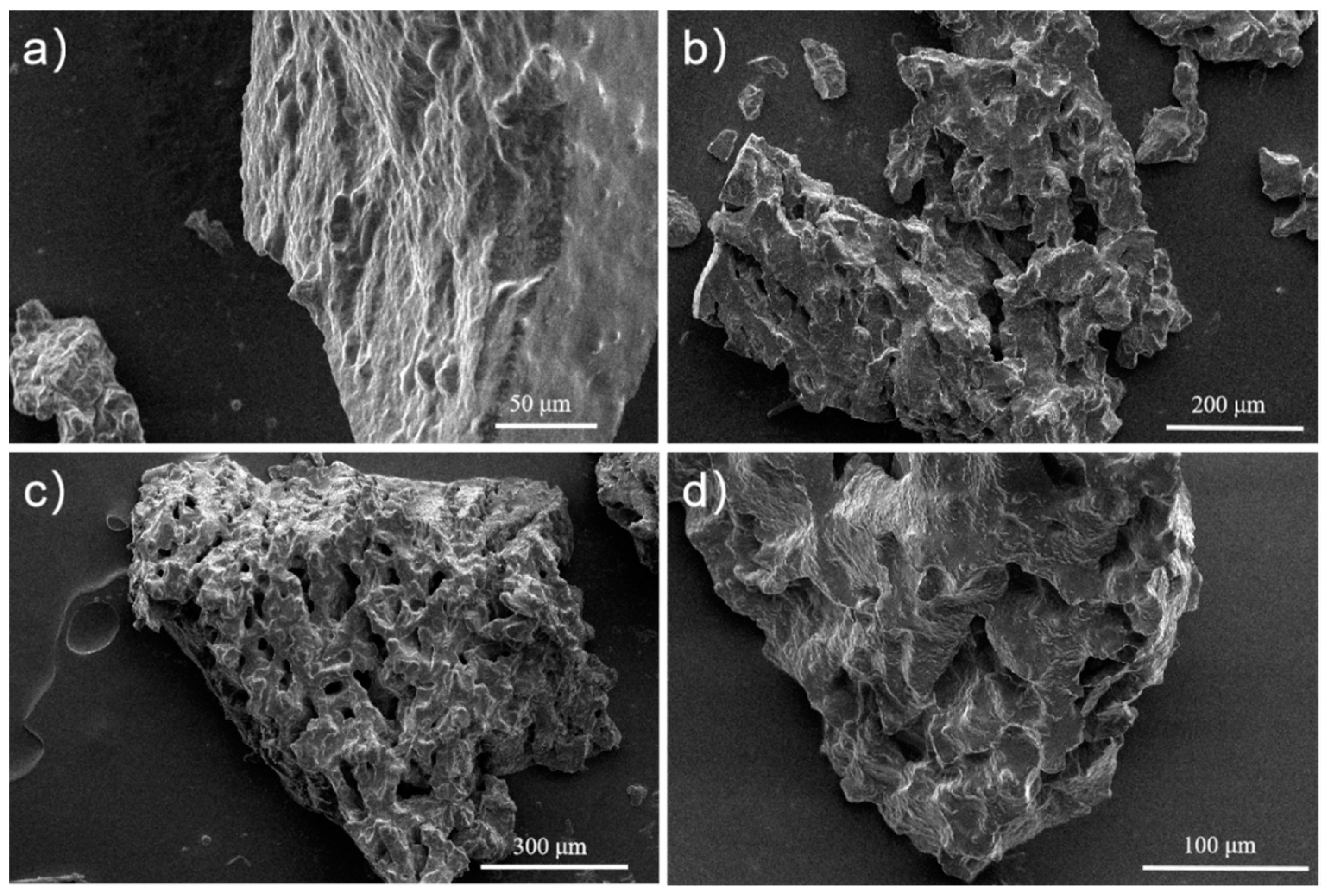
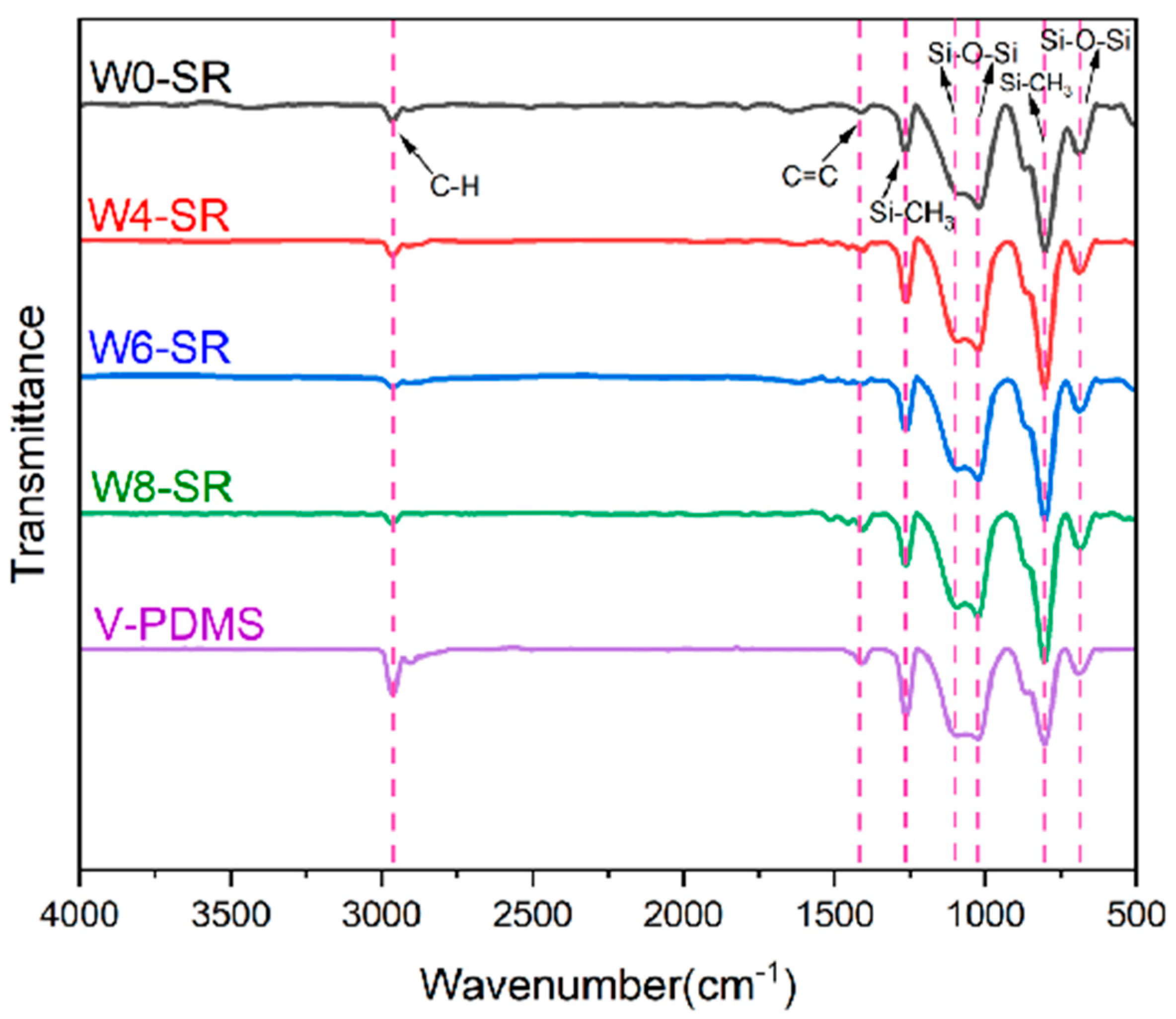
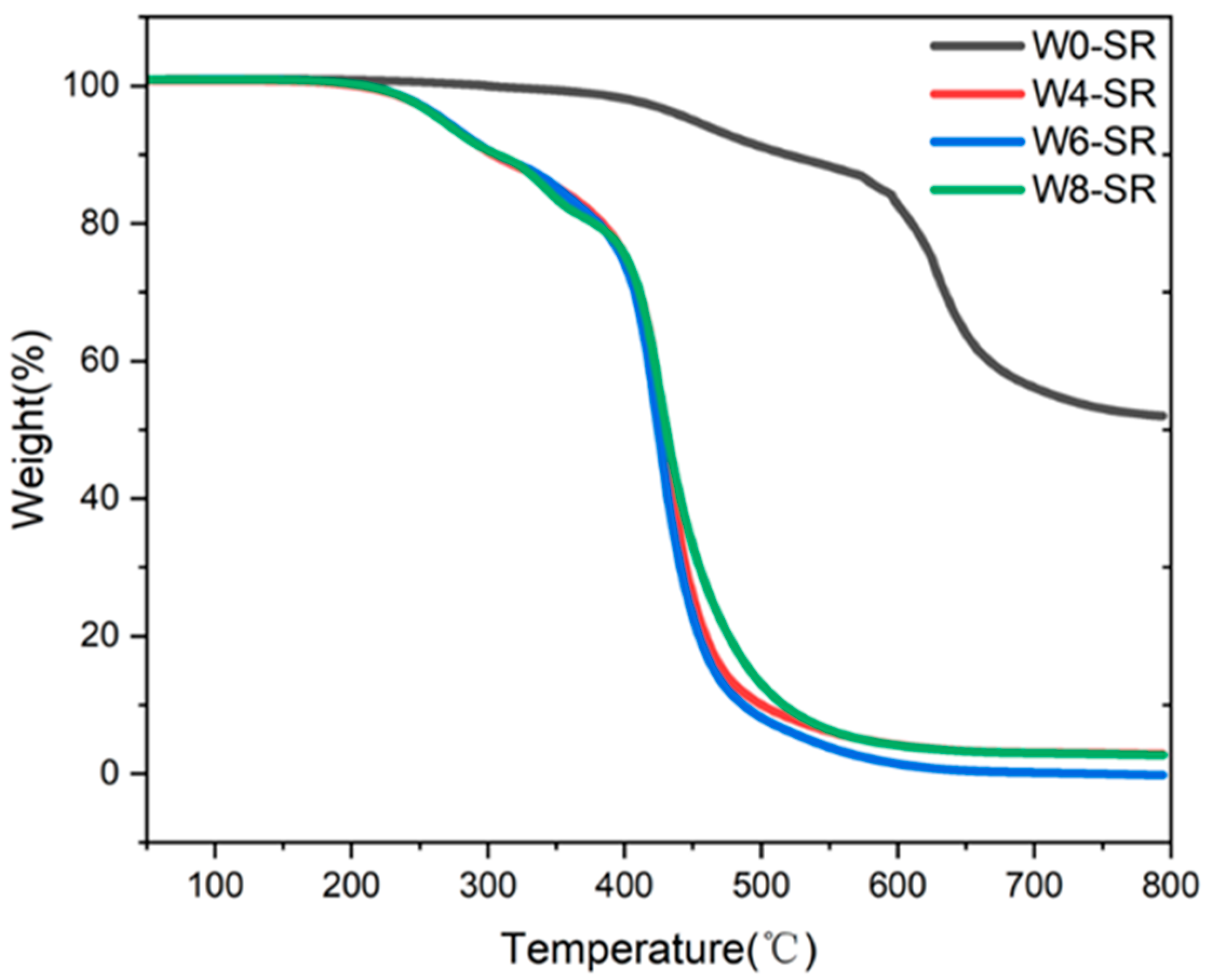
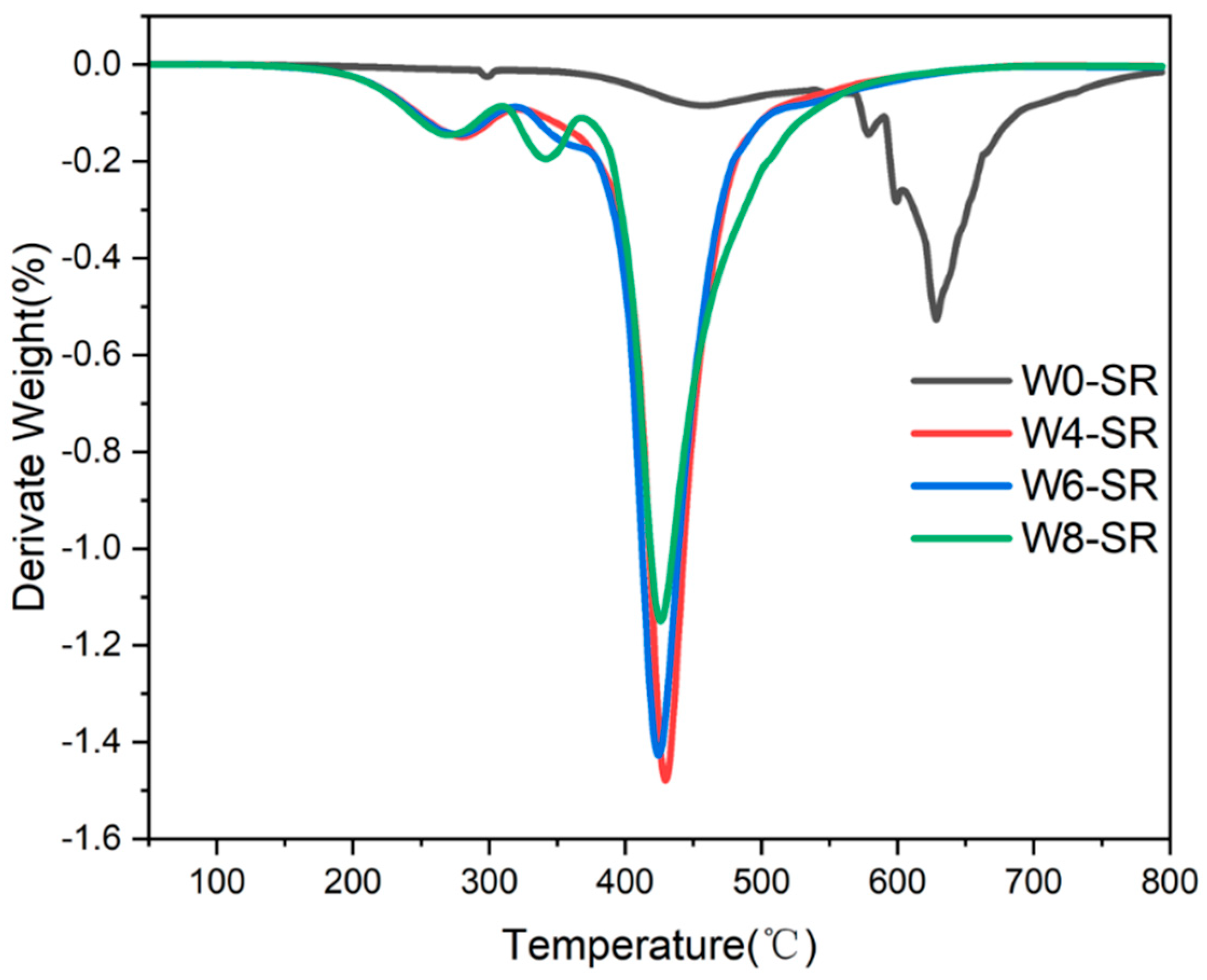
3.2. Characterization of Porous Silicone Rubber
3.3. Hydrogen Absorption Properties of Porous Silicone Rubber
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, C.; Szpunar, J.A. Szpunar, Hydrogen Storage Performance in Pd/Graphene Nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 25933–25940. [Google Scholar] [CrossRef] [PubMed]
- Galliez, K.; Deniard, P.; Lambertin, D.; Jobic, S.; Bart, F. Influence of MnO2 polymorphism form on MnO2/Ag2O hydrogen getter. J. Nucl. Mater. 2013, 438, 261–267. [Google Scholar] [CrossRef]
- Wang, M.; Akiyama, E.; Tsuzaki, K. Effect of hydrogen on the fracture behavior of high strength steel during slow strain rate test. Corros. Sci. 2007, 49, 4081–4097. [Google Scholar] [CrossRef]
- Takagi, S.; Toji, Y.; Yoshino, M.; Hasegawa, K. Hydrogen Embrittlement Resistance Evaluation of Ultra High Strength Steel Sheets for Automobiles. ISIJ Int. 2012, 52, 316–322. [Google Scholar] [CrossRef]
