Research on Hydrogen-Induced Induced Cracking Sensitivity of X80 Pipeline Steel under Different Heat Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Test Methods
2.2.1. Heat Treatment
2.2.2. Microstructure Observation and Hardness Test
2.2.3. Slow Strain Rate Tensile Test with Pre-Charged Hydrogen
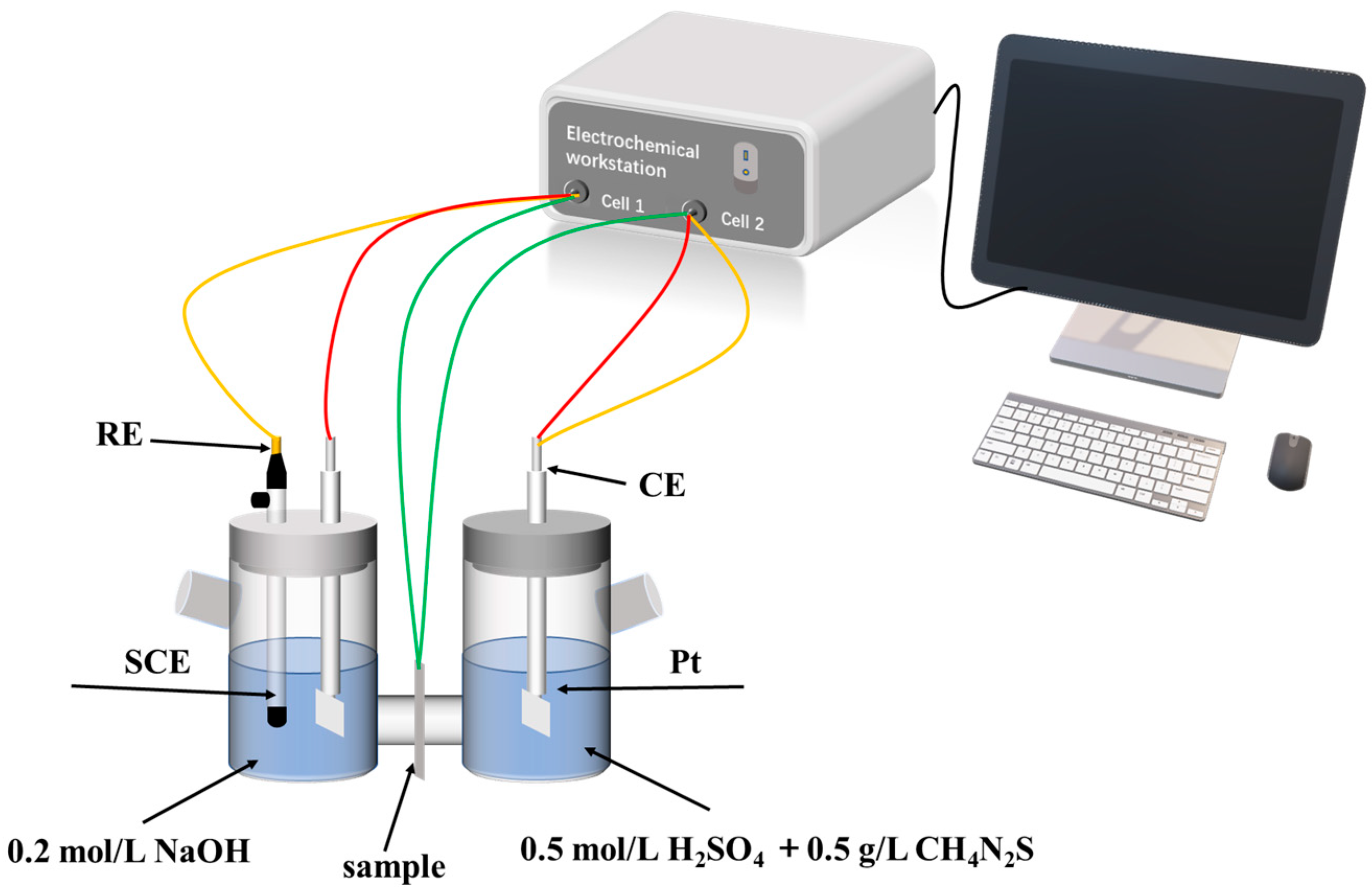
2.2.4. Hydrogen Permeation Test
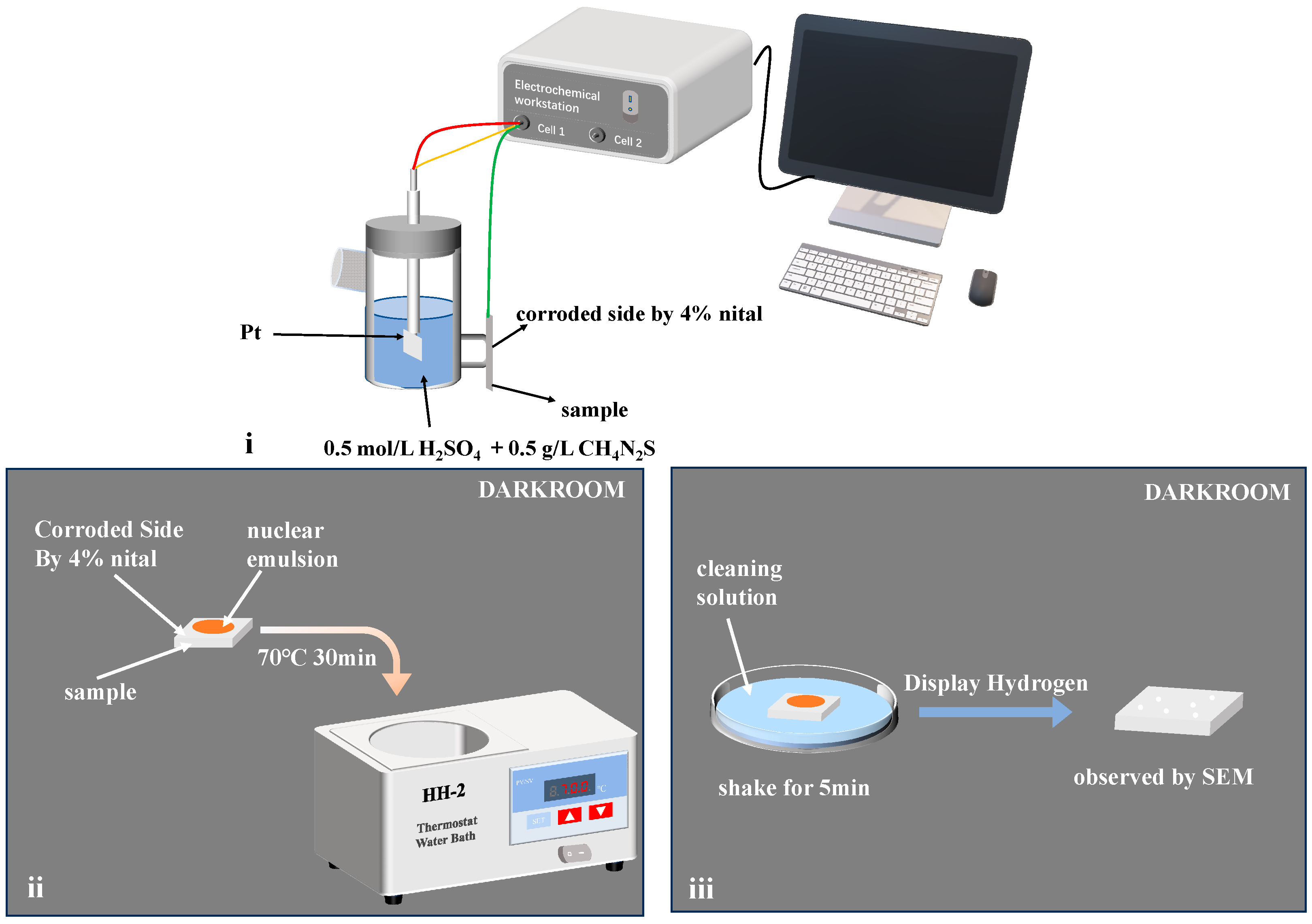
2.2.5. Hydrogenation Cracking Test
2.2.6. Hydrogen Microprint Technology
3. Results and Discussion
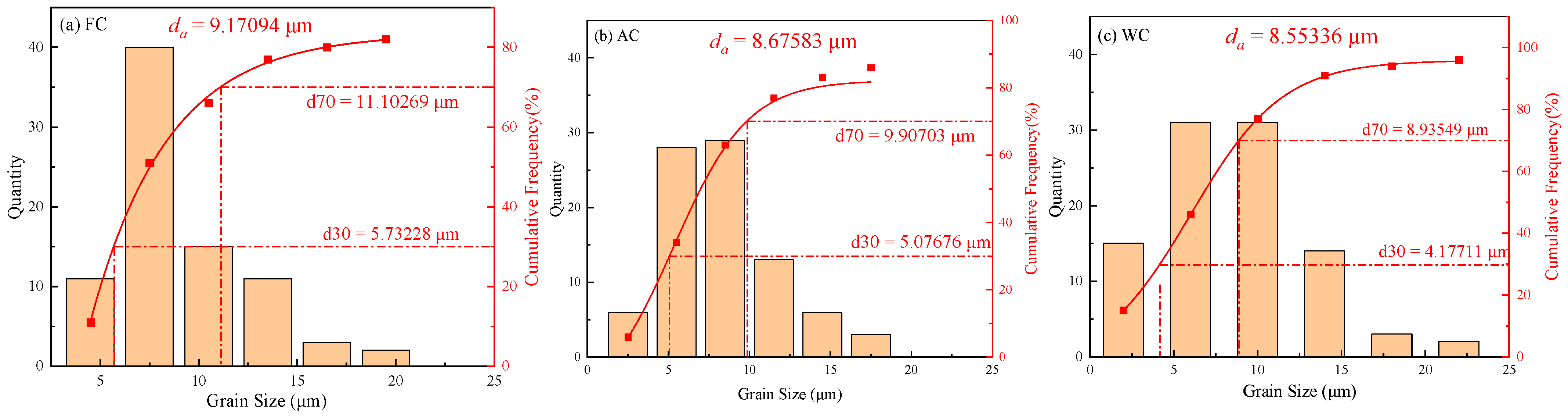
3.1. Microstructures and Hardness
3.2. SSRT with Pre-Charged Hydrogen
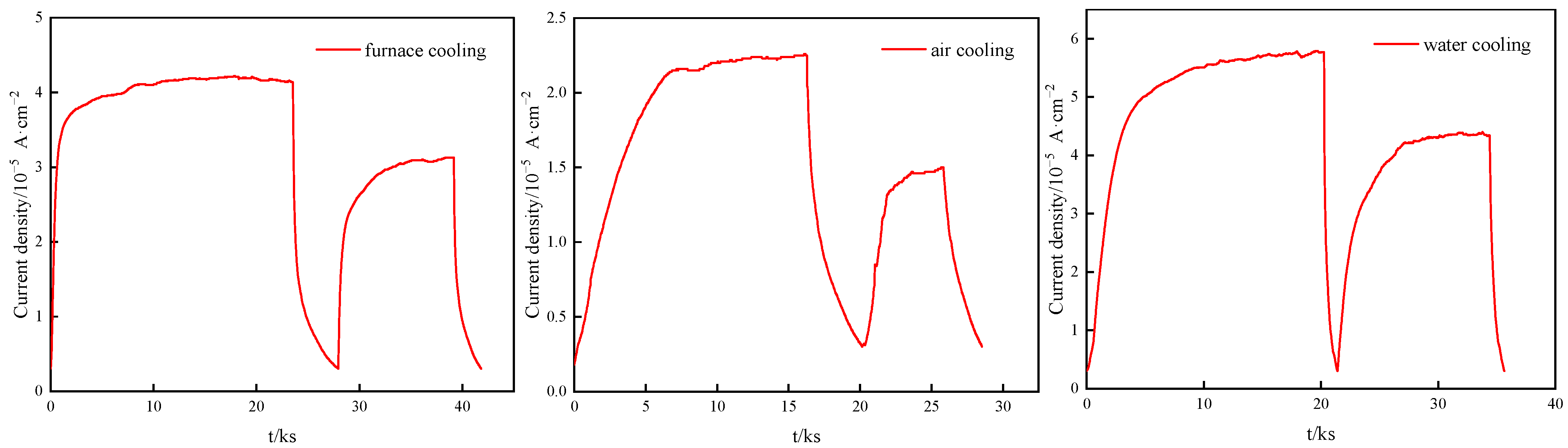
3.3. Hydrogen Permeation
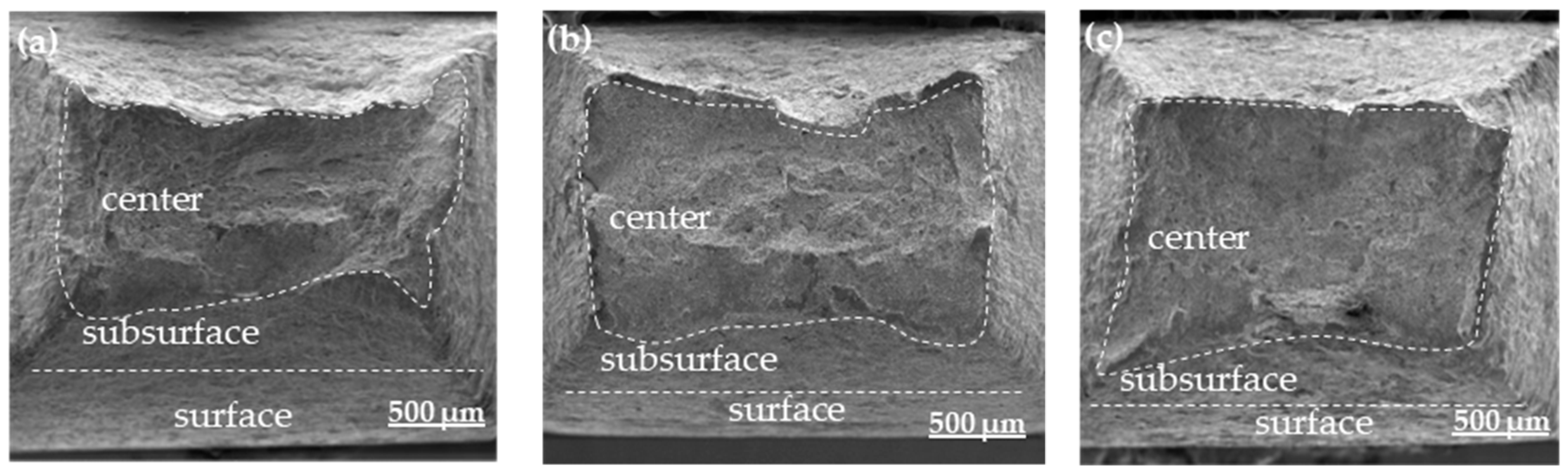
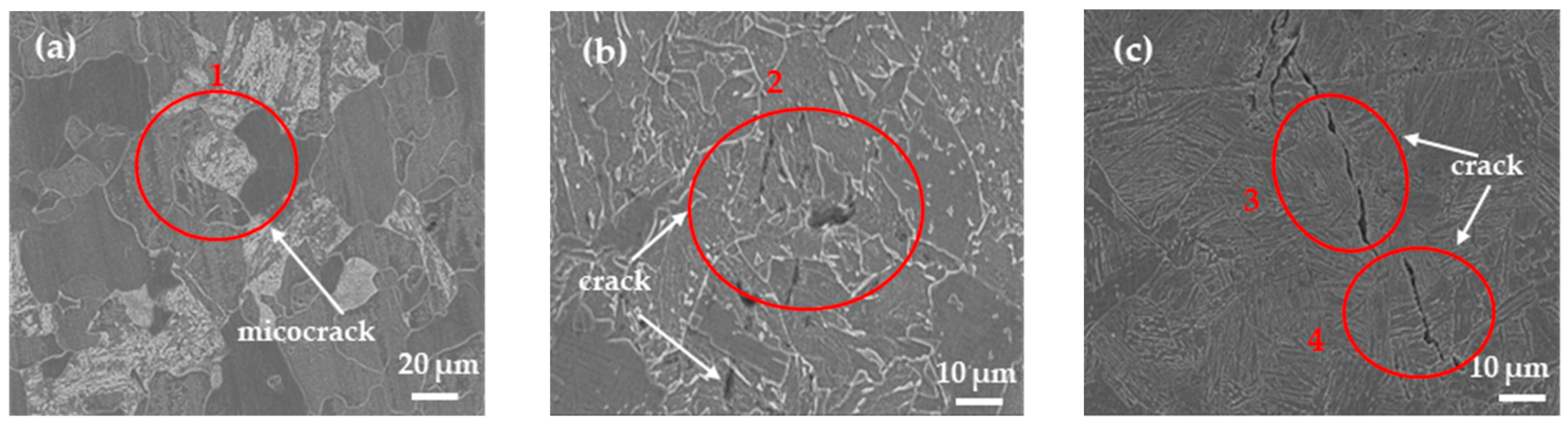
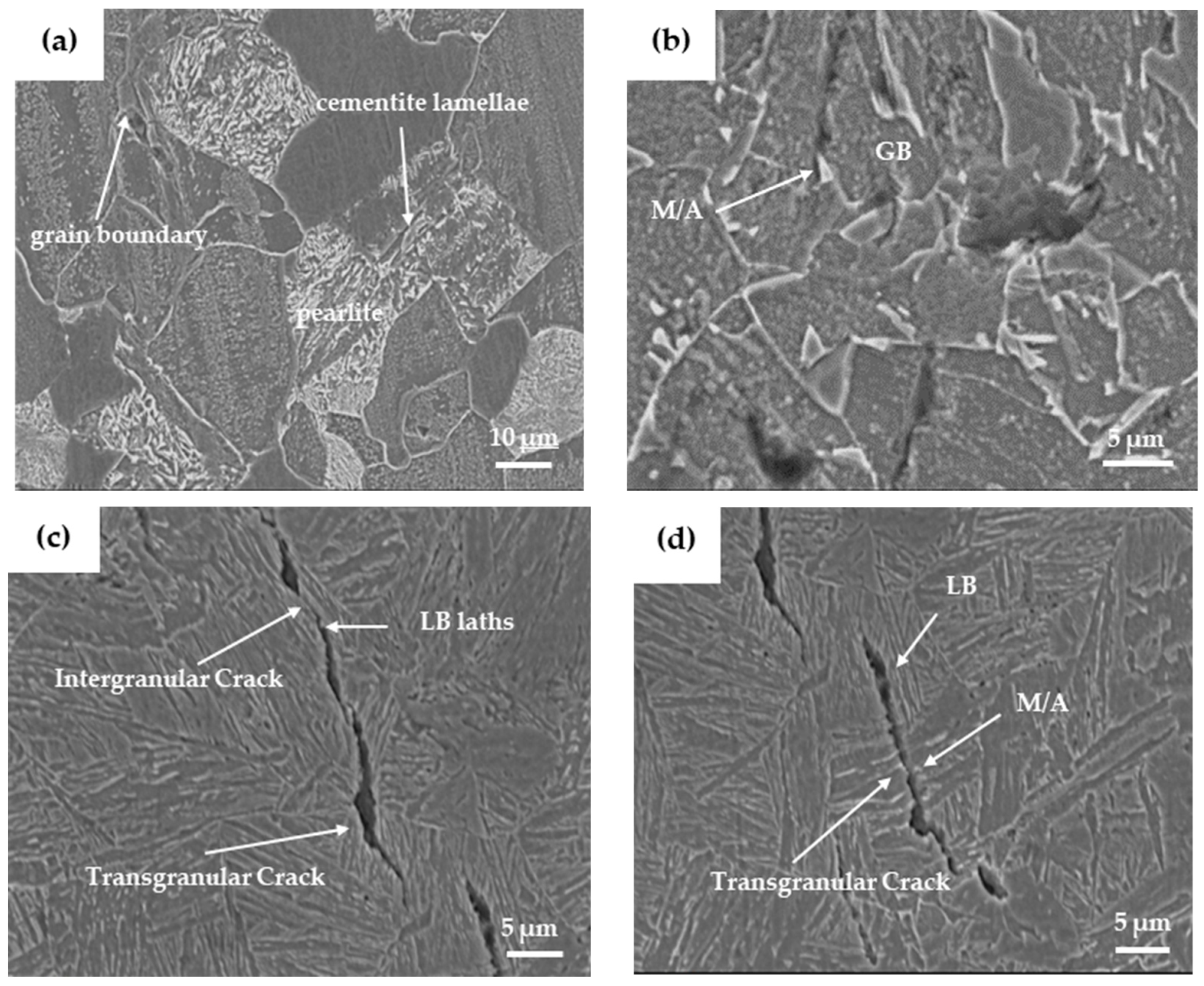
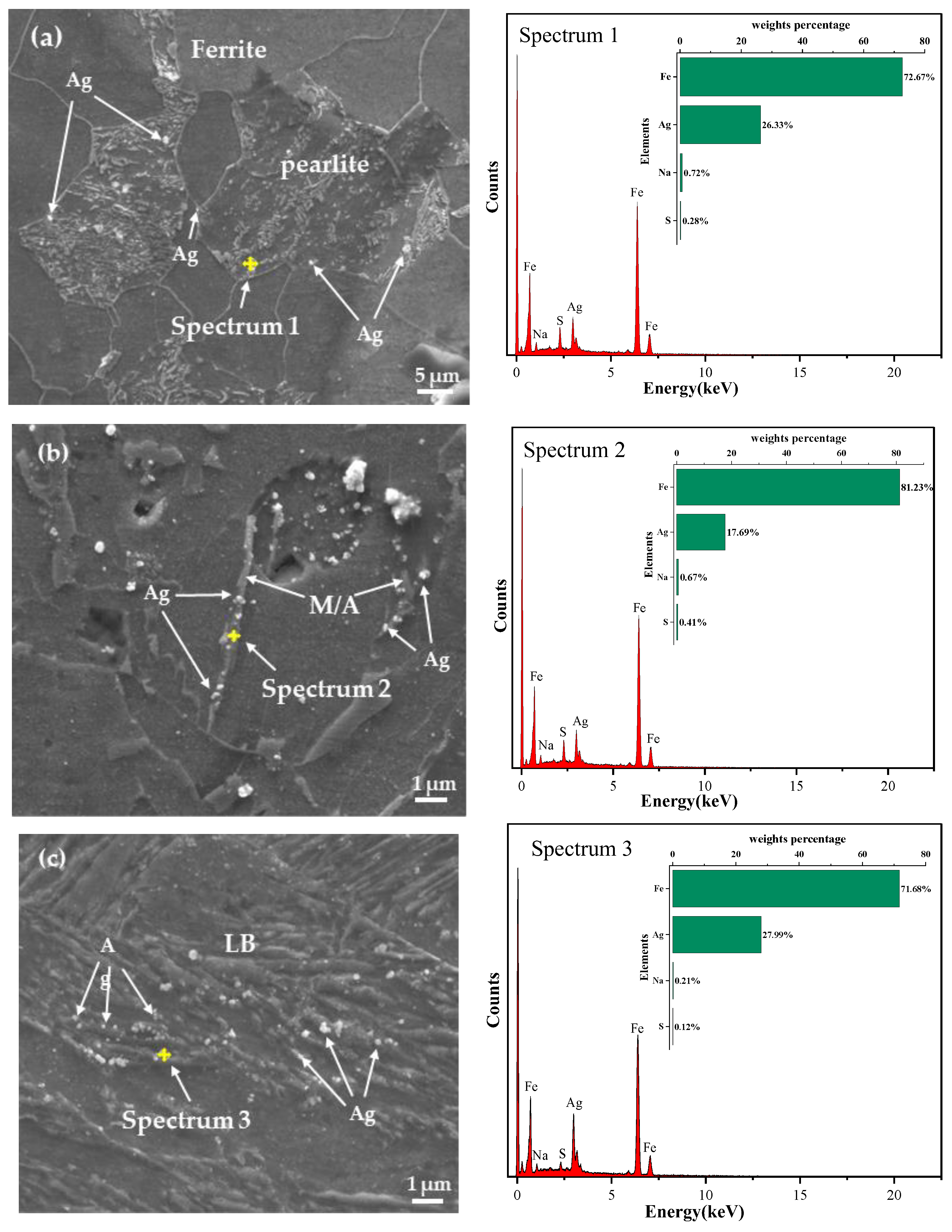
3.4. Hydrogenation Cracking and Hydrogen Microprint Technology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, M.C.; Yang, K.; Shan, Y.Y. The effects of thermo-mechanical control process on microstructures and mechanical properties of a commercial pipeline steel. Mater. Sci. Eng. A 2002, 335, 14–20. [Google Scholar] [CrossRef]
- Omalea, J.I.; Ohaeri, E.G.; Tiamiyu, A.A.; Eskandari, M.; Mostafijur, K.M.; Szpunar, J.A. Microstructure, texture evolution and mechanical properties of X70 pipeline steel after different thermomechanical treatments. Mater. Sci. Eng. A 2017, 703, 477–485. [Google Scholar] [CrossRef]
