Using RAFT Polymerization Methodologies to Create Branched and Nanogel-Type Copolymers
Abstract
:1. Introduction
2. RAFT Polymerization
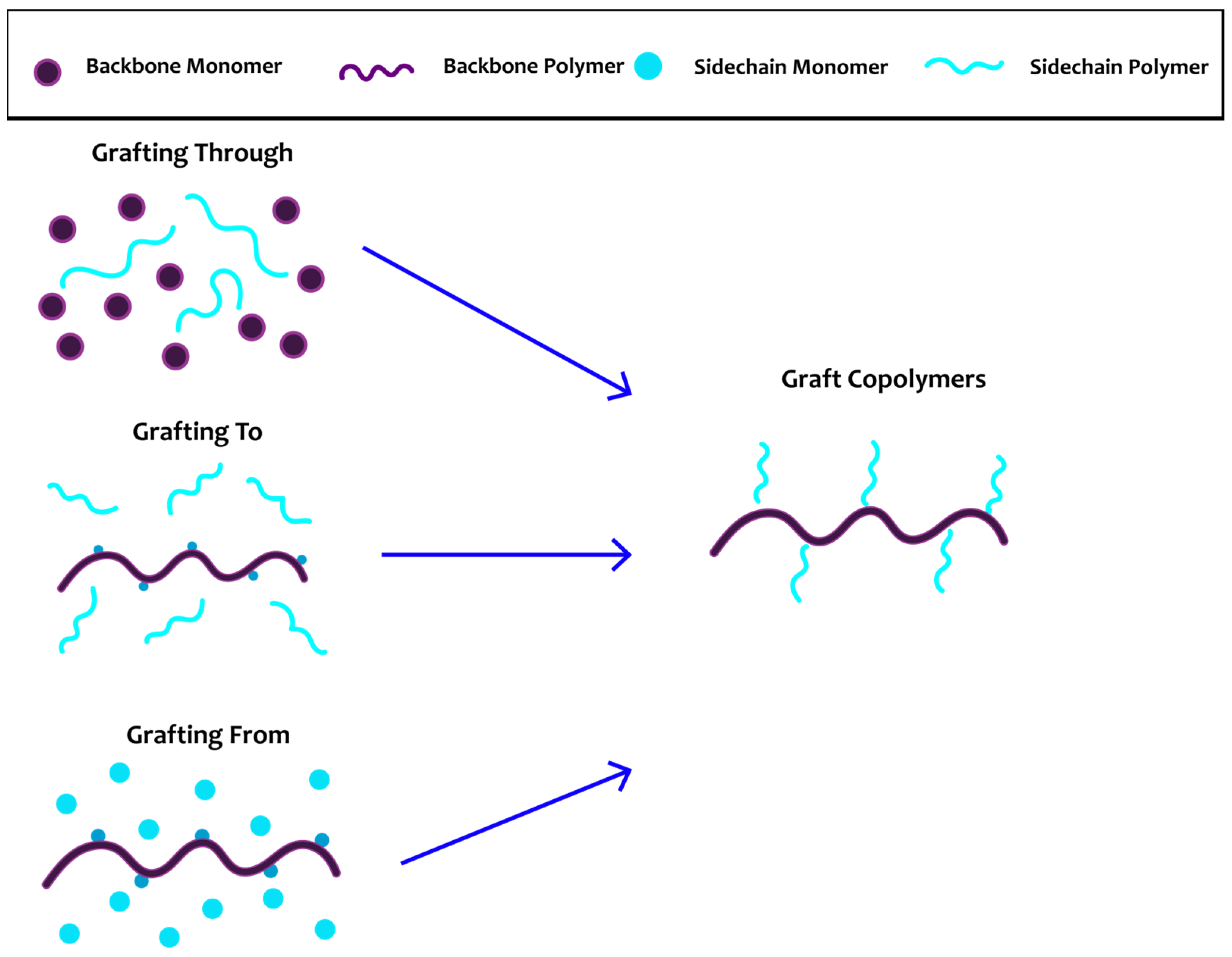
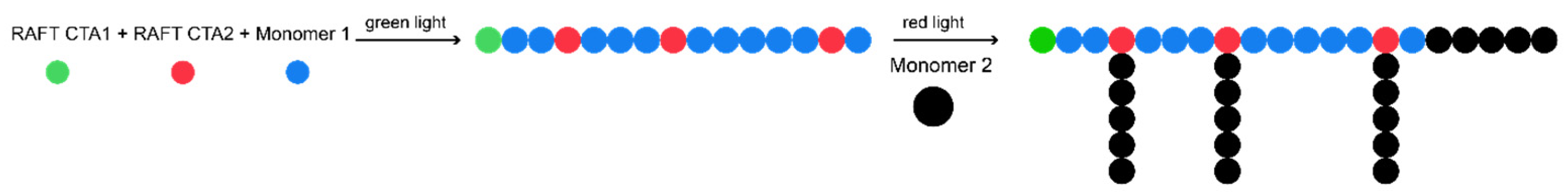
3. Graft Copolymers
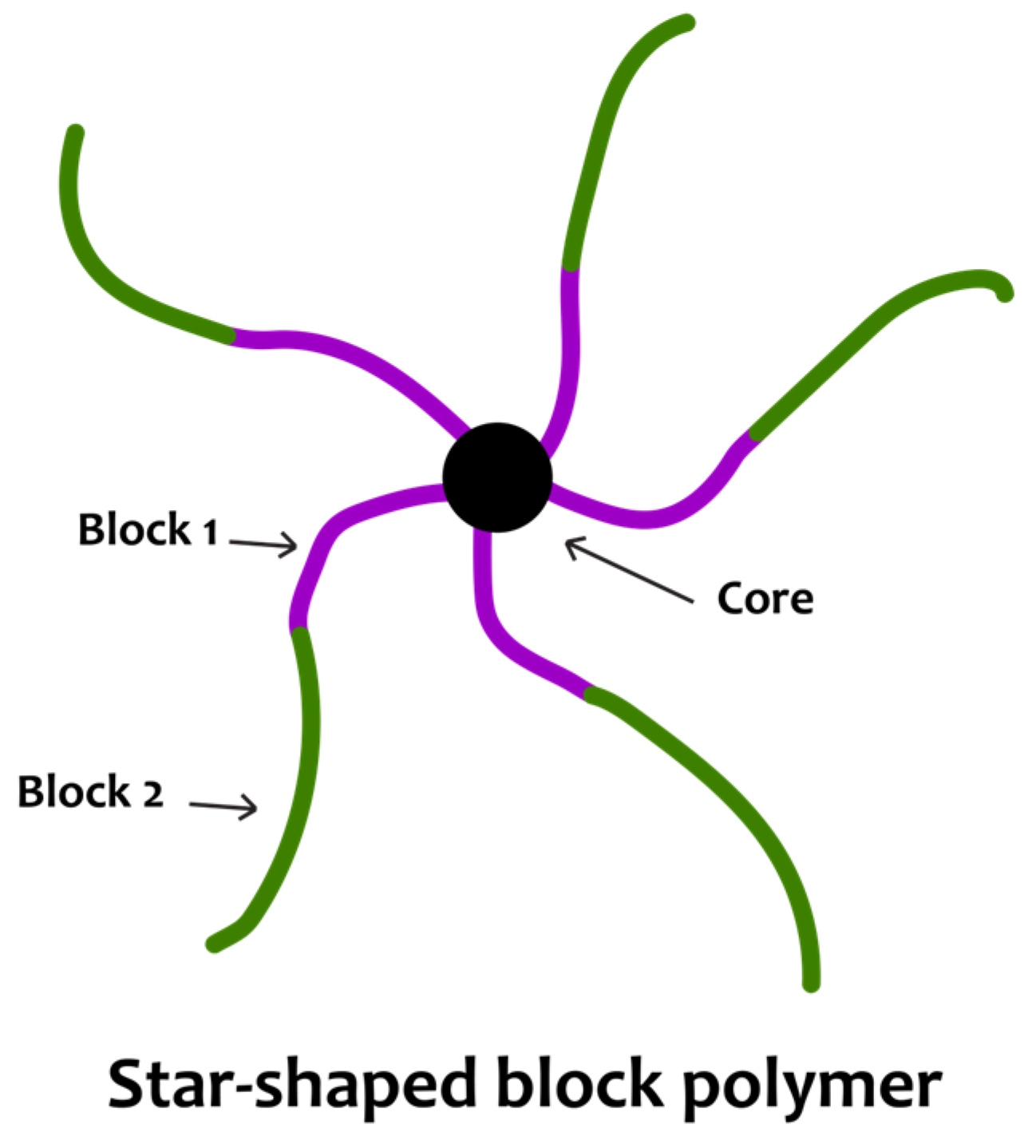
4. Star Copolymers
5. Hyperbranched Copolymers
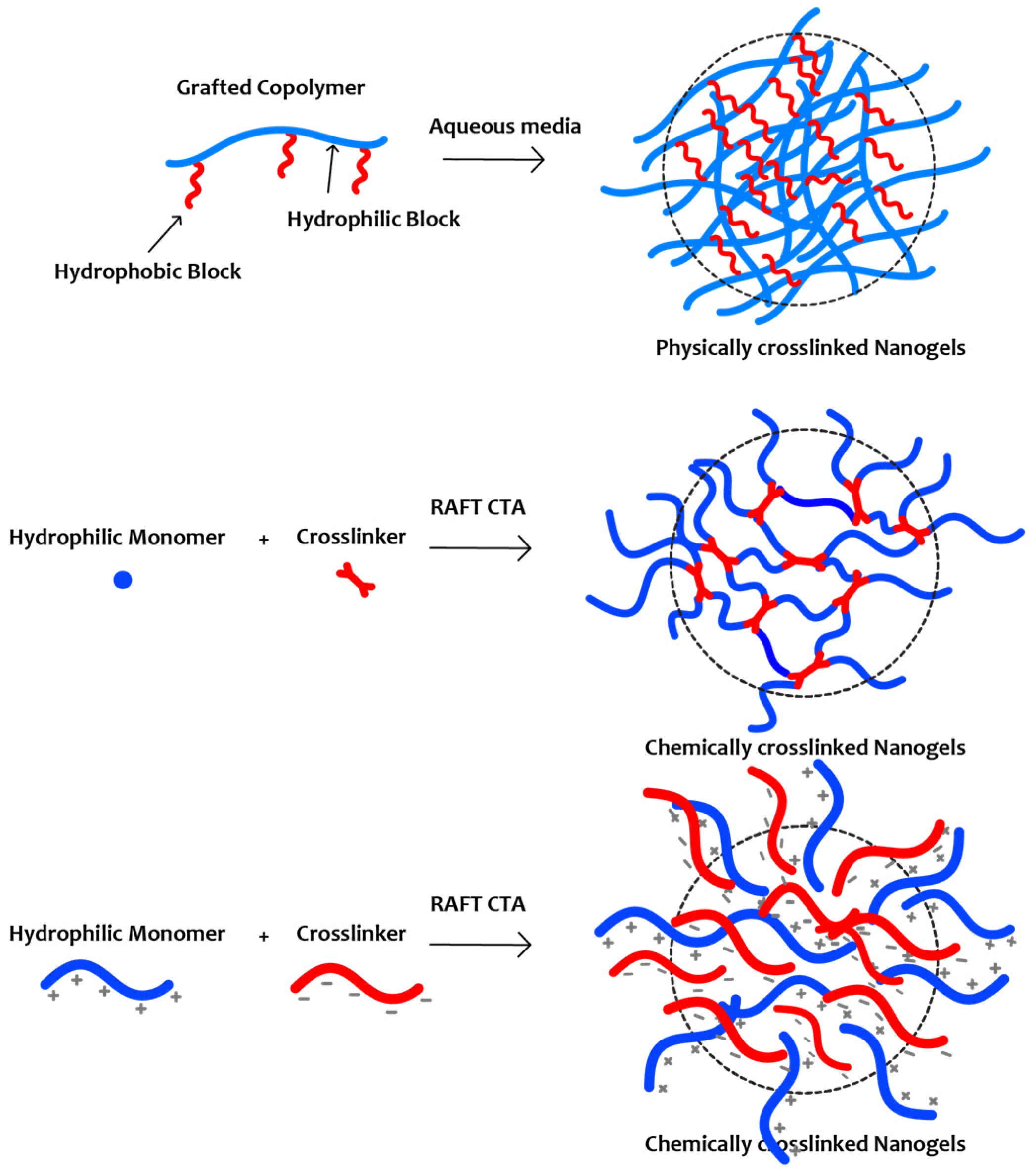
6. Nanogels
6.1. Chemically Crosslinked Nanogels
6.2. Nanogels Formed by Hydrophobic Interactions
6.3. Nanogels Formed by Complexation or Coacervation
| Polymer Name | Macromolecular Architecture | RAFT Agent | Synthetic Method | Citation |
|---|---|---|---|---|
| P((MMA-statBTPEMA)-block-PDMAm)-graft-PDMAm | Graft copolymer | CDTPA and BTPEMA | PET-RAFT | Corrigan et al. [44] |
| P(EGMA-co-BTPEMA)-b-PS-g-PS | Graft copolymer | BTPEMA and CEPA | PET-RAFT | Yang et al. [45] |
| P((OEGMA-co-BTPEMA)-b-NIPAAm)-g-PNIPAAm | Graft copolymer | CPDTC and BTPEMA | PET-RAFT | Xu et al. [46] |
| PMAA-g-PPC | Graft copolymer | CPAD | RAFT | Alagi et al. [47] |
| PHEMA-g-PCL | Graft copolymer | CPADB | RAFT and ROP | Guo et al. [48] |
| POEGMA-b-PMMA)-g-P(GMA-N3) | Graft copolymer | CPADB | RAFT | Thankappan et al. [49] |
| PEHA or PDMA-g-EtOx | Bottlebrush- and comb-like copolymers | BCTPA | RAFT and ROP | Kim et al. [50] |
| (cellulose-4-dimethylaminopyridine) -g-P(AA-co-MMA) | Graft copolymer | Bagasse Cellulose-modified CTA | RAFT and click chemistry | Assen et al. [53] |
| Dextran-g-PHPMA | Graft copolymer | Dextran- modified CTA | RAFT and post-polymerization functionalization | Ikkene et al. [32,54,55] |
| Dextran-g-PHPMA | Graft copolymer | Dextran- modified CTA | RAFT and post-polymerization functionalization | Ferji et al. [56] |
| CS-g-PNIPAAm and Hep-g-PNIPAAm | Graft copolymer | DDMAT | RAFT | Pilipenko et al. [57,58] |
| (PS-b-PI)arms-(DVB-co-PS)core | Star copolymer | CDTP | RAFT | Ge et al. [64] |
| (PMMA-b-TMSPMA)arms-(Peptides) | Star copolymer | CDB | RAFT | Volski et al. [65] |
| PSarms-PHOcore | Star copolymer | PHO-based CTA | RAFT | Alli et al. [66] |
| (PDMAEMA-b-POEGMA)arms-EGDMAcore | Star copolymer | CPAD | RAFT | Skandalis et al. [67] |
| POEGMAarms-(PHPMA-co-EGDMA)core | Star copolymer | CPAD | RAFT | Sentoukas et al. [69] |
| (PNIPAAm)arms-(PS)core | Star copolymer | TTC | RAFT | Qu et al. [70] |
| (PtBA-b-PDMAEMA)-(allyl-ether)core | Star copolymer | RAFT and click chemistry | Xue et al. [71] | |
| PDMAarms | Star copolymer | TTC | RAFT PISA | Zeng et al. [72] |
| BMA, Econea and Divinyl PCL | Hyperbranched copolymer | CDTP | RAFT | Ai et al. [79] |
| mod-tCBEA-co-MAAh | Hyperbranched copolymer | CPDT | RAFT | Dai et al. [81] |
| DAAH-co-MAAh-co-EGDMA | Hyperbranched copolymer | CPDT | RAFT | Pan et al. [82] |
| POEGMA-co-DIPAEMA | Hyperbranched copolymer | CPAD | RAFT | Selianitis et al. [84] |
| P(AA-co-DMAEMA-co DSDA) | Hyperbranched copolymer | CDCTPA | RAFT | Blackburn et al. [86] |
| mod-MEDMA | Hyperbranched copolymer | CPDBA | RAFT | Chen et al. [87] |
| PPFPA, PTFPA | Hyperbranched copolymer | Modified CTA | SCVP RAFT | Bachler et al. [90] |
| P(AbPA) | Hyperbranched copolymer | Modified CTA | SCVP RAFT | Calvo et al. [91] |
| P(SAA) | Hyperbranched copolymer | Modified CTA | SCVP RAFT | Nandi et al. [95] |
| P(MMA-co-EGDMA) | Hyperbranched copolymer | CDB | RAFT | Lin et al. [99] |
| P(AG) | Hyperbranched copolymers | DBCNT | RAFT | Forrester et al. [100] |
| Linear PEG and nonlinear POEG | Nanogel | TTC and CPAD | RAFT | Shen et al. [108] |
| P(MPC)-PAEMA-PMeODEGMA) | Nanogel | Macro-MPC | RAFT | Bhuchar et al. [109] |
| POEGMA-(LAEMA-co-CL), POEGMA-(GAPMA-co-CL) | Nanogel | CPAD | RAFT | Ahmed et al. [110] |
| HA-P(DEGMA-co-OEGMA) | Nanogel | CPAD | RAFT | Stefanello et al. [114] |
| PAA-b-PNIPAAm | Nanogel | DMP | RAFT | Don et al. [113] |
| PHPMA-PDFEA, PMeOx-PDFEA | Nanogel | CPAD and CDTP | RAFT | Kolouchova et al. [115] |
| PDMA-b-PFPA | Nanogel | PABTC | RAFT | Van Driessche et al. [111] |
| P(VPD-VF) | Nanogel | MECTP | RAFT | Peng et al. [116] |
| MPEGMA-co-ONB | Nanogel | CPDB | RAFT | Xin et al. [112] |
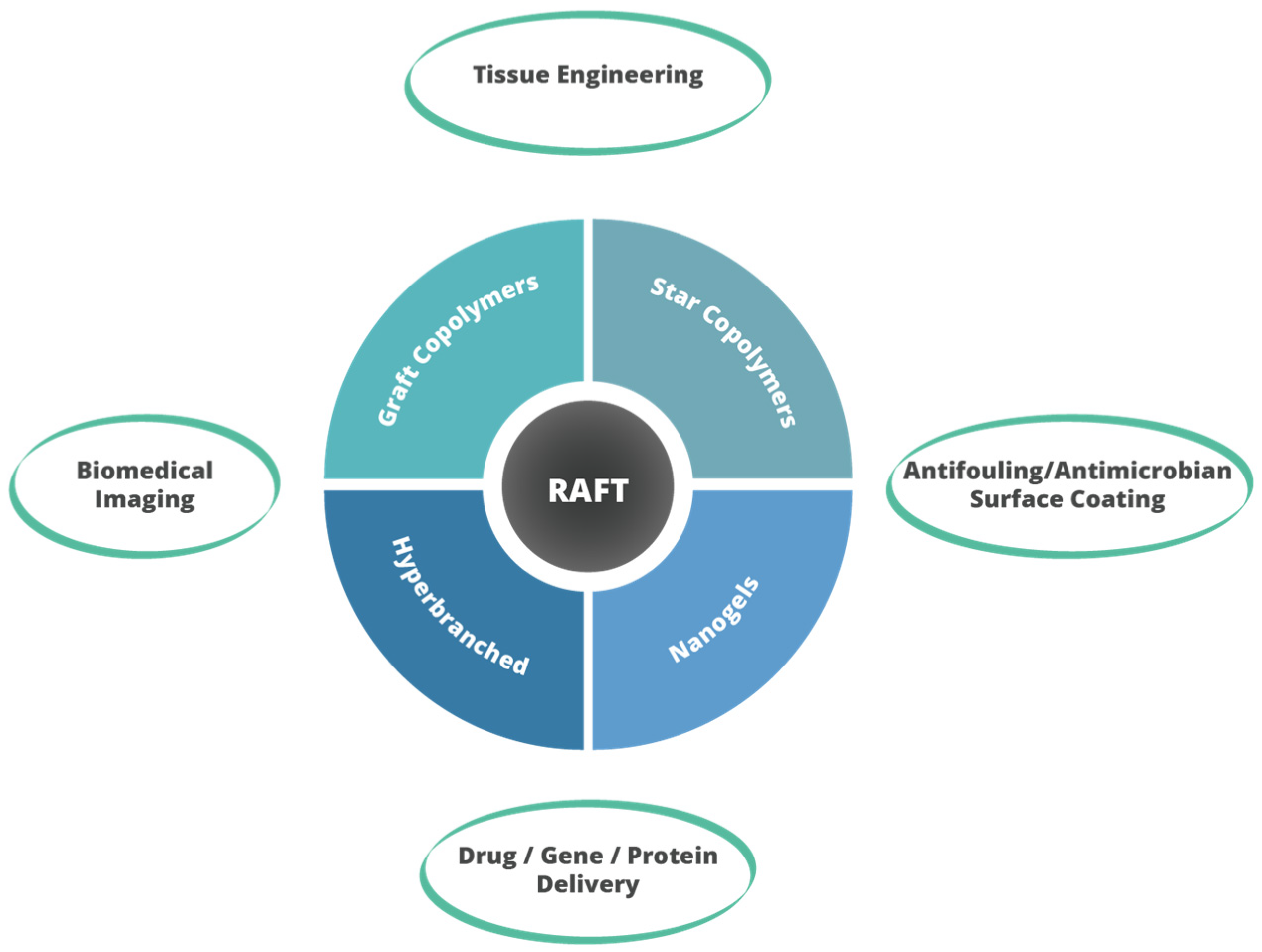
7. Potential Applications of Branched (Co)polymers
8. Challenges and Limitations
