Effect of Adding Al on the Phase Structure and Gettering Performance of TiZrV Non-Evaporable Getter Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Bulk Getter
2.2. Characterization
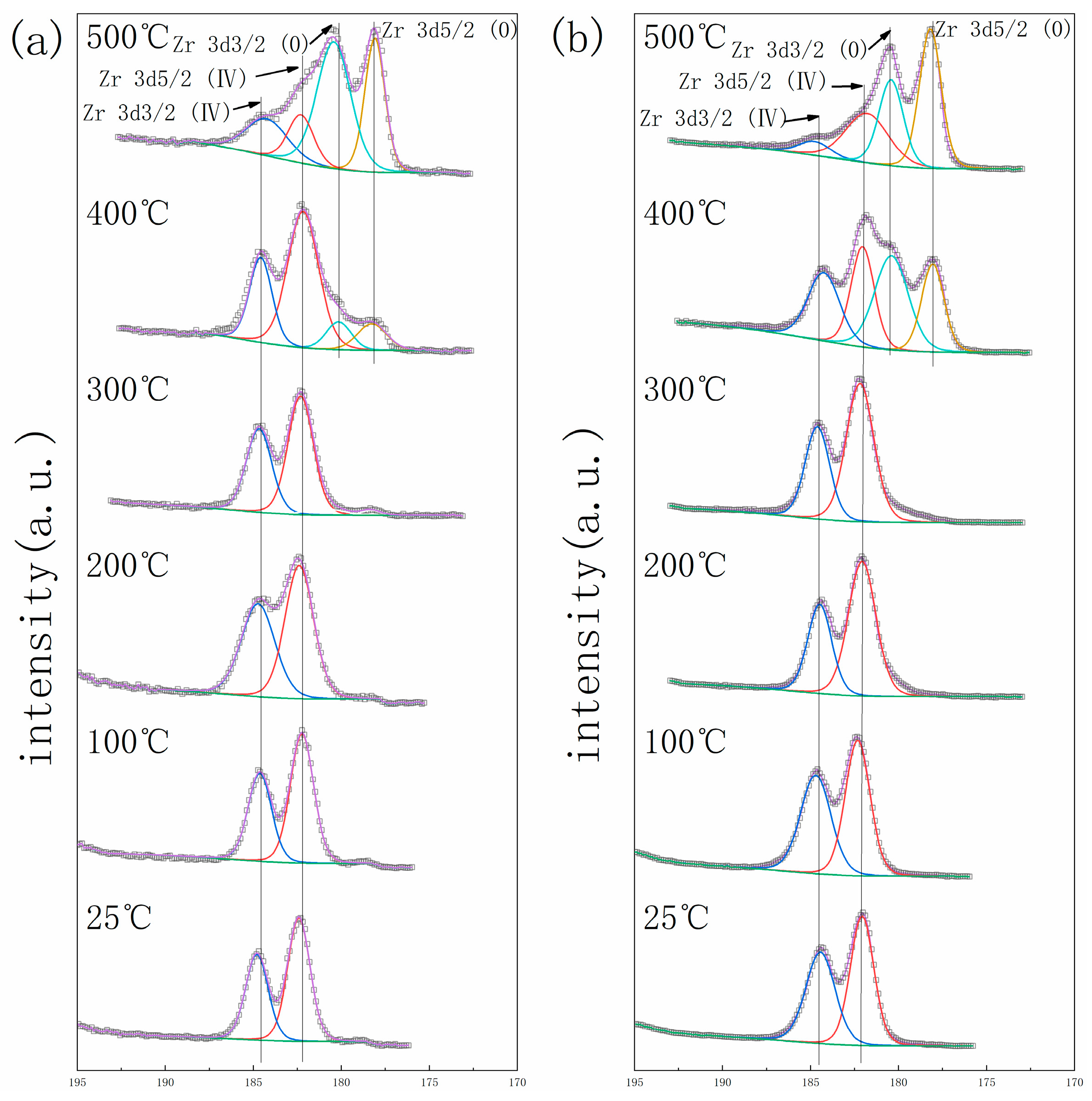
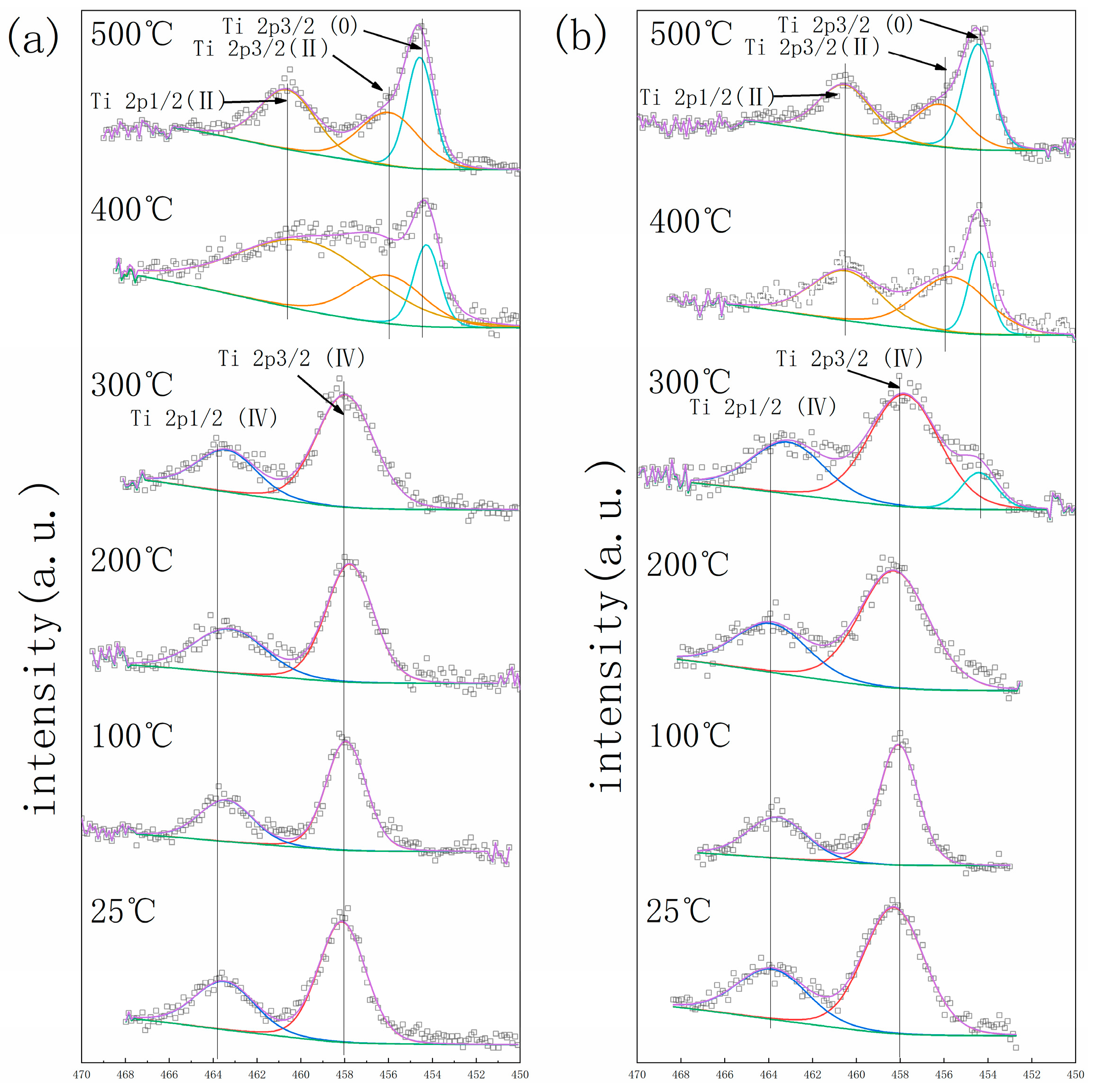
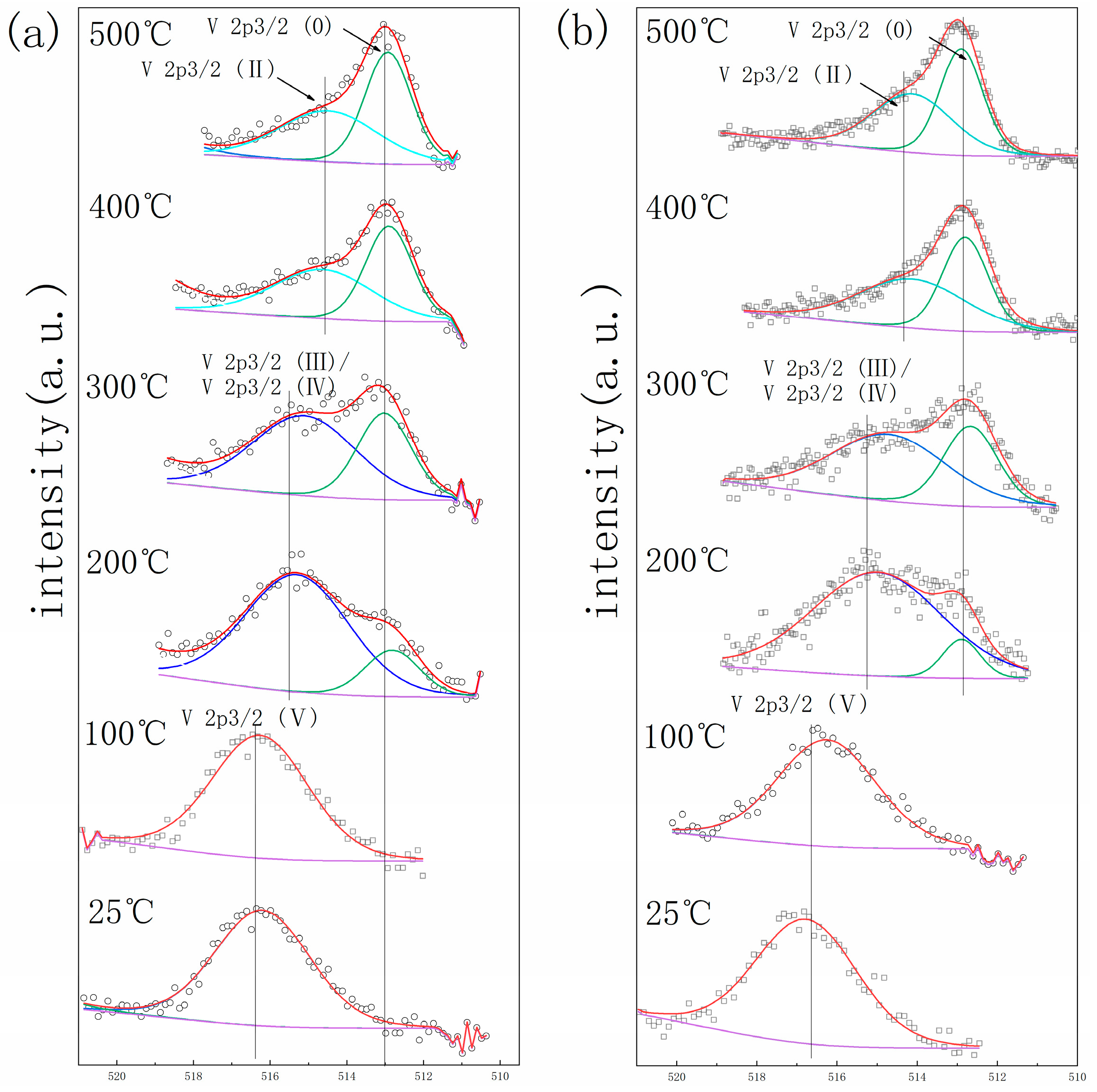
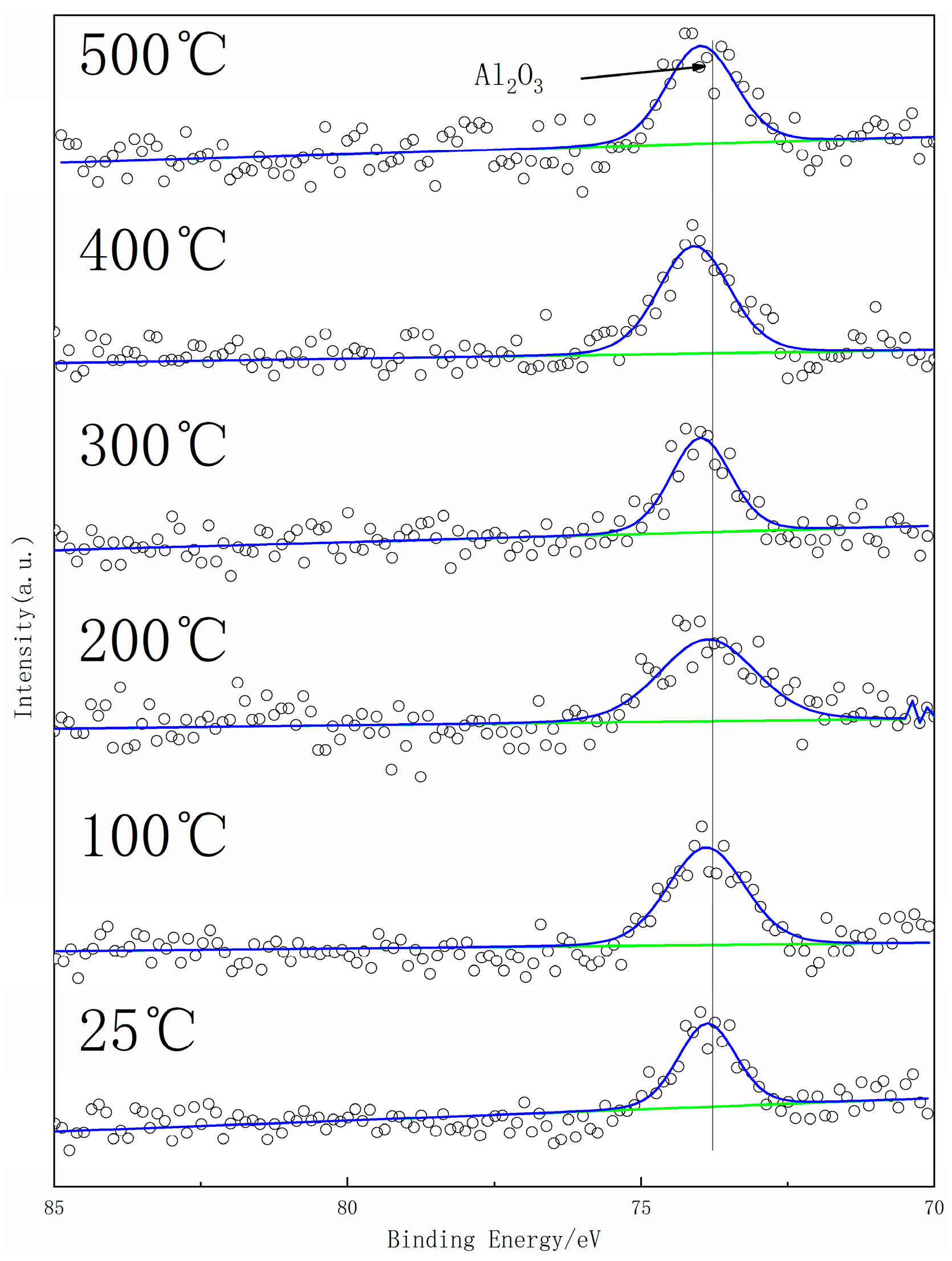
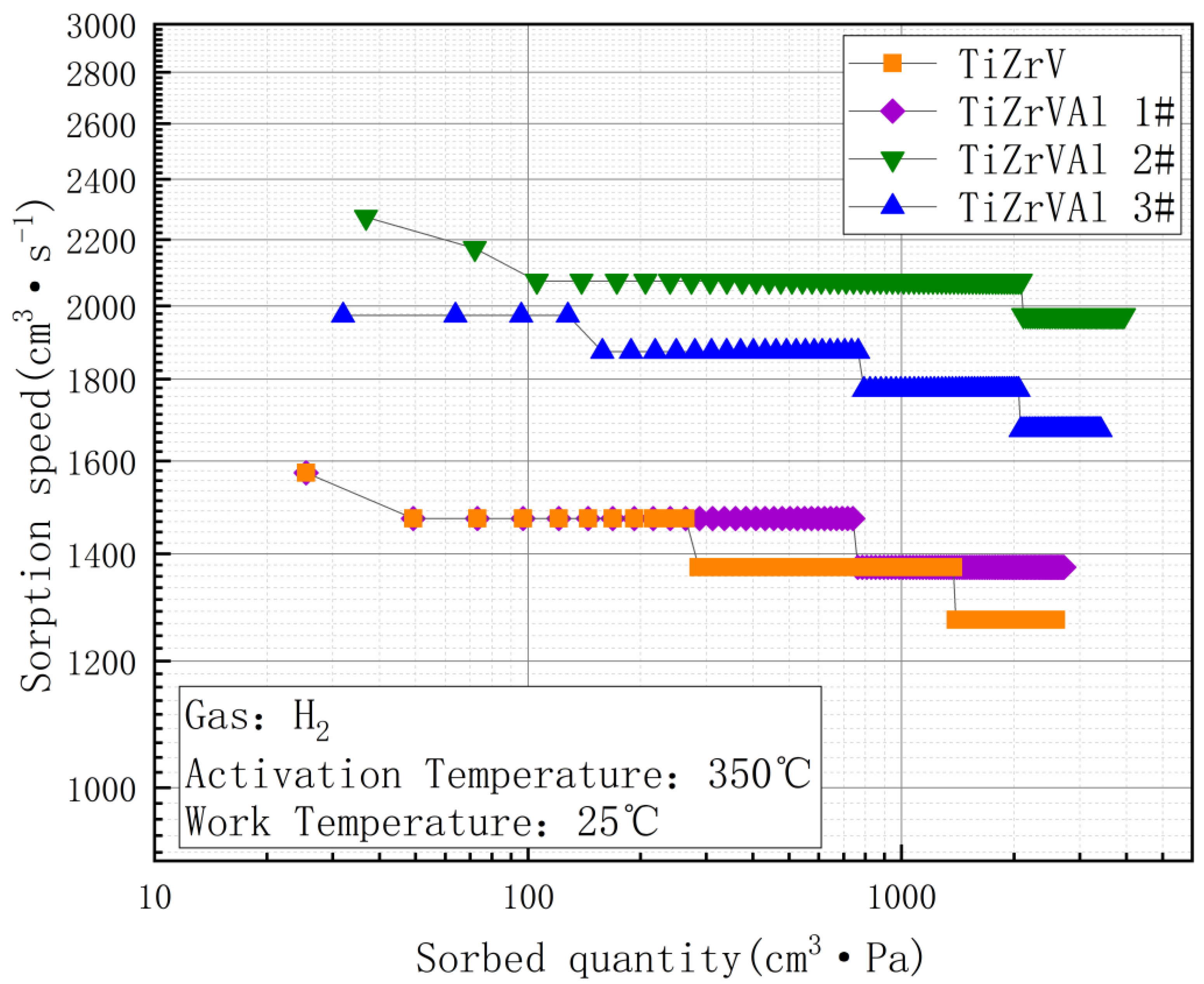
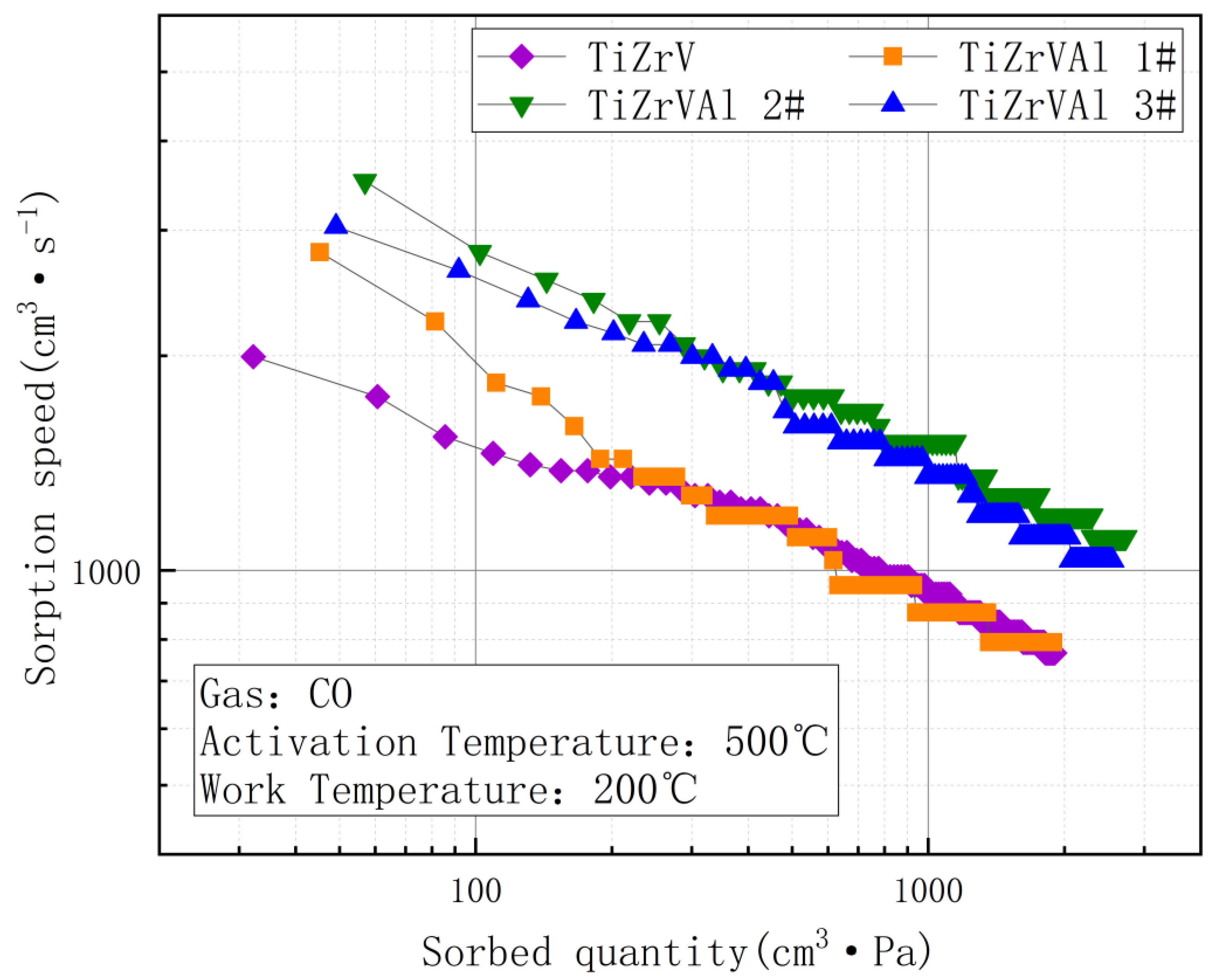
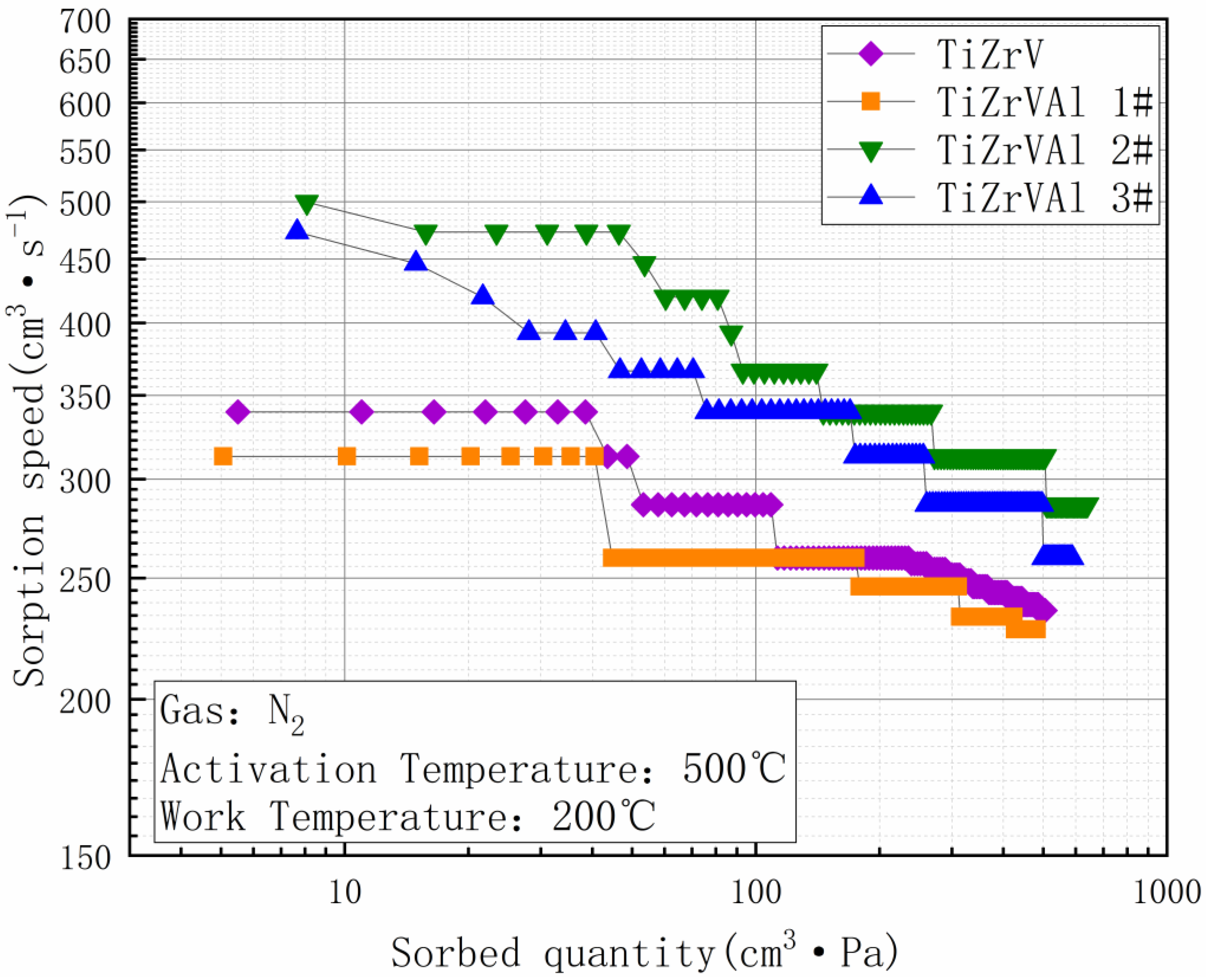
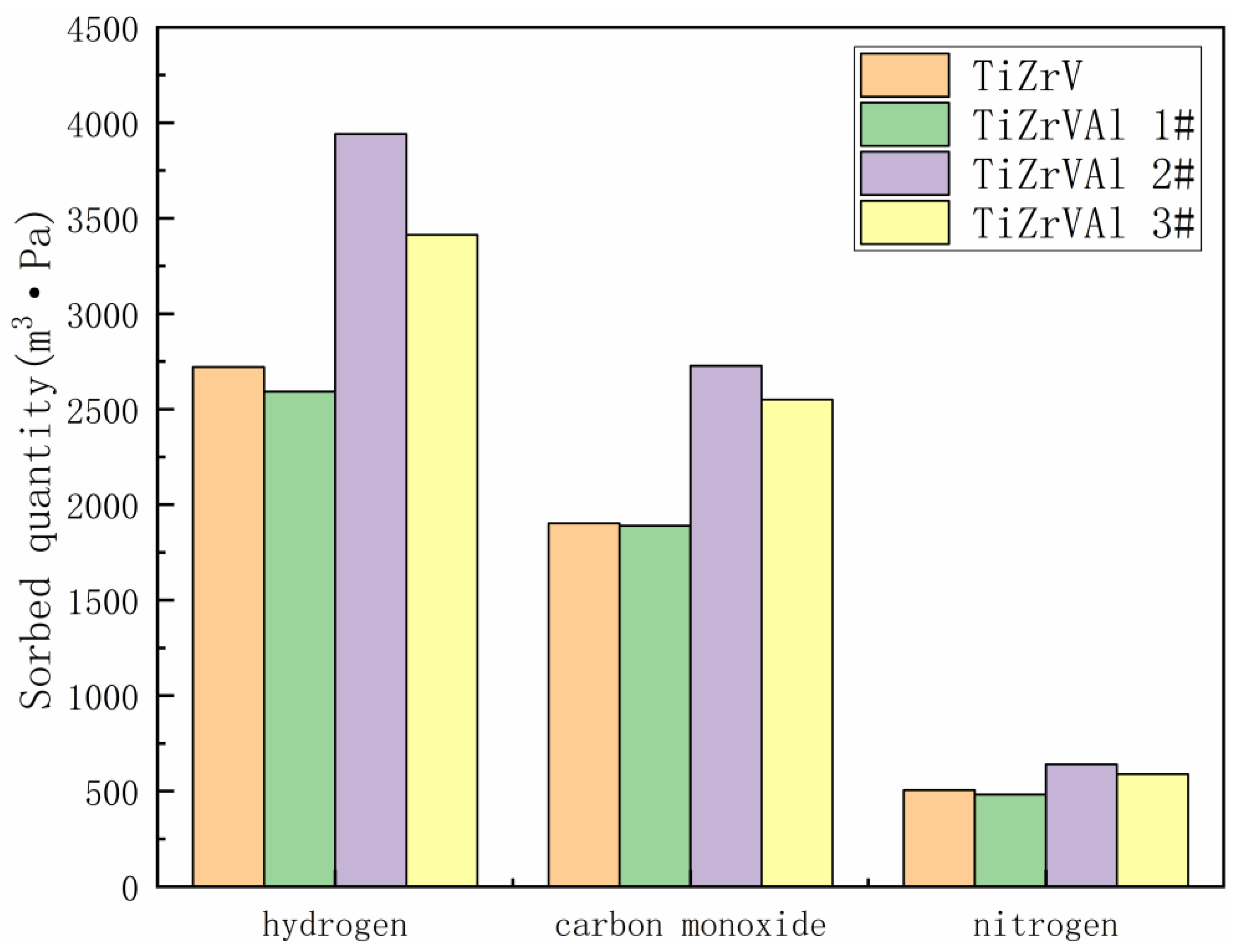
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiggiato, P.; Pinto, P. Ti–Zr–V non-evaporable getter films: From development to large scale production