- Mohtadi-Bonab, M.A.; Masoumi, M. Different aspects of hydrogen diffusion behavior in pipeline steel. J. Mater. Res. Technol. 2023, 24, 4762–4783. [Google Scholar] [CrossRef]
- Shi, X.B.; Yan, W.; Yan, M.C.; Wang, W.; Yang, Z.G.; Shan, Y.Y.; Yang, K. Effect of Cu Addition in Pipeline Steels on Microstructure, Mechanical Properties and Microbiologically Influenced Corrosion. Acta Metall. Sin. (Engl. Lett.) 2017, 30, 601–613. [Google Scholar] [CrossRef]
- Shi, X.B.; Yan, W.; Wang, W.; Shan, Y.Y.; Yang, K. Novel Cu-bearing high-strength pipeline steels with excellent resistance to hydrogen-induced cracking. Mater. Des. 2016, 92, 300–305. [Google Scholar] [CrossRef]
- Hejazi, D.; Haq, A.J.; Yazdipour, N.; Dunne, D.P.; Calka, A.; Pereloma, F.; Barbaro, E.V. Effect of manganese content and microstructure on the susceptibility of X70 pipeline steel to hydrogen cracking. Mater. Sci. Eng. A 2012, 551, 40–49. [Google Scholar] [CrossRef]
- Beidokhti, B.; Koukabi, A.H.; Dolati, A. Effect of titanium addition on the microstructure and inclusion formation in submerged arc welded HSLA pipeline steel. J. Mater. Process. Technol. 2008, 209, 4027–4035. [Google Scholar] [CrossRef]