8.1. Synthesis Complexity and Scalability
8.2. Cost-Effectiveness
8.3. Environmental and Safety Concerns
8.4. Polymerization Control and Predictability
8.5. Biocompatibility and Biodegradation
9. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Glossary—Abbreviations
| AEMA | 2-AminoethylMethacrylamideHydrochloride |
| AG | AcrylateGlycerol |
| ATRP | Atom Transfer RadicalPolymerization |
| BDB | BenzylDithiobenzoate |
| BMA | ButylMethacrylate |
| BSA | BovineSerumAlbumin |
| BzTC | BenzylTrithiocarbonate |
| CL | 22-Dimethacroyloxy-1-Ethoxypropane |
| DAA2H | NN-AdipicBis(DiacetoneAcrylamideHydrazone) |
| DMA | N,N-Dimethylacrylamide |
| DOX | Doxorubicin |
| DSDA | DisulfideDiacrylate |
| DVB | Divinylbenzene |
| ECT | EthylCyanovalericTrithiocarbonate |
| EGDMA | EthyleneGlycolDimethacrylate |
| EHA | 2-EthylhexylAcrylate |
| EGMA | (EthyleneGlycol)MethylEtherMethacrylate |
| FBS | Fetal Bovine Serum |
| GAPMA | 3-GluconamidopropylMethacrylamide |
| HA | Hyaluronic Acid |
| IPrTC | IsopropylTrithiocarbonate |
| LAEMA | 2-LactobionamidoethylMethacrylamide |
| MAAh | MethacrylicAnhydride |
| MEDMA | Methyl-2-Ethylidene-5-Hydroxyhept-6-EnoateMethacrylate |
| MPEGMA | MethoxyPolyethyleneGlycolMethacrylate |
| MPC | MethacryloyloxyethylPhosphorylcholine |
| NMP | Nitroxide-MediatedPolymerization |
| OEGMA | Oligo(EthyleneGlycol)Methacrylate |
| ONB | 5-(Acryloyloxy)-2-NitrobenzylAcrylate |
| PDFEA | Poly(N(22-Difluoroethyl)Acrylamide) |
| PDMAEMA | Poly(2-DimethylaminoEthylMethacrylate) |
| PDMAm | Poly(N,N-Dimethylacrylamide) |
| PEG | Poly(EthyleneGlycol) |
| PEGMA | Poly(EthyleneGlycol)MethylEtherMethacrylate |
| PFPA | Pentafluorophenylacrylate |
| PHO | Poly(3-HydroxyOctanoate) |
| PHPMA | Poly(N-(2-Hydroxypropyl)Methacrylamide) |
| PISA | Polymerization-Induced Self-Assembly |
| PMeOx | Poly(2-Methyl-2-Oxazoline) |
| PMMA | Poly(MethylMethacrylate) |
| PMAA | Poly(MethacrylicAcid) |
| PNIPAAm | Poly(N-Isopropylacrylamide) |
| PTFPA | 2356-TetraFluorophenylAcrylate |
| RAFT | Reversible Addition-Fragmentation chain Transfer |
| RDRP | Reversible Deactivation Radical Polymerization |
| ROP | Ring-Opening Polymerization |
| SAA | Stearoyl-Appended Pendant Amino Acid |
References
- Matyjaszewski, K. Advanced Materials by Atom Transfer Radical Polymerization. Adv. Mater. 2018, 30, 1706441. [Google Scholar] [CrossRef]
- Tardy, A.; Nicolas, J.; Gigmes, D.; Lefay, C.; Guillaneuf, Y. Radical Ring-Opening Polymerization: Scope, Limitations, and Application to (Bio)Degradable Materials. Chem. Rev. 2017, 117, 1319–1406. [Google Scholar] [CrossRef]
- Lamontagne, H.R.; Lessard, B.H. Nitroxide-Mediated Polymerization: A Versatile Tool for the Engineering of Next Generation Materials. ACS Appl. Polym. Mater. 2020, 2, 5327–5344. [Google Scholar] [CrossRef]
- Moad, G. RAFT polymerization to form stimuli-responsive polymers. Polym. Chem. 2017, 8, 177–219. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Lee, W. A Review of Polymeric Micelles and Their Applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef]
- Mable, C.J.; Fielding, L.A.; Derry, M.J.; Mykhaylyk, O.O.; Chambon, P.; Armes, S.P. Synthesis and pH-responsive dissociation of framboidal ABC triblock copolymer vesicles in aqueous solution. Chem. Sci. 2018, 9, 1454–1463. [Google Scholar] [CrossRef]
- Blanazs, A.; Armes, S.P.; Ryan, A.J. Self-Assembled Block Copolymer Aggregates: From Micelles to Vesicles and their Biological Applications. Macromol. Rapid Commun. 2009, 30, 267–277. [Google Scholar] [CrossRef]
- Mable, C.J.; Thompson, K.L.; Derry, M.J.; Mykhaylyk, O.O.; Binks, B.P.; Armes, S.P. ABC Triblock Copolymer Worms: Synthesis, Characterization, and Evaluation as Pickering Emulsifiers for Millimeter-Sized Droplets. Macromolecules 2016, 49, 7897–7907. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, T.; Jiang, C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm. Sin. B 2018, 8, 34–50. [Google Scholar] [CrossRef]
- Fus-Kujawa, A.; Sieron, L.; Dobrzynska, E.; Chajec, L.; Mendrek, B.; Jarosz, N.; Glowacki, L.; Dubaj, K.; Dubaj, W.; Kowalczuk, A.; et al. Star Polymers as Non-Viral Carriers for Apoptosis Induction. Biomolecules 2022, 12, 608. [Google Scholar] [CrossRef]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Liu, S. Emerging trends in solution self-assembly of block copolymers. Polymer 2020, 207, 122914. [Google Scholar] [CrossRef]