for the Large Hadron Collider. Thin Solid Films 2006, 515, 382–388. [Google Scholar] [CrossRef]
- Fermin, C.; Michel, L. Intermetallic alloys as hydrogen getters. J. Alloys Compd. 2022, 905, 164173. [Google Scholar]
- Benvenuti, C.; Chiggiato, P.; Cicoira, F.; L’Aminot, Y. Nonevaporable getter films for ultrahigh vacuum applications. J. Vac. Sci. Technol. A 1998, 16, 148–154. [Google Scholar] [CrossRef]
- Šutara, F.; Tsud, N.; Veltruská, K. XPS and ESD study of carbon and oxygen chemistry on TiZrV NEG. Vacuum 2001, 61, 135–139. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Gao, Y.; Hu, Y.; You, Z.; Xie, Y.; Li, H.; Wu, Y.; Yang, S.; Wang, D. The Activation of Ti-Zr-V-Hf Non-Evaporable Getter Films with Open-Cell Copper Metal Foam Substrates. Materials 2020, 13, 4650. [Google Scholar] [CrossRef]
- Shi, X.; Xiong, Y.; Wu, H. Influence of Barrier Layers on ZrCoCe Getter Film Performance. Materials 2023, 16, 2916. [Google Scholar] [CrossRef]
- Zhang, J.; Song, H.; Fang, J.; Hou, X.; Huang, S.; Xiang, J.; Lu, T.; Zhou, C. Study on Coated Zr-V-Cr Getter with Pore Gradient Structure for Hydrogen Masers. Materials 2022, 15, 6147. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Gao, Y.; Hu, Y.; Xie, Y.; You, Z.; Wang, S. Influence of Film Coating Thickness on Secondary Electron Emission Characteristics