Preparation of a (Ca,Sr,Ba)ZrO3 Crucible by Slip Casting for the Vacuum Induction Melting of NiTi Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the (Ca,Sr,Ba)ZrO3 Crucible
2.2. Melting Experiment
2.3. Analysis Method
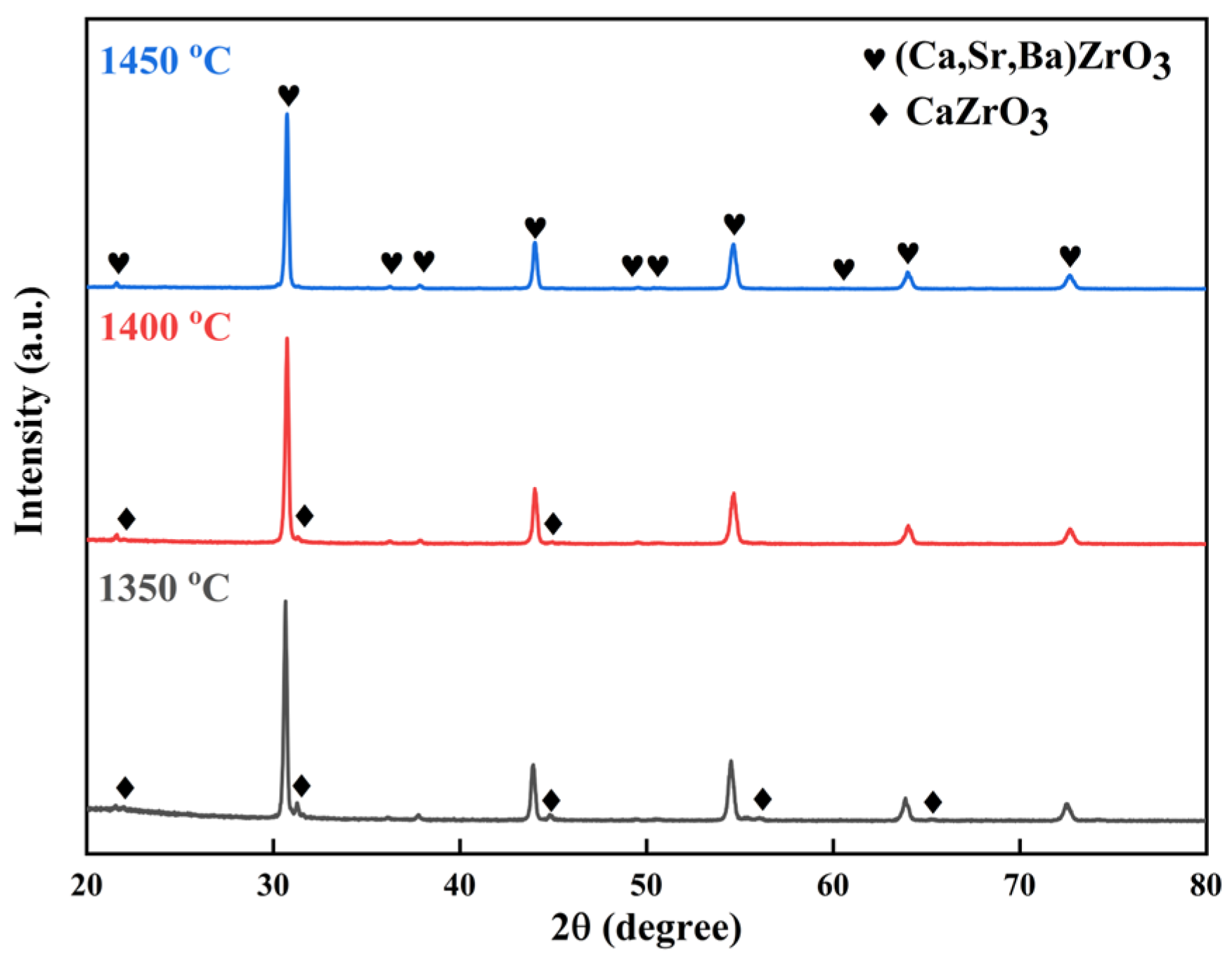
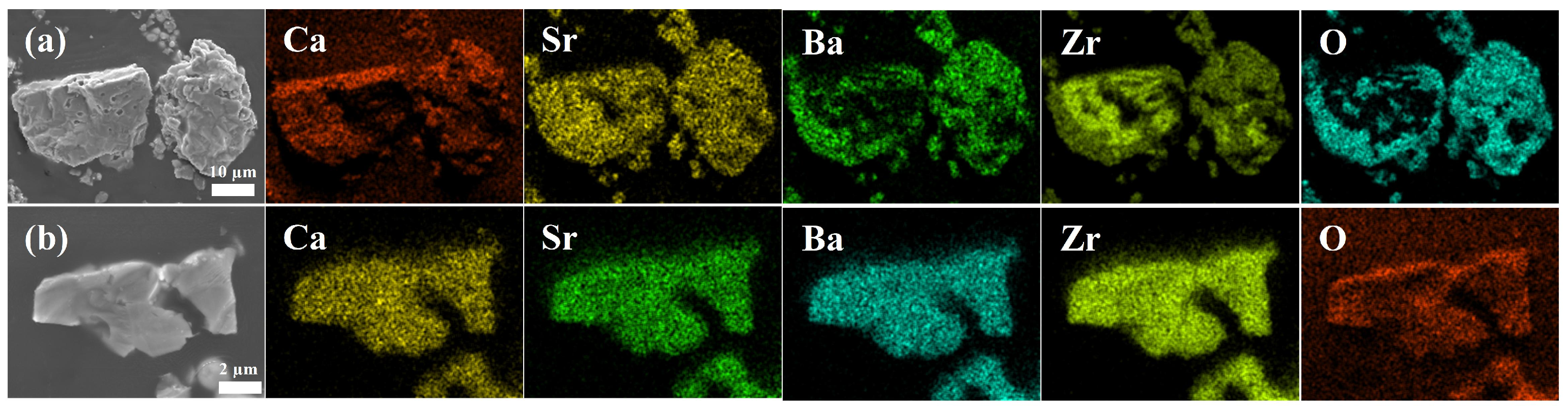
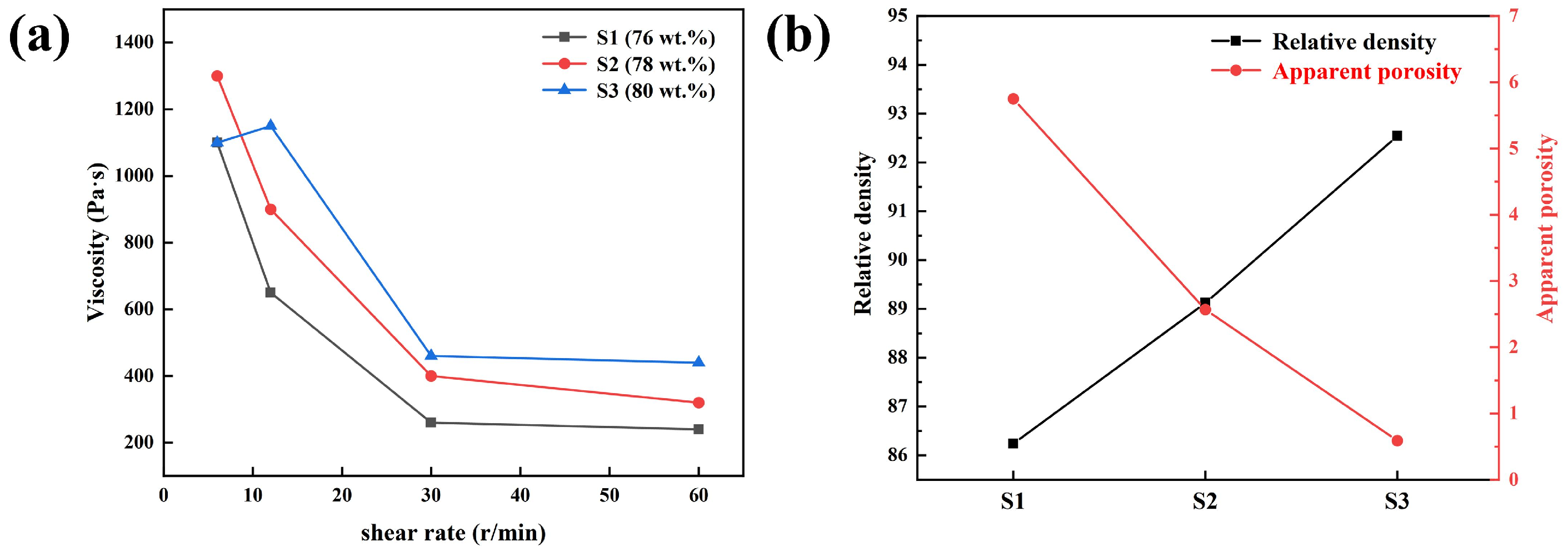
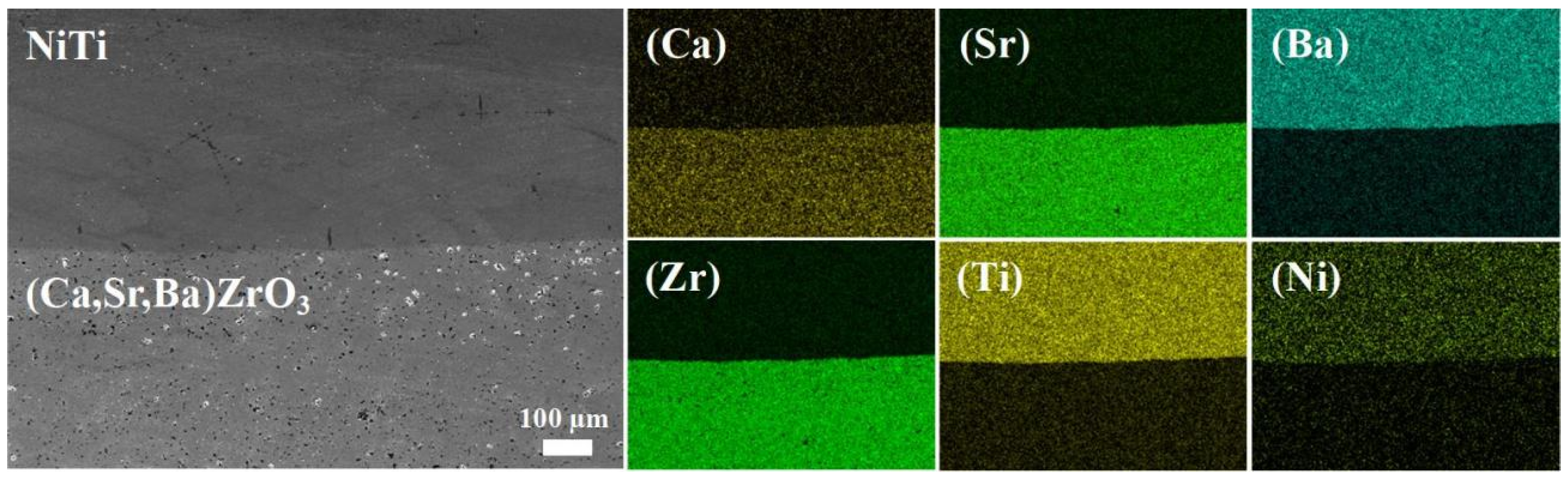
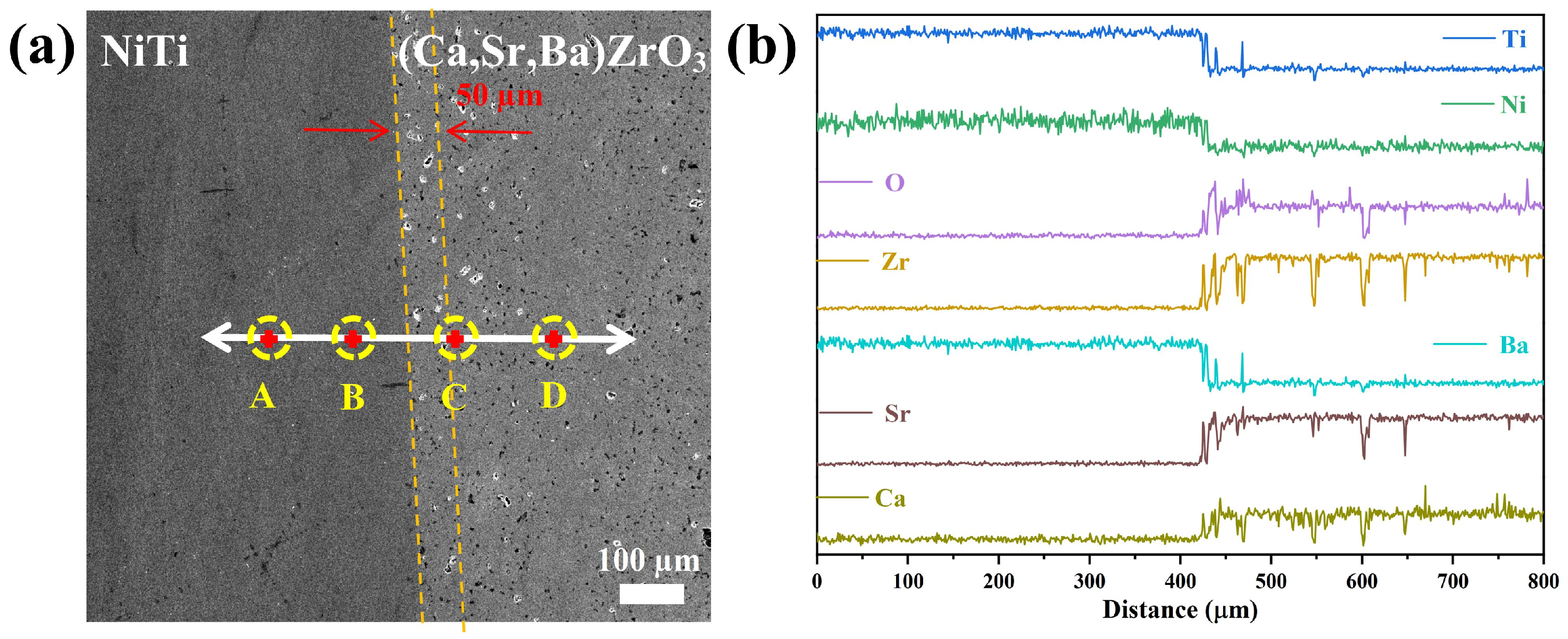
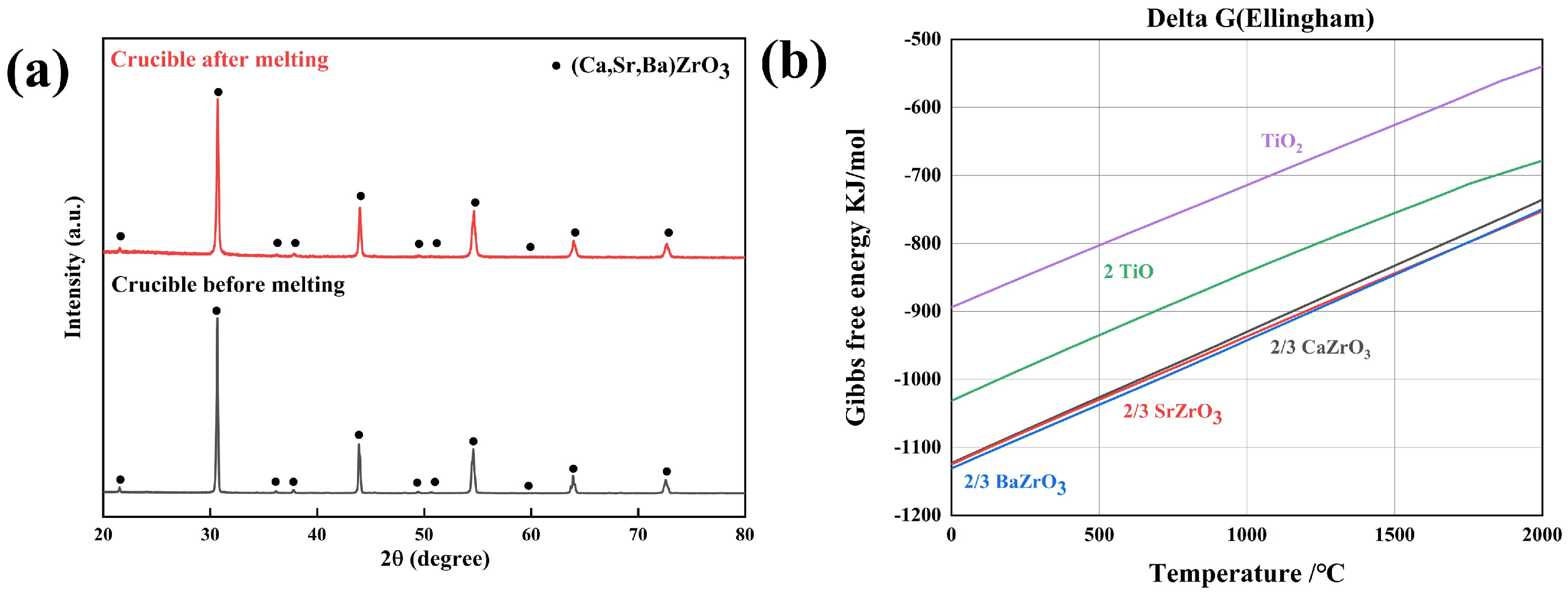
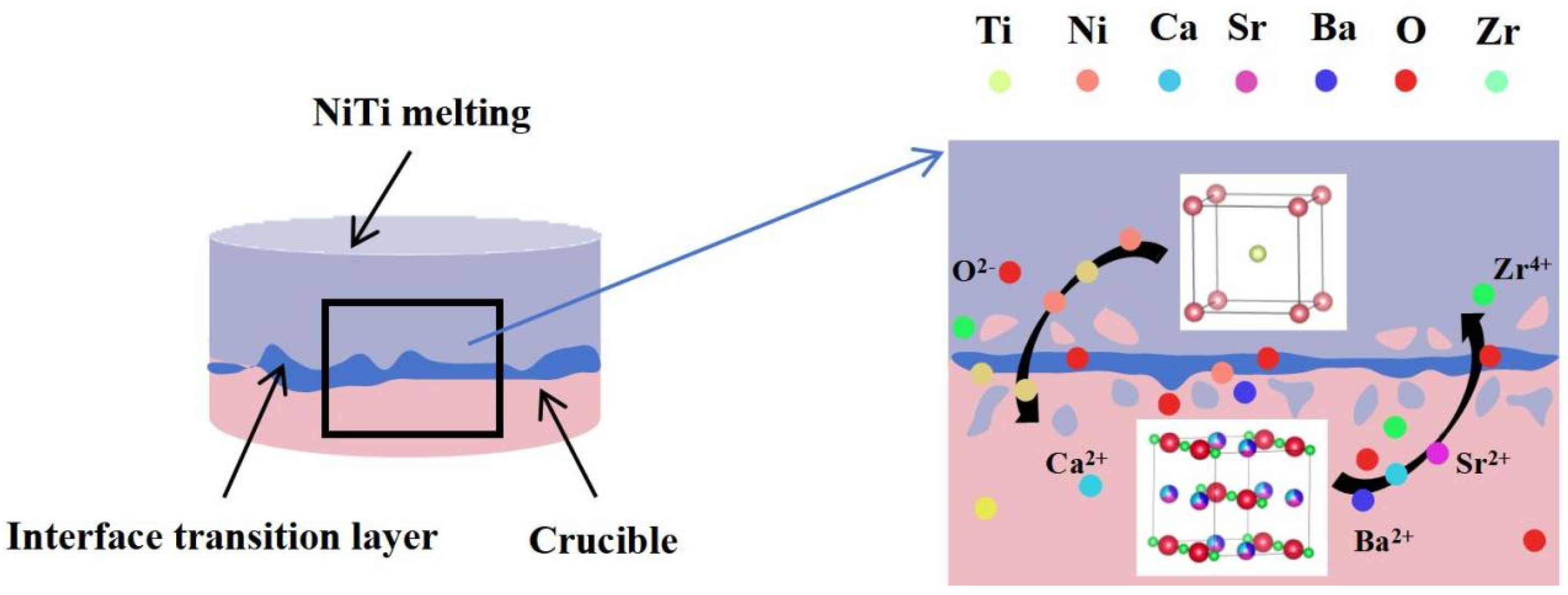
3. Results and Discussion
4. Conclusions
- (1)
- X-ray diffraction and scanning electron microscopy analyses collectively indicated that a single solid solution was formed with a homogeneous distribution of metal elements after sintering at 1500 °C.
- (2)
- There was no new phase on the surface of the (Ca,Sr,Ba)ZrO3 crucible after melting the NiTi alloy, and the interface between NiTi and (Ca,Sr,Ba)ZrO3 was very clear. Most importantly, the total content of oxygen and nitrogen elements was 0.0173 wt.% after vacuum induction melting, which was beneficial for the performance of the titanium alloy.
- (3)