- Karayianni, M.; Pispas, S. Self-Assembly of Amphiphilic Block Copolymers in Selective Solvents. In Fluorescence Studies of Polymer Containing Systems; Springer Series on Fluorescence; Springer: Berlin/Heidelberg, Germany, 2016; pp. 27–63. [Google Scholar]
- Hu, S.; Yan, J.; Yang, G.; Ma, C.; Yin, J. Self-Assembled Polymeric Materials: Design, Morphology, and Functional-Oriented Applications. Macromol. Rapid Commun. 2022, 43, e2100791. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, T.; Gebka, K.; Stolarczyk, A. Recent Advances in Conjugated Graft Copolymers: Approaches and Applications. Molecules 2019, 24, 3019. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, C.; Momin, M.; Gharat, S.; Omri, A. Functionalized and graft copolymers of chitosan and its pharmaceutical applications. Expert. Opin. Drug Deliv. 2017, 14, 1189–1204. [Google Scholar] [CrossRef]
- Purohit, P.; Bhatt, A.; Mittal, R.K.; Abdellattif, M.H.; Farghaly, T.A. Polymer Grafting and its chemical reactions. Front. Bioeng. Biotechnol. 2023, 10, 1044927. [Google Scholar] [CrossRef]
- Ren, J.M.; McKenzie, T.G.; Fu, Q.; Wong, E.H.; Xu, J.; An, Z.; Shanmugam, S.; Davis, T.P.; Boyer, C.; Qiao, G.G. Star Polymers. Chem. Rev. 2016, 116, 6743–6836. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, S.; Weng, Z.; Gao, C. Hyperbranched polymers: Advances from synthesis to applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef] [PubMed]
- Karg, M.; Pich, A.; Hellweg, T.; Hoare, T.; Lyon, L.A.; Crassous, J.J.; Suzuki, D.; Gumerov, R.A.; Schneider, S.; Potemkin, I.I.; et al. Nanogels and Microgels: From Model Colloids to Applications, Recent Developments, and Future Trends. Langmuir 2019, 35, 6231–6255. [Google Scholar] [CrossRef]
- Cook, A.B.; Perrier, S. Branched and Dendritic Polymer Architectures: Functional Nanomaterials for Therapeutic Delivery. Adv. Funct. Mater. 2020, 30, 1901001. [Google Scholar] [CrossRef]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.-C. Polysaccharide based nanogels in the drug delivery system: Application as the carrier of pharmaceutical agents. Mater. Sci. Eng. C 2016, 68, 964–981. [Google Scholar] [CrossRef]
- Mendrek, B.; Sieroń, Ł.; Libera, M.; Smet, M.; Trzebicka, B.; Sieroń, A.L.; Dworak, A.; Kowalczuk, A. Polycationic star polymers with hyperbranched cores for gene delivery. Polymer 2014, 55, 4551–4562. [Google Scholar] [CrossRef]
- Nicolle, L.; Casper, J.; Willimann, M.; Journot, C.M.A.; Detampel, P.; Einfalt, T.; Grisch-Chan, H.M.; Thöny, B.; Gerber-Lemaire, S.; Huwyler, J. Development of Covalent Chitosan-Polyethylenimine Derivatives as Gene Delivery Vehicle: Synthesis, Characterization, and Evaluation. Int. J. Mol. Sci. 2021, 22, 3828. [Google Scholar] [CrossRef]
- Seo, S.E.; Hawker, C.J. The Beauty of Branching in Polymer Science. Macromolecules 2020, 53, 3257–3261. [Google Scholar] [CrossRef]
- Moad, C.L.; Moad, G. Fundamentals of reversible addition–fragmentation chain transfer (RAFT). Chem. Teach. Int. 2021, 3, 3–17. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living Radical Polymerization by the RAFT Process—A Second Update. Aust. J. Chem. 2009, 62, 1402–1472. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Radical addition–fragmentation chemistry in polymer synthesis. Polymer 2008, 49, 1079–1131. [Google Scholar] [CrossRef]
- Wan, J.; Fan, B.; Thang, S.H. RAFT-mediated polymerization-induced self-assembly (RAFT-PISA): Current status and future directions. Chem. Sci. 2022, 13, 4192–4224. [Google Scholar] [CrossRef] [PubMed]
- Neal, T.J.; Penfold, N.J.W.; Armes, S.P. Reverse Sequence Polymerization-Induced Self-Assembly in Aqueous Media. Angew. Chem. Int. Ed. Engl. 2022, 61, e202207376. [Google Scholar] [CrossRef] [PubMed]
- Ikkene, D.; Arteni, A.A.; Ouldali, M.; Six, J.-L.; Ferji, K. Self-assembly of amphiphilic copolymers containing polysaccharide: PISA versus nanoprecipitation, and the temperature effect. Polym. Chem. 2020, 11, 4729–4740. [Google Scholar] [CrossRef]