- Bonagani, S.K.; Vishwanadh, B.S.; Tenneti, S.; Kumar, N.N.; Kain, V. Influence of tempering treatments on mechanical properties and hydrogen embrittlement of 13 wt% Cr martensitic stainless steel. Int. J. Press. Vessel. Pip. 2019, 176, 103969. [Google Scholar] [CrossRef]
- Shanmu, S.; Misra, R.D.K.; Hartmann, J.; Jansto, S.G. Microstructure of high strength niobium-containing pipeline steel. Mater. Sci. Eng. A 2006, 441, 215–229. [Google Scholar] [CrossRef]
- Huang, F.; Liu, J.; Deng, Z.J.; Cheng, J.H.; Lu, Z.H.; Li, X.G. Effect of microstructure and inclusions on hydrogen induced cracking susceptibility and hydrogen trapping efficiency of X120 pipeline steel. Mater. Sci. Eng. A 2010, 527, 6997–7001. [Google Scholar] [CrossRef]
- Gan, L.J.; Huang, F.; Zhao, X.Y.; Liu, J.; Cheng, Y.F. Hydrogen trapping and hydrogen induced cracking of welded X100 pipeline steel in H2S environments. Int. J. Hydrogen Energy 2018, 43, 2293–2306. [Google Scholar] [CrossRef]
- Nayak, S.S.; Misra, R.D.K.; Hartmann, J.; Siciliano, F.; Gray, J.M. Microstructure and properties of low manganese and niobium containing HIC pipeline steel. Mater. Sci. Eng. A 2008, 494, 456–463. [Google Scholar] [CrossRef]
- Shi, X.B.; Yan, W.; Yan, M.C.; Wang, W.; Yang, Z.G.; Shan, Y.Y.; Yang, K. Effect of Microstructure on Hydrogen Induced Cracking Behavior of a High Deformability Pipeline Steel. J. Iron Steel Res. Int. 2015, 22, 937–942. [Google Scholar] [CrossRef]