- Sangalang, E.A.; Sharma, H.N.; Saw, C.K.; Gollott, R.; Matt, S.M.; Wilson, T.S.; McLean, W.; Maxwell, R.S.; Dinh, L.N. Hydrogen Uptake Kinetics of 1,4-Bis(phenylethynyl)benzene (DEB) Rubberized Coating on Silicone Foam Substrate. ACS Appl. Mater. Interfaces 2020, 12, 3993–4001. [Google Scholar] [CrossRef] [PubMed]
- Reeb, C.; Davy, C.; Pierlot, C.; Lambertin, D. Trapping performance of alkali-activated materials incorporating a hydrogen/tritium getter for the conditioning of tritiated organic liquids. J. Nucl. Mater. 2023, 576, 154272. [Google Scholar] [CrossRef]
- Powell, G.L. The hydriding kinetics of organic hydrogen getters. In Advanced Materials for Energy Conversion II; Wiley: Hoboken, NJ, USA, 2004; pp. 467–475. [Google Scholar]
- Ortiz-Acosta, D.; Moore, T.; Safarik, D.J.; Hubbard, K.M.; Janicke, M. 3D-Printed Silicone Materials with Hydrogen Getter Capability. Adv. Funct. Mater. 2018, 28, 1707285. [Google Scholar] [CrossRef]
- Shit, S.C.; Shah, P. A Review on Silicone Rubber. Natl. Acad. Sci. Lett. 2013, 36, 355–365. [Google Scholar] [CrossRef]
- Huang, G.; Yang, Q.; Xu, Q.; Yu, S.; Jiang, H. Polydimethylsiloxane Coating for a Palladium/MOF Composite: Highly Improved Catalytic Performance by Surface Hydrophobization. Angew. Chem.-Int. Ed. 2016, 55, 7379–7383. [Google Scholar] [CrossRef]