- The good resistance of the (Ca,Sr,Ba)ZrO3 crucible to molten NiTi has a relationship with the sluggish diffusion effect of high-entropy ceramics. The combination of the properties indicates that (Ca,Sr,Ba)ZrO3 is a potential crucible material for high-reactivity titanium alloys through vacuum induction melting.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bil, C.; Massey, K.; Abdullah, E.J. Wing morphing control with shape memory alloy actuators. J. Intell. Mater. Syst. Struct. 2013, 24, 879–898. [Google Scholar] [CrossRef]
- Morgan, N.B. Medical shape memory alloy applications—The market and its products. Mater. Sci. Eng. A 2004, 378, 16–23. [Google Scholar] [CrossRef]
- Frenzel, J.; Zhang, Z.; Somsen, C.; Neuking, K.; Eggeler, G. Influence of carbon on martensitic phase transformations in NiTi shape memory alloys. Acta Mater. 2007, 55, 1331–1341. [Google Scholar] [CrossRef]
- Nayan, N.; Govind; Saikrishna, C.N.; Ramaiah, K.V.; Bhaumik, S.K.; Nair, K.S.; Mittal, M.C. Vacuum induction melting of NiTi shape memory alloys in graphite crucible. Mater. Sci. Eng. A 2007, 465, 44–48. [Google Scholar] [CrossRef]
- Kartavykh, A.V.; Tcherdyntsev, V.V.; Zollinger, J. TiAl–Nb melt interaction with pyrolytic boron nitride crucibles. Mater. Chem. Phys. 2010, 119, 347–350. [Google Scholar] [CrossRef]