- Ramírez, E.; González-Rodriguez, J.G.; Torres-Islas, A.; Serna, S.; Campillo, B.; Dominguez-Patino, G.; Juarez-Islas, J.A. Effect of microstructure on the sulphide stress cracking susceptibility of a high strength pipeline steel. Corros. Sci. 2008, 50, 3534–3541. [Google Scholar] [CrossRef]
- Xue, H.B.; Cheng, Y.F. Characterization of inclusions of X80 pipeline steel and its correlation with hydrogen-induced cracking. Corros. Sci. 2011, 53, 1201–1208. [Google Scholar] [CrossRef]
- Ohaeri, E.; Szpunar, J.; Fazeli, F.; Arafin, M. Hydrogen induced cracking susceptibility of API 5L X70 pipeline steel in relation to microstructure and crystallographic texture developed after different thermomechanical treatments. Mater. Charact. 2018, 145, 142–156. [Google Scholar] [CrossRef]
- Magazzù, A.; Marcuello, C. Investigation of soft matter nanomechanics by atomic force microscopy and optical tweezers: A comprehensive review. Nanomaterials 2023, 13, 963. [Google Scholar] [CrossRef] [PubMed]
- Zhan, N.; Hu, Z.; Zhang, X. Experimental investigation of fatigue crack growth behavior in banded structure of pipeline steel. Metals 2020, 10, 1193. [Google Scholar] [CrossRef]
- Latifi, V.A.; Miresmaeili, R.; Abdollah-Zadeh, A. The mutual effects of hydrogen and microstructure on hardness and impact energy of SMA welds in X65 steel. Mater. Sci. Eng. A 2017, 679, 87–94. [Google Scholar] [CrossRef]