- Canning, S.L.; Smith, G.N.; Armes, S.P. A Critical Appraisal of RAFT-Mediated Polymerization-Induced Self-Assembly. Macromolecules 2016, 49, 1985–2001. [Google Scholar] [CrossRef]
- Hartlieb, M. Photo-Iniferter RAFT Polymerization. Macromol. Rapid Commun. 2022, 43, 2100514. [Google Scholar] [CrossRef]
- Wang, J.; Hu, X.; Zhu, N.; Guo, K. Continuous flow photo-RAFT and light-PISA. Chem. Eng. J. 2021, 420, 127663. [Google Scholar] [CrossRef]
- Tkachenko, V.; Matei Ghimbeu, C.; Vaulot, C.; Vidal, L.; Poly, J.; Chemtob, A. RAFT-photomediated PISA in dispersion: Mechanism, optical properties and application in templated synthesis. Polym. Chem. 2019, 10, 2316–2326. [Google Scholar] [CrossRef]
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization—A User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- McCormick, C.L.; Lowe, A.B. Aqueous RAFT Polymerization: Recent Developments in Synthesis of Functional Water-Soluble (Co)polymers with Controlled Structures. Acc. Chem. Res. 2004, 37, 312–325. [Google Scholar] [CrossRef]
- Majewski, A.P.; Stahlschmidt, U.; Jerome, V.; Freitag, R.; Muller, A.H.; Schmalz, H. PDMAEMA-grafted core-shell-corona particles for nonviral gene delivery and magnetic cell separation. Biomacromolecules 2013, 14, 3081–3090. [Google Scholar] [CrossRef]
- Uhrig, D.; Mays, J. Synthesis of well-defined multigraft copolymers. Polym. Chem. 2011, 2, 69–76. [Google Scholar] [CrossRef]
- Carvalho, L.T.; Teixeira, A.J.R.M.; Moraes, R.M.; Barbosa, R.F.S.; Queiroz, R.C.; Tada, D.B.; Mulinari, D.R.; Rosa, D.S.; Ré, M.I.; Medeiros, S.F. Preparation and characterization of cationic pullulan-based polymers with hydrophilic or amphiphilic characteristics for drug delivery. React. Funct. Polym. 2022, 181, 105441. [Google Scholar] [CrossRef]
- Jha, S.; Malviya, R.; Fuloria, S.; Sundram, S.; Subramaniyan, V.; Sekar, M.; Sharma, P.K.; Chakravarthi, S.; Wu, Y.S.; Mishra, N.; et al. Characterization of Microwave-Controlled Polyacrylamide Graft Copolymer of Tamarind Seed Polysaccharide. Polymers 2022, 14, 1037. [Google Scholar] [CrossRef]
- Kumar, D.; Gihar, S.; Shrivash, M.K.; Kumar, P.; Kundu, P.P. A review on the synthesis of graft copolymers of chitosan and their potential applications. Int. J. Biol. Macromol. 2020, 163, 2097–2112. [Google Scholar] [CrossRef]
- Corrigan, N.; Trujillo, F.J.; Xu, J.; Moad, G.; Hawker, C.J.; Boyer, C. Divergent Synthesis of Graft and Branched Copolymers through Spatially Controlled Photopolymerization in Flow Reactors. Macromolecules 2021, 54, 3430–3446. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, L.; Chen, Y.; Tan, J. Combining Green Light-Activated Photoiniferter RAFT Polymerization and RAFT Dispersion Polymerization for Graft Copolymer Assemblies. Macromolecules 2022, 55, 8472–8484. [Google Scholar] [CrossRef]
- Xu, J.; Abetz, V. Double thermoresponsive graft copolymers with different chain ends: Feasible precursors for covalently crosslinked hydrogels. Soft Matter 2022, 18, 2082–2091. [Google Scholar] [CrossRef]
- Alagi, P.; Zapsas, G.; Hadjichristidis, N.; Hong, S.C.; Gnanou, Y.; Feng, X. All-Polycarbonate Graft Copolymers with Tunable Morphologies by Metal-Free Copolymerization of CO2 with Epoxides. Macromolecules 2021, 54, 6144–6152. [Google Scholar] [CrossRef]
- Guo, K.; Li, S.; Chen, G.; Wang, J.; Wang, Y.; Xie, X.; Xue, Z. One-Pot Synthesis of Polyester-Based Linear and Graft Copolymers for Solid Polymer Electrolytes. CCS Chem. 2022, 4, 3134–3149. [Google Scholar] [CrossRef]
- Thankappan, H.; Semsarilar, M.; Li, S.; Chang, Y.; Bouyer, D.; Quemener, D. Synthesis of Block Copolymer Brush by RAFT and Click Chemistry and Its Self-Assembly as a Thin Film. Molecules 2020, 25, 4774. [Google Scholar] [CrossRef]
- Kim, J.; Cattoz, B.; Leung, A.H.M.; Parish, J.D.; Becer, C.R. Enabling Reversible Addition-Fragmentation Chain-Transfer Polymerization for Brush Copolymers with a Poly(2-oxazoline) Backbone. Macromolecules 2022, 55, 4411–4419. [Google Scholar] [CrossRef]
- Naguib, M.; Nixon, K.L.; Keddie, D.J. Effect of radical copolymerization of the (oxa)norbornene end-group of RAFT-prepared macromonomers on bottlebrush copolymer synthesis via ROMP. Polym. Chem. 2022, 13, 1401–1410. [Google Scholar] [CrossRef]