- Goyal, A.; Mohl, M.; Kumar, A.; Puskas, R.; Kukovecz, A.; Konya, Z.; Kiricsi, I.; Ajayan, P.M. In situ synthesis of catalytic metal nanoparticle-PDMS membranes by thermal decomposition process. Compos. Sci. Technol. 2011, 71, 129–133. [Google Scholar] [CrossRef]
- Edlová, T.; Normand, A.T.; Cattey, H.; Brandès, S.; Wu, Y.; Antonangelo, A.; Théron, B.; Bonnin, Q.; Carta, M.; Le Gendre, P. Hydrosilylation and Silane Polymerization Catalyzed by Group 4 Amidometallocene Cations. Organometallics 2023, 42, 1166–1178. [Google Scholar] [CrossRef]
- Zeng, X.; Kang, X.; Chen, H.; Xu, J.; Zhang, Z.; Chen, S. Research of nano-materials modified silicone rubber. New Chem. Mater. 2010, 38, 17. [Google Scholar]
- Yoo, B.R.; Jung, I.N. Synthesis of organosilicon compounds by new direct reactions. Adv. Organomet. Chem. 2004, 50, 145–177. [Google Scholar] [CrossRef]
- van Dalen, M.J.; van den Berg, P.J. Thermodynamics of Silicon Compounds. 2. Ethylchlorosilanes and Phenylchlorosilanes. J. Organomet. Chem. 1970, 24, 277–283. [Google Scholar] [CrossRef]