- Beidokhti, B.; Dolati, A.; Koukabi, A.H. Effects of alloying elements and microstructure on the susceptibility of the welded HSLA steel to hydrogen-induced cracking and sulfide stress cracking. Mater. Sci. Eng. A 2008, 507, 167–173. [Google Scholar] [CrossRef]
- Arafin, M.A.; Szpunar, J.A. Effect of bainitic microstructure on the susceptibility of pipeline steels to hydrogen induced cracking. Mater. Sci. Eng. A 2011, 528, 4927–4940. [Google Scholar] [CrossRef]
- Li, J.; Gao, X.H.; Du, L.X.; Liu, Z.G. Relationship between microstructure and hydrogen induced cracking behavior in a low alloy pipeline steel. J. Mater. Sci. Technol. 2017, 33, 1504–1512. [Google Scholar] [CrossRef]
- Anijdan, S.H.M.; Arab, G.; Sabzi, M.; Sadeghi, M.; Eivani, A.R.; Jafarian, H.R. Sensitivity to hydrogen induced cracking, and corrosion performance of an API X65 pipeline steel in H2S containing environment: Influence of heat treatment and its subsequent microstructural changes. J. Mater. Res. Technol. 2021, 15, 1–16. [Google Scholar] [CrossRef]
- Huang, M.; Fu, Q.G.; Wang, Y.; Zhong, W.W. Corrosion Resistant Ceramic coating for X80 pipeline steel by low-temperature pack aluminizing and oxidation treatment. Surf. Rev. Lett. 2013, 20, 1350063. [Google Scholar] [CrossRef]