- Gegenhuber, T.; Müllner, M. Molecular Polymer Brushes Made via Ring-Opening Metathesis Polymerization from Cleavable RAFT Macromonomers. Macromol. Chem. Phys. 2021, 222, 2100077. [Google Scholar] [CrossRef]
- Assem, Y.; Abu-Zeid, R.; Ali, K.; Kamel, S. Synthesis of Acrylate-Modified Cellulose via Raft Polymerization and Its Application as Efficient Metal Ions Adsorbent. Egypt. J. Chem. 2019, 62, 85–96. [Google Scholar] [CrossRef]
- Ikkene, D.; Arteni, A.A.; Song, H.; Laroui, H.; Six, J.L.; Ferji, K. Synthesis of dextran-based chain transfer agent for RAFT-mediated polymerization and glyco-nanoobjects formulation. Carbohydr. Polym. 2020, 234, 115943. [Google Scholar] [CrossRef] [PubMed]
- Ikkene, D.; Arteni, A.A.; Ouldali, M.; Francius, G.; Brûlet, A.; Six, J.-L.; Ferji, K. Direct Access to Polysaccharide-Based Vesicles with a Tunable Membrane Thickness in a Large Concentration Window via Polymerization-Induced Self-Assembly. Biomacromolecules 2021, 22, 3128–3137. [Google Scholar] [CrossRef] [PubMed]
- Ferji, K.; Venturini, P.; Cleymand, F.; Chassenieux, C.; Six, J.-L. In situ glyco-nanostructure formulation via photo-polymerization induced self-assembly. Polym. Chem. 2018, 9, 2868–2872. [Google Scholar] [CrossRef]
- Pilipenko, I.M.; Korzhikov-Vlakh, V.A.; Zakharova, N.V.; Urtti, A.; Tennikova, T.B. Thermo- and pH-sensitive glycosaminoglycans derivatives obtained by controlled grafting of poly(N-isopropylacrylamide). Carbohydr. Polym. 2020, 248, 116764. [Google Scholar] [CrossRef] [PubMed]
- Pilipenko, I.; Korzhikov-Vlakh, V.; Valtari, A.; Anufrikov, Y.; Kalinin, S.; Ruponen, M.; Krasavin, M.; Urtti, A.; Tennikova, T. Mucoadhesive properties of nanogels based on stimuli-sensitive glycosaminoglycan-graft-pNIPAAm copolymers. Int. J. Biol. Macromol. 2021, 186, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, A.; Sentoukas, T.; Giaouzi, D.; Kafetzi, M.; Pispas, S. Latest advances on the synthesis of linear abc-type triblock terpolymers and star-shaped polymers by raft polymerization. Polymers 2021, 13, 1698. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Matyjaszewski, K. Arm-first method as a simple and general method for synthesis of miktoarm star copolymers. J. Am. Chem. Soc. 2007, 129, 11828–11834. [Google Scholar] [CrossRef]
- Gao, H.; Matyjaszewski, K. Synthesis of star polymers by a new “core-first” method: Sequential polymerization of cross-linker and monomer. Macromolecules 2008, 41, 1118–1125. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. RAFT polymerization and some of its applications. Chem. An. Asian J. 2013, 8, 1634–1644. [Google Scholar] [CrossRef]
- Allison-Logan, S.; Karimi, F.; Nothling, M.D.; Qiao, G.G. Star polymers by RAFT polymerization. RAFT Polym. Methods Synth. Appl. 2021, 2, 983–1015. [Google Scholar]
- Ge, H.; Shi, W.; He, C.; Feng, A.; Thang, S.H. Star-Shaped Thermoplastic Elastomers Prepared via RAFT Polymerization. Polymers 2023, 15, 2002. [Google Scholar] [CrossRef]
- Volsi, A.L.; Tallia, F.; Iqbal, H.; Georgiou, T.K.; Jones, J.R. Enzyme degradable star polymethacrylate/silica hybrid inks for 3D printing of tissue scaffolds. Mater. Adv. 2020, 1, 3189–3199. [Google Scholar] [CrossRef]
- Allı, A.; Allı, S.; Hazer, B.; Zinn, M. Synthesis and characterization of star-shaped block copolymers composed of poly (3-hydroxy octanoate) and styrene via RAFT polymerization. J. Macromol. Sci. Part A 2022, 59, 526–536. [Google Scholar] [CrossRef]
- Skandalis, A.; Pispas, S. Synthesis of (AB) n-, AnBn-, and AxBy-type amphiphilic and double-hydrophilic star copolymers by RAFT polymerization. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1771–1783. [Google Scholar] [CrossRef]
- Haladjova, E.; Panseri, S.; Montesi, M.; Rossi, A.; Skandalis, A.; Pispas, S.; Rangelov, S. Influence of DNA Type on the Physicochemical and Biological Properties of Polyplexes Based on Star Polymers Bearing Different Amino Functionalities. Polymers 2023, 15, 894. [Google Scholar] [CrossRef]
- Sentoukas, T.; Forys, A.; Marcinkowski, A.; Otulakowski, L.; Pispas, S.; Trzebicka, B. Poly(oligoethylene glycol methacrylate) Star-Shaped Copolymers with Hydroxypropyl Methacrylate Cores. Macromol. Chem. Phys. 2022, 224, 2200115. [Google Scholar] [CrossRef]