of Non-Evaporable Getter Ti-Hf-V-Zr Coated Open-Cell Copper Foam Substrates. Materials 2022, 15, 2185. [Google Scholar] [CrossRef]
- Wu, L.; Cheng, C.; Shueh, C. Synthesis of Ti-Zr-V Non-Evaporable Getter Thin Films Grown on Al Alloy and CuCrZr Alloy. Key Eng. Mater. 2017, 730, 87–94. [Google Scholar] [CrossRef]
- Adhikari, S.; Safarik, D.; Stockdale, J. Additively Manufactured Silicone Polymer Composite with High Hydrogen Getter Content and Hydrogen Absorption Capacity. ACS Omega 2024, 9, 15547–15555. [Google Scholar] [CrossRef]
- Benvenuti, C.; Chiggiato, P.; Mongelluzzo, A.; Prodromides, A.; Ruzinov, V.; Scheuerlein, C.; Taborelli, M.; Lévy, F. Influence of the elemental composition and crystal structure on the vacuum properties of Ti-Zr-V non-evaporable getter films. J. Vac. Sci. Technol. 2001, 19, 2925. [Google Scholar] [CrossRef]
- Hideya, Y.; Daisuke, O. Study of long-term performance of vacuum insulation panels containing getter materials in building environment. Energy Build. 2022, 255, 111648. [Google Scholar]
- Tripathi, A.; Singh, N.; Avasthi, D. Hydrogen intake capacity of ZrVFe alloy bulk getters. Vacuum 1997, 48, 1023–1025. [Google Scholar] [CrossRef]
- Changyul, S. Activation and Hydrogen Sorption Characteristics of a Ti0.3Zr0.2V0.5 Alloy Getter. Korean J. Mater. Res. 2005, 15, 79–84. [Google Scholar]
- Zhao, Z.; Wei, X.; Xiong, Y. Preparation of Ti-Mo getters by injection molding. Rare Metals 2009, 28, 147–150. [Google Scholar] [CrossRef]
- Cecchi, J.; LaMarche, P.; Dylla, H. Technique for in vacuo passivation of ZrAl alloy bulk getters. J. Vac. Sci. Technol. A Vac. Surf. Film. 1985, 3, 487–490. [Google Scholar] [CrossRef]
- Ferrario, B.; Figini, A.; Borghi, M. A new generation of porous non-evaporable getters. Vacuum 1985, 35, 13–17. [Google Scholar] [CrossRef]
- GB/T 8763-2020; Test Methods for the Gas Absorption Characteristic of Non-Evaporation Getter Materials and Products. State Administration for Market Regulation: Beijing, China, 2020.