- Frenzel, J.; Zhang, Z.; Neuking, K.; Eggeler, G. High quality vacuum induction melting of small quantities of NiTi shape memory alloys in graphite crucibles. J. Alloys Compd. 2004, 385, 214–223. [Google Scholar] [CrossRef]
- Gomes, F.; Barbosa, J.; Ribeiro, C.S. Induction melting of γ-TiAl in CaO crucibles. Intermetallics 2008, 16, 1292–1297. [Google Scholar] [CrossRef]
- Li, C.-h.; He, J.; Zhang, Z.; Yang, B.; Leng, H.-y.; Lu, X.-g.; Li, Z.-l.; Wu, Z.; Wang, H.-b. Preparation of TiFe based alloys melted by CaO crucible and its hydrogen storage properties. J. Alloys Compd. 2015, 618, 679–684. [Google Scholar] [CrossRef]
- Gao, M.; Cui, R.; Ma, L.; Zhang, H.; Tang, X.; Zhang, H. Physical erosion of yttria crucibles in Ti–54Al alloy casting process. J. Mater. Process. Technol. 2011, 211, 2004–2011. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, X.; Zhou, C.; Zhang, H.; Zhang, S. Comparison of directional solidification of γ-TiAl alloys in conventional Al2O3 and novel Y2O3-coated Al2O3 crucibles. J. Eur. Ceram. Soc. 2013, 33, 925–934. [Google Scholar] [CrossRef]