- Qu, Y.; Chang, X.; Chen, S.; Zhang, W. In situ synthesis of thermoresponsive 4-arm star block copolymer nano-assemblies by dispersion RAFT polymerization. Polym. Chem. 2017, 8, 3485–3496. [Google Scholar] [CrossRef]
- Xue, Y.; Huang, D.; Wang, X.; Zhang, C. A Study on the Dual Thermo-and pH-Responsive Behaviors of Well-Defined Star-like Block Copolymers Synthesize by Combining of RAFT Polymerization and Thiol-Ene Click Reaction. Polymers 2022, 14, 1695. [Google Scholar] [CrossRef]
- Zeng, R.; Chen, Y.; Zhang, L.; Tan, J. R-RAFT or Z-RAFT? Well-defined star block copolymer nano-objects prepared by RAFT-mediated polymerization-induced self-assembly. Macromolecules 2020, 53, 1557–1566. [Google Scholar] [CrossRef]
- Polanowski, P.; Hałagan, K.; Sikorski, A. Dendrimers vs. Hyperbranched Polymers: Studies of the Polymerization Process Based on Monte Carlo Simulations. Comput. Methods Sci. Technol. 2022, 28, 109–117. [Google Scholar]
- Chen, B.; Syed, M.N.; Daymon, S.P.; Olson, B.G.; Kareem, O.O.; Giesen, J.A.; Fahs, G.B.; Moore, R.R.; Grayson, S.M.; Nazarenko, S. Insights and comparison of structure–bulk property relationships in low generation hydroxylated polyester dendrimer and hyperbranched polymer prepared from bis-MPA monomer. Polymer 2021, 231, 124097. [Google Scholar] [CrossRef]
- Saadati, A.; Hasanzadeh, M.; Seidi, F. Biomedical application of hyperbranched polymers: Recent Advances and challenges. TrAC Trends Anal. Chem. 2021, 142, 116308. [Google Scholar] [CrossRef]
- Gregory, A.; Stenzel, M.H. Complex polymer architectures via RAFT polymerization: From fundamental process to extending the scope using click chemistry and nature’s building blocks. Prog. Polym. Sci. 2012, 37, 38–105. [Google Scholar] [CrossRef]
- Alfurhood, J.A.; Bachler, P.R.; Sumerlin, B.S. Hyperbranched polymers via RAFT self-condensing vinyl polymerization. Polym. Chem. 2016, 7, 3361–3369. [Google Scholar] [CrossRef]
- Li, Z.; Yong, H.; Wang, K.; Zhou, Y.-N.; Lyu, J.; Liang, L.; Zhou, D. (Controlled) Free radical (co) polymerization of multivinyl monomers: Strategies, topological structures and biomedical applications. Chem. Commun. 2023, 59, 4142–4157. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Mei, L.; Ma, C.; Zhang, G. Degradable hyperbranched polymer with fouling resistance for antifouling coatings. Prog. Org. Coat. 2021, 153, 106141. [Google Scholar] [CrossRef]
- Mei, L.; Ai, X.; Ma, C.; Zhang, G. Surface-fragmenting hyperbranched copolymers with hydrolysis-generating zwitterions for antifouling coatings. J. Mater. Chem. B 2020, 8, 5434–5440. [Google Scholar] [CrossRef]
- Dai, G.; Ai, X.; Mei, L.; Ma, C.; Zhang, G. Kill–resist–renew trinity: Hyperbranched polymer with self-regenerating attack and defense for antifouling coatings. ACS Appl. Mater. Interfaces 2021, 13, 13735–13743. [Google Scholar] [CrossRef]
- Pan, J.; Mei, L.; Zhou, H.; Zhang, C.; Xie, Q.; Ma, C. Self-regenerating zwitterionic hyperbranched polymer with tunable degradation for anti-biofouling coatings. Prog. Org. Coat. 2022, 163, 106674. [Google Scholar] [CrossRef]
- Balafouti, A.; Pispas, S. Hyperbranched Copolymers of Methacrylic Acid and Lauryl Methacrylate HP (MAA-co-LMA): Synthetic Aspects and Interactions with Biorelevant Compounds. Pharmaceutics 2023, 15, 1198. [Google Scholar] [CrossRef] [PubMed]
- Selianitis, D.; Pispas, S. Multi-responsive poly (oligo (ethylene glycol) methyl methacrylate)-co-poly (2-(diisopropylamino) ethyl methacrylate) hyperbranched copolymers via reversible addition fragmentation chain transfer polymerization. Polym. Chem. 2021, 12, 6582–6593. [Google Scholar] [CrossRef]
- Selianitis, D.; Katifelis, H.; Gazouli, M.; Pispas, S. Novel Multi-Responsive Hyperbranched Polyelectrolyte Polyplexes as Potential Gene Delivery Vectors. Pharmaceutics 2023, 15, 1627. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, C.; Tai, H.; Salerno, M.; Wang, X.; Hartsuiker, E.; Wang, W. Folic acid and rhodamine labelled pH responsive hyperbranched polymers: Synthesis, characterization and cell uptake studies. Eur. Polym. J. 2019, 120, 109259. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Yue, S.; Ling, J.; Ni, X.; Shen, Z. Chemoselective RAFT polymerization of a trivinyl monomer derived from carbon dioxide and 1, 3-butadiene: From linear to hyperbranched. Macromolecules 2017, 50, 9598–9606. [Google Scholar] [CrossRef]