- Sartori, E.; Siragusa, M.; Berton, G. Design of a large nonevaporable getter pump for the full size ITER beam source prototype. J. Vac. Sci. Technol. B 2023, 41, 034202. [Google Scholar] [CrossRef]
- Jiang, X.; Yu, G.; Feng, Z. Abnormal β-phase stability in TiZrAl alloys. J. Alloys Compd. 2016, 699, 256–261. [Google Scholar] [CrossRef]
- Vogel, U.; Brachmann, E.; Oswald, S.; Menzel, S.; Gemming, T.; Eckert, J. Evaluation of a mobile vacuum transfer system for in vacuo XPS analysis using as-deposited Ti thin-films. Vacuum 2015, 117, 81–84. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, Y.; Feng, Z. Influence of Zr content on β-phase stability in α-type Ti–Al alloys. Mater. Sci. Eng. A 2015, 639, 407–411. [Google Scholar] [CrossRef]
- Machado, F.; Ney, L. Study of electronic properties of Al3Ti, AlTi and AlTi3 intermetallic compounds using DFT-FPLAPW. J. Comput. Methods Sci. Eng. 2014, 14, 53–71. [Google Scholar] [CrossRef]
- Lain Amador, L. Production of Ultra-High-Vacuum Chambers with Integrated Getter Thin-Film Coatings by Electroforming. Ph.D. Dissertation, Université Bourgogne Franche-Comté, Besançon, France, 2019. [Google Scholar]