- Watanabe, H.; Asami, M.; Nagai, Y. Convenient Laboratory Synthesis of Vinylic Silicon-Compounds Via the Reactions of Acetylene with Hydrosilanes Catalyzed by Group-Viii Metal Phosphine Complexes. J. Organomet. Chem. 1980, 195, 363–373. [Google Scholar] [CrossRef]
- Trujillo, R.E.; Courtney, R.L. Organic Hydrogen Getters. 2. Hydrogenation Rates of Solid Alkynes on Palladium-Calcium Carbonate Catalysts. J. Mater. Sci. 1977, 12, 937–945. [Google Scholar] [CrossRef]
- Turnbull, A. Perspectives on hydrogen uptake, diffusion and trapping. Int. J. Hydrogen Energy 2015, 40, 16961–16970. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, G.; Dong, G.; Mu, Y.; Park, C.B.; Wang, G. Lightweight, super-elastic, and thermal-sound insulation bio-based PEBA foams fabricated by high-pressure foam injection molding with mold-opening. Eur. Polym. J. 2018, 103, 68–79. [Google Scholar] [CrossRef]
- Tessanan, W.; Phinyocheep, P.; Daniel, P.; Gibaud, A. Microcellular natural rubber using supercritical CO2 technology. J. Supercrit. Fluids 2019, 149, 70–78. [Google Scholar] [CrossRef]
- Shao, J.; Qiu, J.; Chen, W.; Wang, H.; Zhang, K.; Wu, J.; Yan, L. Self-assembled monolayers modified and further silanized graphene nanosheets reinforced silicone rubber with highly mechanical performance. Compos. Commun. 