- Sims, M.B. Controlled radical copolymerization of multivinyl crosslinkers: A robust route to functional branched macromolecules. Polym. Int. 2021, 70, 14–23. [Google Scholar] [CrossRef]
- Cai, W.; Yang, S.; Zhang, L.; Chen, Y.; Zhang, L.; Tan, J. Efficient Synthesis and Self-Assembly of Segmented Hyperbranched Block Copolymers via RAFT-Mediated Dispersion Polymerization Using Segmented Hyperbranched Macro-RAFT Agents. Macromolecules 2022, 55, 5775–5787. [Google Scholar] [CrossRef]
- Bachler, P.R.; Forry, K.E.; Sparks, C.A.; Schulz, M.D.; Wagener, K.B.; Sumerlin, B.S. Modular segmented hyperbranched copolymers. Polym. Chem. 2016, 7, 4155–4159. [Google Scholar] [CrossRef]
- Calvo, P.R.; Sparks, C.A.; Hochberg, J.; Wagener, K.B.; Sumerlin, B.S. Hyperbranched Bisphosphonate-Functional Polymers via Self-Condensing Vinyl Polymerization and Postpolymerization Multicomponent Reactions. Macromol. Rapid Commun. 2021, 42, 2000578. [Google Scholar] [CrossRef]
- Rahemipoor, S.; Kohestanian, M.; Pourjavadi, A.; Vazifehkhorani, H.H.; Mehrali, M. Synthesis and Properties of Multi-stimuli-Responsive Water-Soluble Hyperbranched Polymers Prepared Via Reversible Addition–Fragmentation Chain Transfer Self-Condensing Vinyl Polymerization. ACS Appl. Polym. Mater. 2022, 4, 692–702. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, H.; Zheng, L.; Wu, Z.; Zhang, X.; Zhang, X. One-Pot Synthesis of Dual-Responsive Hyperbranched Polymeric Prodrugs Using an All-in-One Chain Transfer Monomer. ACS Macro Lett. 2018, 7, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wang, Y.; Zhang, X.; Ma, L.; Wang, B.; Ji, X.; Wei, H. Fabrication of Hyperbranched Block-Statistical Copolymer-Based Prodrug with Dual Sensitivities for Controlled Release. Bioconjugate Chem. 2018, 29, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Nandi, M.; Banerjee, S.; De, P. Stearoyl-appended pendant amino acid-based hyperbranched polymers for selective gelation of oil from oil/water mixtures. Polym. Chem. 2019, 10, 1795–1805. [Google Scholar] [CrossRef]
- Rikkou-Kalourkoti, M.; Elladiou, M.; Patrickios, C.S. Synthesis and characterization of hyperbranched amphiphilic block copolymers prepared via self-condensing RAFT polymerization. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1310–1319. [Google Scholar] [CrossRef]
- Wang, X.; Gao, H. Recent progress on hyperbranched polymers synthesized via radical-based self-condensing vinyl polymerization. Polymers 2017, 9, 188. [Google Scholar] [CrossRef]
- Cuneo, T.; Gao, H. Recent advances on synthesis and biomaterials applications of hyperbranched polymers. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1640. [Google Scholar] [CrossRef]
- Lin, F.-Y.; Yan, M.; Cochran, E.W. Gelation suppression in RAFT polymerization. Macromolecules 2019, 52, 7005–7015. [Google Scholar] [CrossRef]
- Forrester, M.; Becker, A.; Hohmann, A.; Hernandez, N.; Lin, F.-Y.; Bloome, N.; Johnson, G.; Dietrich, H.; Marcinko, J.; Williams, R.C. RAFT thermoplastics from glycerol: A biopolymer for development of sustainable wood adhesives. Green. Chem. 2020, 22, 6148–6156. [Google Scholar] [CrossRef]
- Xu, P.; Huang, X.; Pan, X.; Li, N.; Zhu, J.; Zhu, X. Hyperbranched polycaprolactone through RAFT polymerization of 2-methylene-1, 3-dioxepane. Polymers 2019, 11, 318. [Google Scholar] [CrossRef]
- Kohestanian, M.; Keshavarzi, N.; Pourjavadi, A.; Rahmani, F. Fabrication of pH and thermal dual-responsive hyperbranched copolymer grafted magnetic graphene oxide via surface-initiated RAFT-SCVP for controlled release of DOX. Mater. Today Commun. 2023, 35, 105504. [Google Scholar] [CrossRef]
- Kavand, A.; Blanck, C.; Przybilla, F.; Mély, Y.; Anton, N.; Vandamme, T.; Serra, C.A.; Chan-Seng, D. Investigating the growth of hyperbranched polymers by self-condensing vinyl RAFT copolymerization from the surface of upconversion nanoparticles. Polym. Chem. 2020, 11, 4313–4325. [Google Scholar] [CrossRef]