- Kostov, A.; Friedrich, B. Predicting thermodynamic stability of crucible oxides in molten titanium and titanium alloys. Comp. Mater. Sci. 2006, 38, 374–385. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, G.; Yu, F.; Hou, X.; Zhang, Y.; Zou, X.; Lu, X.; Li, C. Preparation of a novel Sr-Zr oxide refractory for induction melting of high-activity alloy. J. Eur. Ceram. Soc. 2021, 41, 6738–6743. [Google Scholar] [CrossRef]
- Tetsui, T.; Kobayashi, T.; Mori, T.; Kishimoto, T.; Harada, H. Evaluation of Yttria Applicability as a Crucible for Induction Melting of TiAl Alloy. Mater. Trans. 2010, 51, 1656–1662. [Google Scholar] [CrossRef]
- Song, Q.; Liang, T.; Qian, K.; Xing, W.; Zha, X.; Chen, B.; Ma, Y.; Liu, K. Corrosion resistance of calcium zirconate crucible to titanium-copper melts. J. Eur. Ceram. Soc. 2022, 42, 3321–3331. [Google Scholar] [CrossRef]
- Meng, D.Z.; Chen, G.Y.; Zhang, R.L.; Li, C.H. Preparation of Y2O3 Doped SrZrO3 Refractory and Study on its Interface Reaction with Molten TiNi Alloys. Key Eng. Mater. 2018, 768, 256–260. [Google Scholar] [CrossRef]
- Chen, G.; Gao, P.; Kang, J.; Li, B.; Ali, W.; Qin, Z.; Lu, X.; Li, C. Improved stability of BaZrO3 refractory with Y2O3 additive and its interaction with titanium melts. J. Alloys Compd. 2017, 726, 403–409. [Google Scholar] [CrossRef]