2021, 24, 100666. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, T.; Li, W.; Zhang, C.; Zhang, D.; Pang, Z. Carbon Supported Oxide-Rich Pd-Cu Bimetallic Electrocatalysts for Ethanol Electrooxidation in Alkaline Media Enhanced by Cu/CuOx. Catalysts 2016, 6, 62. [Google Scholar] [CrossRef]
- Cao, L.; Sinha, T.K.; Tao, L.; Li, H.; Zong, C.; Kim, J.K. Synergistic reinforcement of silanized silica-graphene oxide hybrid in natural rubber for tire-tread fabrication: A latex based facile approach. Compos. Part B-Eng. 2019, 161, 667–676. [Google Scholar] [CrossRef]
- Kang, H.; Tang, Y.; Yao, L.; Yang, F.; Fang, Q.; Hui, D. Fabrication of graphene/natural rubber nanocomposites with high dynamic properties through convenient mechanical mixing. Compos. Part B-Eng. 2017, 112, 1–7. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, S.-H.; Zou, Y.-F.; Ma, W.-S.; Huang, G.-J.; Li, M.-D. Improving the Thermal Conductivity and Mechanical Properties of Two-component Room Temperature Vulcanized Silicone Rubber by Filling with Hydrophobically Modified SiO2-Graphene Nanohybrids. Chin. J. Polym. Sci. 2019, 37, 189–196. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, T.; Deng, Y.; Zhang, K.; Wu, Y.; Yan, L. Silanized Graphene Oxide-Supported Pd Nanoparticles and Silicone Rubber for Enhanced Hydrogen Elimination. Materials 2022, 15, 4578. [Google Scholar] [CrossRef]


| Sample | Hydrogen Absorption | Improved Capacity | Theoretical Hydrogen Absorption | Yield Rate |
|---|---|---|---|---|
| W0-SR | 13.6 mL | 0% | 52.2 mL | 26.1% |
| W4-SR | 19.7 mL | 44.9% | 37.3% | |
| W6-SR | 40.8 mL | 200.0% | 78.2% | |
| W8-SR | 46.7 mL | 243.4% | 89.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xing, T.; Yan, L. Porous Silicone Rubber Composite Supported 1,4-Diphenylethynyl Benzene for Hydrogen Absorption with Pd/C Catalyst. Materials 2024, 17, 1921. https://doi.org/10.3390/ma17081921
Wang Y, Xing T, Yan L. Porous Silicone Rubber Composite Supported 1,4-Diphenylethynyl Benzene for Hydrogen Absorption with Pd/C Catalyst. Materials. 2024; 17(8):1921. https://doi.org/10.3390/ma17081921
Chicago/Turabian StyleWang, Yu, Tao Xing, and Lifeng Yan. 2024. "Porous Silicone Rubber Composite Supported 1,4-Diphenylethynyl Benzene for Hydrogen Absorption with Pd/C Catalyst" Materials 17, no. 8: 1921. https://doi.org/10.3390/ma17081921