- Xue, H.B.; Cheng, Y.F. Hydrogen Permeation and Electrochemical Corrosion Behavior of the X80 Pipeline Steel Weld. J. Mater. Eng. Perform. 2013, 22, 170–175. [Google Scholar] [CrossRef]
- Li, L.F.; Song, B.; Cai, Z.Y.; Liu, Z.; Cui, X.K. Influence of Tempering Treatment on Precipitation Behavior, Microstructure, Dislocation Density and Hydrogen-Induced Ductility Loss in High-Vanadium Hot-Rolled X80 Pipeline Steel. In Tms 2019 148th Annual Meeting & Exhibition Supplemental Proceedings; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 1111–1122. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Gao, H.L.; Zhang, X.Q.; Yang, Y. Effect of volume fraction of bainite on microstructure and mechanical properties of X80 pipeline steel with excellent deformability. Mater. Sci. Eng. A 2011, 531, 84–90. [Google Scholar] [CrossRef]
- Aydin, H.; Nelson, W.T. Microstructure and mechanical properties of hard zone in friction stir welded X80 pipeline steel relative to different heat input. Mater. Sci. Eng. A 2013, 586, 313–322. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Vishwakarma, M. Effect of hydrogen in advanced high strength steel materials. Int. J. Hydrogen Energy 2019, 44, 28007–28030. [Google Scholar] [CrossRef]
- Venezuela, J.; Liu, Q.L.; Zhang, M.X.; Zhou, Q.J.; Atrens, A. A review of hydrogen embrittlement of martensitic advanced high- strength steels. Corros. Rev. 2016, 34, 153–186. [Google Scholar] [CrossRef]