- Akrami, S.; Edalati, P.; Fuji, M.; Edalati, K. High-entropy ceramics: Review of principles, production and applications. Mat. Sci. Eng. R 2021, 146, 100644. [Google Scholar] [CrossRef]
- Qiu, S.; Li, M.; Shao, G.; Wang, H.; Zhu, J.; Liu, W.; Fan, B.; Xu, H.; Lu, H.; Zhou, Y.; et al. (Ca,Sr,Ba)ZrO3: A promising entropy-stabilized ceramic for titanium alloys smelting. J. Mater. Sci. Technol. 2021, 65, 82–88. [Google Scholar] [CrossRef]
- Shafeiey, A.; Enayati, M.H.; Al-Haji, A. The effect of slip casting parameters on the green density of MgAl2O4 spinel. Ceram. Int. 2017, 43, 6069–6074. [Google Scholar] [CrossRef]
- Deng, Z.; Wei, Y.; Zhou, H.; Tuo, K.; Wang, Y.; Zhang, Y. Influences of solid content of slurry on the microstructure of CaO crucible prepared by slip casting. Int. J. Appl. Ceram. Technol. 2023, 20, 1547–1556. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, L.; Chen, J.; Guo, W. Fabrication of Y2O3 transparent ceramics by pressure-assisted alcoholic slip casting. Ceram. Int. 2023, 49, 37729–37736. [Google Scholar] [CrossRef]
- Michálek, M.; Bodišová, K.; Michálková, M.; Sedláček, J.; Galusek, D. Alumina/MWCNTs composites by aqueous slip casting and pressureless sintering. Ceram. Int. 2013, 39, 6543–6550. [Google Scholar] [CrossRef]