- Rong, L.-H.; Cheng, X.; Ge, J.; Krebs, O.K.; Capadona, J.R.; Caldona, E.B.; Advincula, R.C. Synthesis of hyperbranched polymer films via electrodeposition and oxygen-tolerant surface-initiated photoinduced polymerization. J. Colloid. Interface Sci. 2023, 637, 33–40. [Google Scholar] [CrossRef]
- Huang, J.; Li, D.; Liang, H.; Lu, J. Synthesis of Photocrosslinkable and Amine Containing Multifunctional Nanoparticles via Polymerization-Induced Self-Assembly. Macromol. Rapid Commun. 2017, 38, 1700202. [Google Scholar] [CrossRef]
- Rauschenbach, M.; Lawrenson, S.B.; Taresco, V.; Pearce, A.K.; O’Reilly, R.K. Antimicrobial hyperbranched polymer–usnic acid complexes through a combined ROP-RAFT strategy. Macromol. Rapid Commun. 2020, 41, 2000190. [Google Scholar] [CrossRef]
- Rong, L.H.; Cheng, X.; Ge, J.; Caldona, E.B.; Advincula, R.C. Synthesis of Hyperbranched Polymers via PET-RAFT Self-Condensing Vinyl Polymerization in a Flow Reactor. Macromol. Chem. Phys. 2022, 223, 2100342. [Google Scholar] [CrossRef]
- Shen, W.; Chang, Y.; Liu, G.; Wang, H.; Cao, A.; An, Z. Biocompatible, antifouling, and thermosensitive core−shell nanogels synthesized by RAFT aqueous dispersion polymerization. Macromolecules 2011, 44, 2524–2530. [Google Scholar] [CrossRef]
- Bhuchar, N.; Sunasee, R.; Ishihara, K.; Thundat, T.; Narain, R. Degradable thermoresponsive nanogels for protein encapsulation and controlled release. Bioconjugate Chem. 2012, 23, 75–83. [Google Scholar] [CrossRef]
- Ahmed, M.; Narain, R. Intracellular delivery of DNA and enzyme in active form using degradable carbohydrate-based nanogels. Mol. Pharm. 2012, 9, 3160–3170. [Google Scholar] [CrossRef]
- Van Driessche, A.; Kocere, A.; Everaert, H.; Nuhn, L.; Van Herck, S.; Griffiths, G.; Fenaroli, F.; De Geest, B.G. pH-sensitive hydrazone-linked doxorubicin nanogels via polymeric-activated Ester scaffolds: Synthesis, assembly, and in vitro and in vivo evaluation in tumor-bearing zebrafish. Chem. Mater. 2018, 30, 8587–8596. [Google Scholar] [CrossRef]
- Xin, F.; Wei, M.; Jiang, S.; Gao, Y.; Nie, J.; Wu, Y.; Sun, F. Design of hydrophilic photocleavage o-nitrobenzyl acrylate-modified nanogels with outstanding biocompatibility prepared by RAFT polymerization for drug carrier. Eur. Polym. J. 2020, 122, 109364. [Google Scholar] [CrossRef]
- Don, T.-M.; Lu, K.-Y.; Lin, L.-J.; Hsu, C.-H.; Wu, J.-Y.; Mi, F.-L. Temperature/pH/Enzyme triple-responsive cationic protein/PAA-b-PNIPAAm nanogels for controlled anticancer drug and photosensitizer delivery against multidrug resistant breast cancer cells. Mol. Pharm. 2017, 14, 4648–4660. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, T.F.; Szarpak-Jankowska, A.; Appaix, F.; Louage, B.; Hamard, L.; De Geest, B.G.; van der Sanden, B.; Nakamura, C.V.; Auzély-Velty, R. Thermoresponsive hyaluronic acid nanogels as hydrophobic drug carrier to macrophages. Acta Biomater. 2014, 10, 4750–4758. [Google Scholar] [CrossRef] [PubMed]
- Kolouchova, K.; Sedlacek, O.; Jirak, D.; Babuka, D.; Blahut, J.; Kotek, J.; Vit, M.; Trousil, J.; Konefał, R.; Janouskova, O. Self-assembled thermoresponsive polymeric nanogels for 19F MR imaging. Biomacromolecules 2018, 19, 3515–3524. [Google Scholar] [CrossRef]
- Peng, H.; Huang, X.; Melle, A.; Karperien, M.; Pich, A. Redox-responsive degradable prodrug nanogels for intracellular drug delivery by crosslinking of amine-functionalized poly (N-vinylpyrrolidone) copolymers. J. Colloid. Interface Sci. 2019, 540, 612–622. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skandalis, A.; Sentoukas, T.; Selianitis, D.; Balafouti, A.; Pispas, S. Using RAFT Polymerization Methodologies to Create Branched and Nanogel-Type Copolymers. Materials 2024, 17, 1947. https://doi.org/10.3390/ma17091947
Skandalis A, Sentoukas T, Selianitis D, Balafouti A, Pispas S. Using RAFT Polymerization Methodologies to Create Branched and Nanogel-Type Copolymers. Materials. 2024; 17(9):1947. https://doi.org/10.3390/ma17091947
Chicago/Turabian StyleSkandalis, Athanasios, Theodore Sentoukas, Dimitrios Selianitis, Anastasia Balafouti, and Stergios Pispas. 2024. "Using RAFT Polymerization Methodologies to Create Branched and Nanogel-Type Copolymers" Materials 17, no. 9: 1947. https://doi.org/10.3390/ma17091947