- Neeraj, T.; Srinivasan, R.; Li, J. Hydrogen embrittlement of ferritic steels: Observations on deformation microstructure, nanoscale dimples and failure by nanovoiding. Acta Mater. 2012, 60, 5160–5171. [Google Scholar] [CrossRef]
- Gesnouin, C.; Hazarabedian, A.; Bruzzoni, P.; Ovejero-García, J.; Bilmes, P.; Llorente, C. Effect of post-weld heat treatment on the microstructure and hydrogen permeation of 13CrNiMo steels. Corros. Sci. 2004, 46, 1633–1647. [Google Scholar] [CrossRef]
- Park, G.T.; Koh, S.U.; Jung, H.G.; Kim, K.Y. Effect of microstructure on the hydrogen trapping efficiency and hydrogen induced cracking of linepipe steel. Corros. Sci. 2008, 50, 1865–1871. [Google Scholar] [CrossRef]
- Jack, T.A.; Pourazizi, R.; Ohaeri, E.; Szpunar, J.; Zhang, J.M.; Qu, J.B. Investigation of the hydrogen induced cracking behaviour of API 5L X65 pipeline steel. Int. J. Hydrogen Energy 2020, 45, 17671–17684. [Google Scholar] [CrossRef]









| C | S | Mn | Si | Cu | Nb | Cr | Ni | P | Ti | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.048 | 0.003 | 1.559 | 0.208 | 0.182 | 0.041 | 0.044 | 0.250 | 0.025 | 0.017 | Balance |
| HP Parameters | Primary Hydrogen Permeation | Secondary Hydrogen Permeation | ||||
|---|---|---|---|---|---|---|
| FC | AC | WC | FC | AC | WC | |
| J∞/mol·cm−2·s−1 | 5.617 × 10−10 | 3.441 × 10−10 | 7.244 × 10−10 | 4.487 × 10−10 | 2.954 × 10−10 | 5.804 × 10−10 |