- Fatimi, A.; Tassin, J.-F.; Axelos, M.A.V.; Weiss, P. The stability mechanisms of an injectable calcium phosphate ceramic suspension. J. Mater. Sci. Mater. Med. 2010, 21, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Zhang, F.; Cheng, F.; Hou, J.; Ren, B.; Miao, Y.; Xin, L.; Wang, X. The effect of plasma-assisted ball milling on preparation and sintering behavior of (Zr0.1429Hf0.1429Ce0.1429Y0.2857La0.2857)O2-δ high entropy fluorite oxide. Ceram. Int. 2023, 49, 13092–13101. [Google Scholar] [CrossRef]
- Huo, D.; Zheng, Y.; Sun, X.; Li, X.; Liu, S. Preparation of transparent Y2O3 ceramic by slip casting and vacuum sintering. J. Rare Earths 2012, 30, 57–62. [Google Scholar] [CrossRef]
- Fashu, S.; Lototskyy, M.; Davids, M.W.; Pickering, L.; Linkov, V.; Tai, S.; Renheng, T.; Fangming, X.; Fursikov, P.V.; Tarasov, B.P. A review on crucibles for induction melting of titanium alloys. Mater. Design. 2020, 186, 108295. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Wang, E.; Chen, G.; Xu, E.; Zhao, F.; Zhao, Y.; Li, C.; Hou, X. Interaction between CA6-MA crucible and molten wrought Ni-based superalloys. J. Eur. Ceram. Soc. 2023, 43, 1714–1722. [Google Scholar] [CrossRef]
- Otubo, J.; Rigo, O.D.; Neto, C.M.; Mei, P.R. The effects of vacuum induction melting and electron beam melting techniques on the purity of NiTi shape memory alloys. Mater. Sci. Eng. A 2006, 438–440, 679–682. [Google Scholar] [CrossRef]
- Gao, P.; Liu, Y.; Ren, Y.; Chen, G.; Lan, B.; Lu, X.; Li, C. Evaluation of the microstructure and property of TiNi SMA prepared using VIM in BaZrO3 crucible. Vacuum 2019, 168, 108843. [Google Scholar] [CrossRef]
- Qiu, S.; Xiang, H.; Dai, F.-Z.; Wang, H.; Huang, M.; Wan, C.; Meng, Q.; Li, J.; Wang, X.; Zhou, Y. Medium-entropy (Me,Ti)0.1(Zr,Hf,Ce)0.9O2 (Me = Y and Ta): Promising thermal barrier materials for high-temperature thermal radiation shielding and CMAS blocking. J. Mater. Sci. Technol. 2022, 123, 144–153. [Google Scholar] [CrossRef]
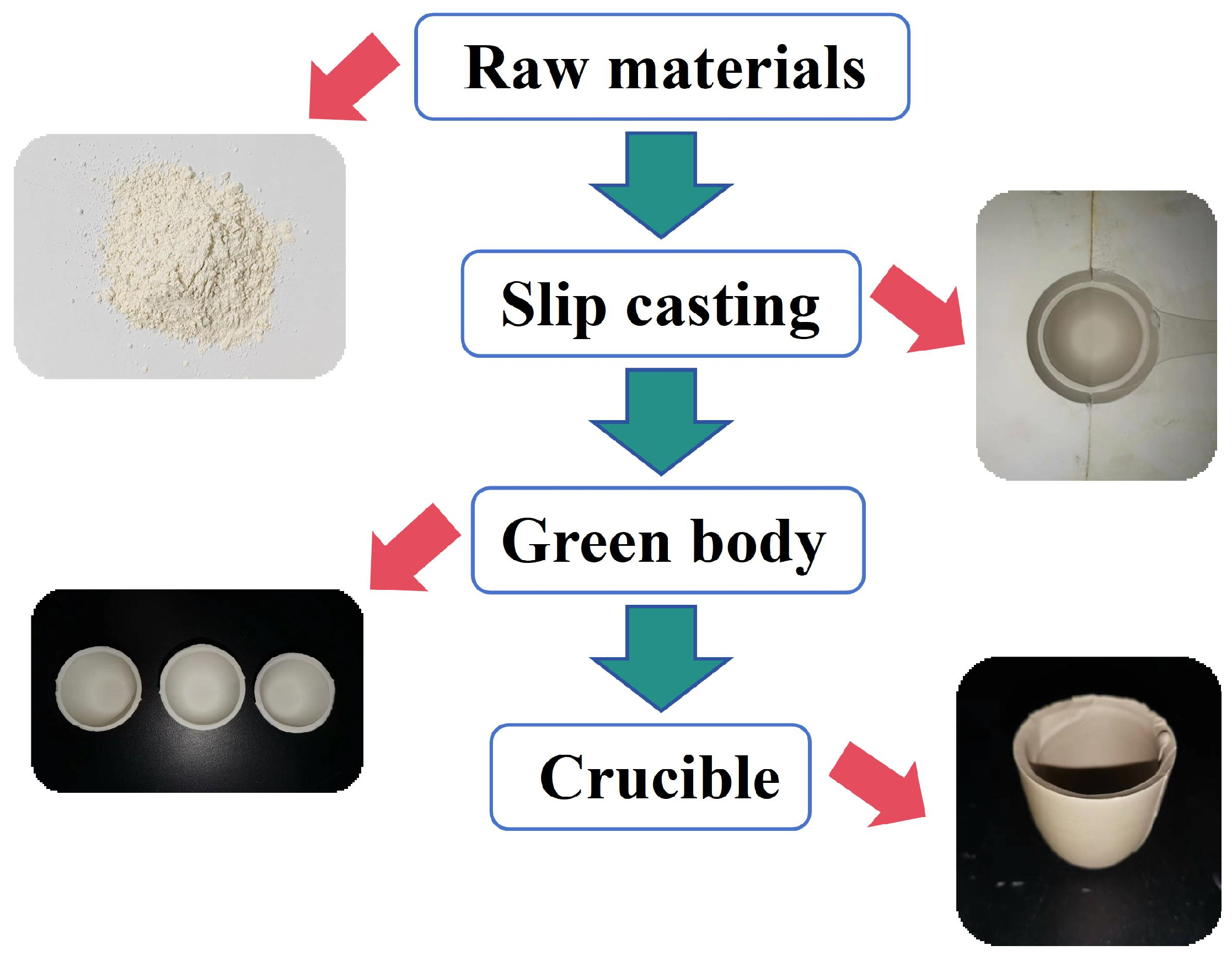

| Grinding Time (h) | Average Particle Size (µm) | D10 (µm) | D50 (µm) |
|---|---|---|---|
| 6 | 1.535 | 0.658 | 2.089 |
| 8 | 1.521 | 0.650 | 2.054 |
| 10 | 1.621 | 0.696 | 2.399 |
| Sample | Solid Content of Slurry (wt.%) | Gum Arabic Content (wt.%) |
|---|---|---|
| S1 | 76 | 1.2 |
| S2 | 78 | 1.1 |
| S3 | 80 | 1.0 |