| Deff/cm-2·s−1 | 2.863 × 10−6 | 7.466 × 10−7 | 5.790 × 10−7 | 4.615 × 10−6 | 1.483 × 10−6 | 1.053 × 10−6 |
| C0/mol·cm−3 | 1.570 × 10−5 | 3.687 × 10−5 | 1.001 × 10−4 | 7.779 × 10−6 | 1.594 × 10−5 | 4.411 × 10−5 |
| NT/mol·cm−3 | 2.287 × 10−4 | 2.095 × 10−3 | 7.342 × 10−3 | 6.932 × 10−5 | 4.533 × 10−4 | 1.773 × 10−3 |
| Parameters | FC | AC | WC |
|---|---|---|---|
| NT/mol·cm−3 | 2.287 × 10−4 | 2.095 × 10−3 | 7.342 × 10−3 |
| Nr/mol·cm−3 | 6.932 × 10−5 | 4.533 × 10−4 | 1.773 × 10−3 |
| Nir/mol·cm−3 | 1.594 × 10−4 | 1.642 × 10−3 | 5.569 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Yan, C.; Zhang, S.; Zhou, L.; Shen, M.; Tian, Z. Research on Hydrogen-Induced Induced Cracking Sensitivity of X80 Pipeline Steel under Different Heat Treatments. Materials 2024, 17, 1953. https://doi.org/10.3390/ma17091953
Wu C, Yan C, Zhang S, Zhou L, Shen M, Tian Z. Research on Hydrogen-Induced Induced Cracking Sensitivity of X80 Pipeline Steel under Different Heat Treatments. Materials. 2024; 17(9):1953. https://doi.org/10.3390/ma17091953
Chicago/Turabian StyleWu, Chen, Chunyan Yan, Shenglin Zhang, Lingchuan Zhou, Mengdie Shen, and Zhanpeng Tian. 2024. "Research on Hydrogen-Induced Induced Cracking Sensitivity of X80 Pipeline Steel under Different Heat Treatments" Materials 17, no. 9: 1953. https://doi.org/10.3390/ma17091953