| Point | Ca (at.%) | Sr (at.%) | Ba (at.%) | Zr (at.%) | O (at.%) | Ti (at.%) | Ni (at.%) | Possible Phase |
|---|---|---|---|---|---|---|---|---|
| A | - | - | - | - | - | 52.43 | 47.57 | NiTi |
| B | - | - | - | - | - | 50.91 | 49.09 | NiTi |
| C | 6.80 | 6.65 | 7.23 | 23.54 | 55.77 | - | - | (Ca,Sr,Ba)ZrO3 |
| D | 6.64 | 6.91 | 7.83 | 23.67 | 54.95 | - | - | (Ca,Sr,Ba)ZrO3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, S.; Li, M.; Wang, H.; Zhu, J.; Shao, G.; Xu, H.; Lu, H.; Zhang, R. Preparation of a (Ca,Sr,Ba)ZrO3 Crucible by Slip Casting for the Vacuum Induction Melting of NiTi Alloy. Materials 2024, 17, 1924. https://doi.org/10.3390/ma17081924
Ding S, Li M, Wang H, Zhu J, Shao G, Xu H, Lu H, Zhang R. Preparation of a (Ca,Sr,Ba)ZrO3 Crucible by Slip Casting for the Vacuum Induction Melting of NiTi Alloy. Materials. 2024; 17(8):1924. https://doi.org/10.3390/ma17081924
Chicago/Turabian StyleDing, Shijia, Mingliang Li, Hailong Wang, Jinpeng Zhu, Gang Shao, Hongliang Xu, Hongxia Lu, and Rui Zhang. 2024. "Preparation of a (Ca,Sr,Ba)ZrO3 Crucible by Slip Casting for the Vacuum Induction Melting of NiTi Alloy" Materials 17, no. 8: 1924. https://doi.org/10.3390/ma17081924