- Yang, Y.; Ma, Y.; Wang, J.; Huang, T.; Liu, B.; Sun, F.; Wang, X.; Chen, S.; Chen, Z.; Peng, X. Activation of Zr, ZrVHf and TiZrV Non-Evaporative Getters Characterized by In Situ Synchrotron Radiation Photoemission Spectroscopy. Appl. Sci. 2021, 11, 4844. [Google Scholar] [CrossRef]
- Morioka, N.; Yamano, Y.; Yamamoto, M. Study on dielectric breakdown in vacuum of TiZrV coated electrode. In Proceedings of the 2023 30th International Symposium on Discharges and Electrical Insulation in Vacuum (ISDEIV), Okinawa, Japan, 25–30 June 2023; pp. 62–64. [Google Scholar]
- Miyazawa, T.; Kurihara, M.; Ohno, S.; Terashima, N.; Natsui, Y.; Kato, H.; Kato, Y.; Hashimoto, A.; Kikuchi, T.; Mase, K. Oxygen-free palladium/titanium coating, a novel nonevaporable getter coating with an activation temperature of 133 °C. J. Vac. Sci. Technol. A 2018, 36, 051601. [Google Scholar] [CrossRef]
- Davis, J.; Beaux, M.; Freye, C. Evaluation of different getter substrates using two-dimensional gas chromatography with time of flight mass spectrometry. J. Chromatogr. A 2023, 1689, 463760. [Google Scholar] [CrossRef]
- Liang, S.; Ma, M.; Jing, R.; Zhang, X.; Liu, R. Microstructure and mechanical properties of hot-rolled ZrTiAlV alloys. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2012, 532, 1–5. [Google Scholar] [CrossRef]
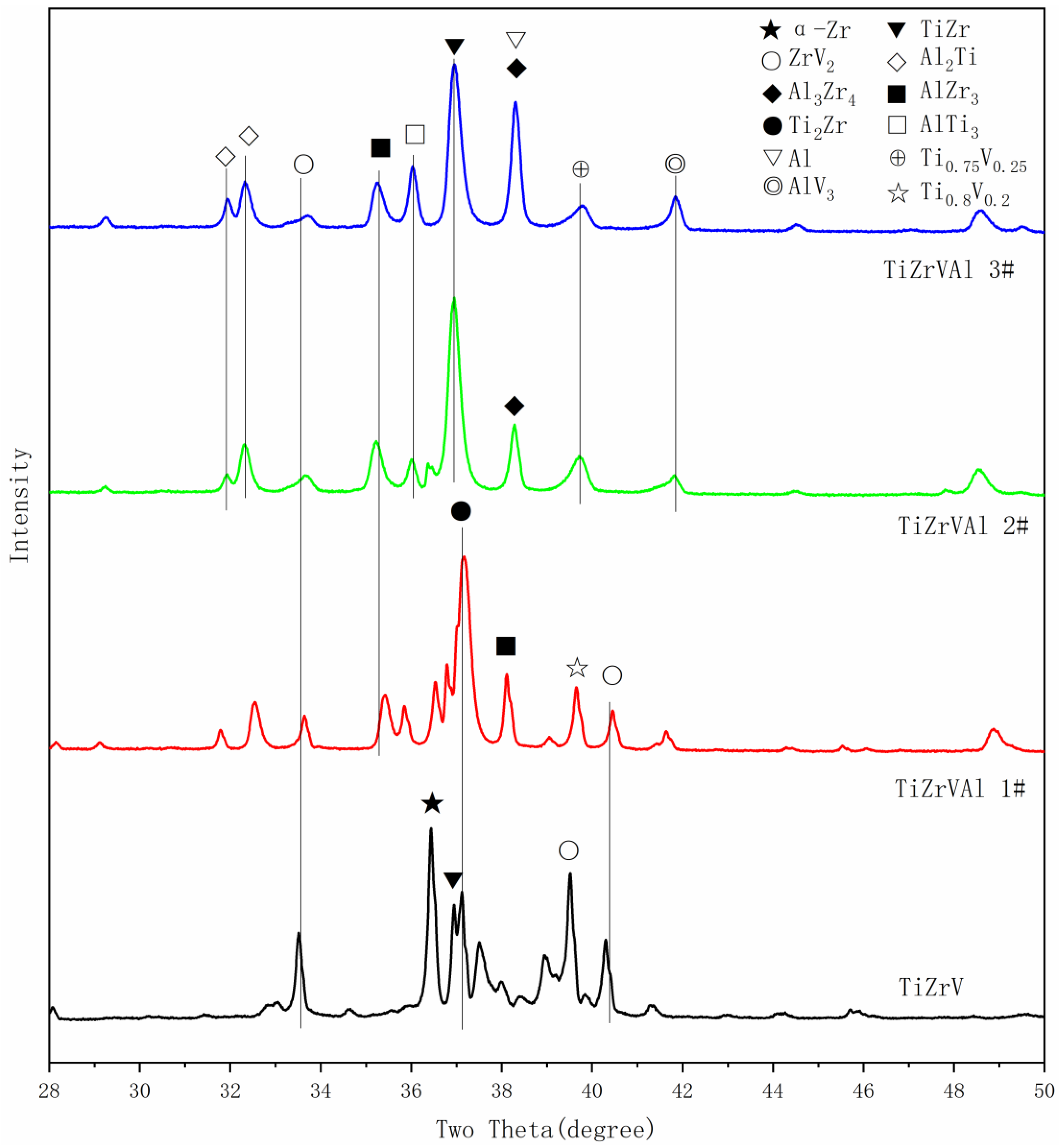
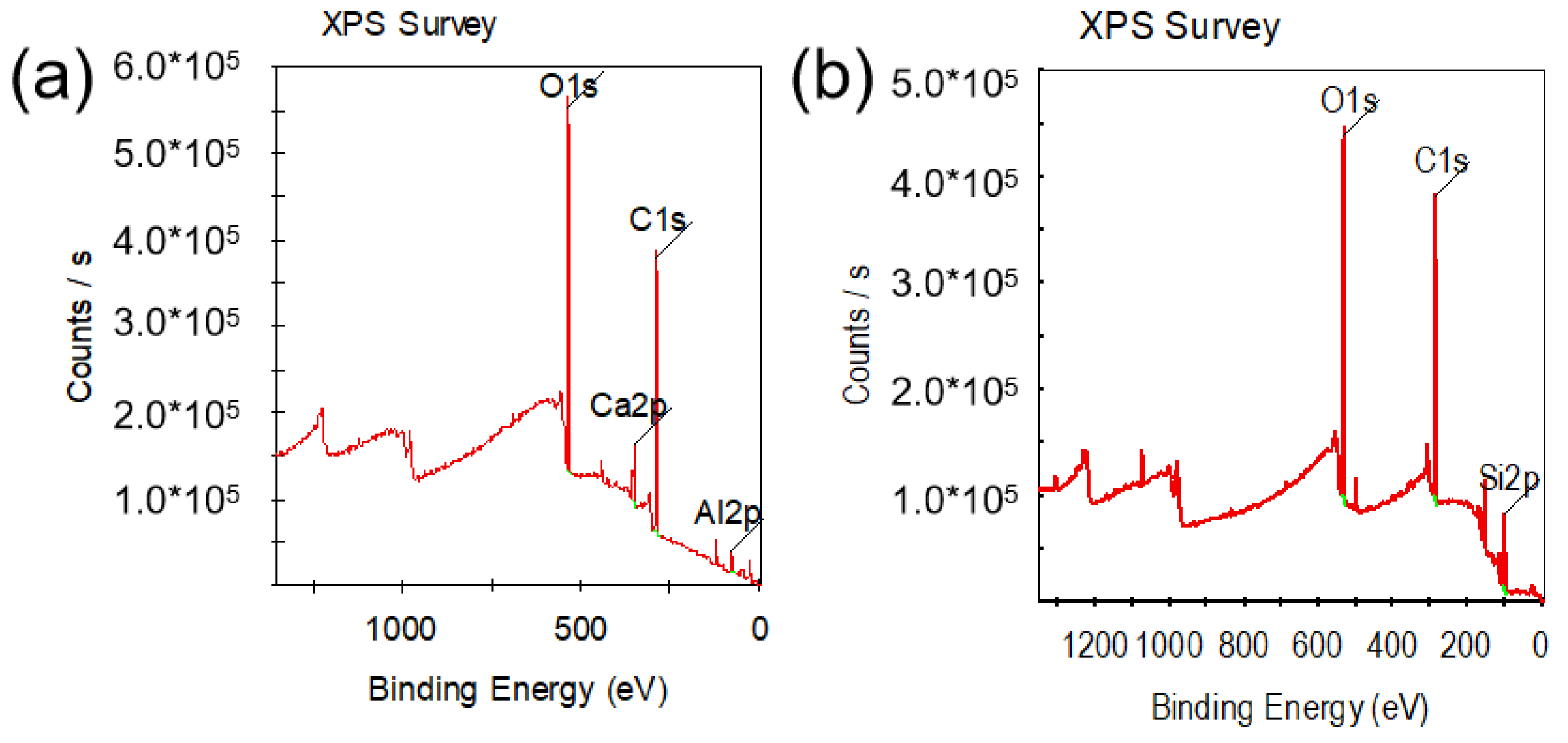
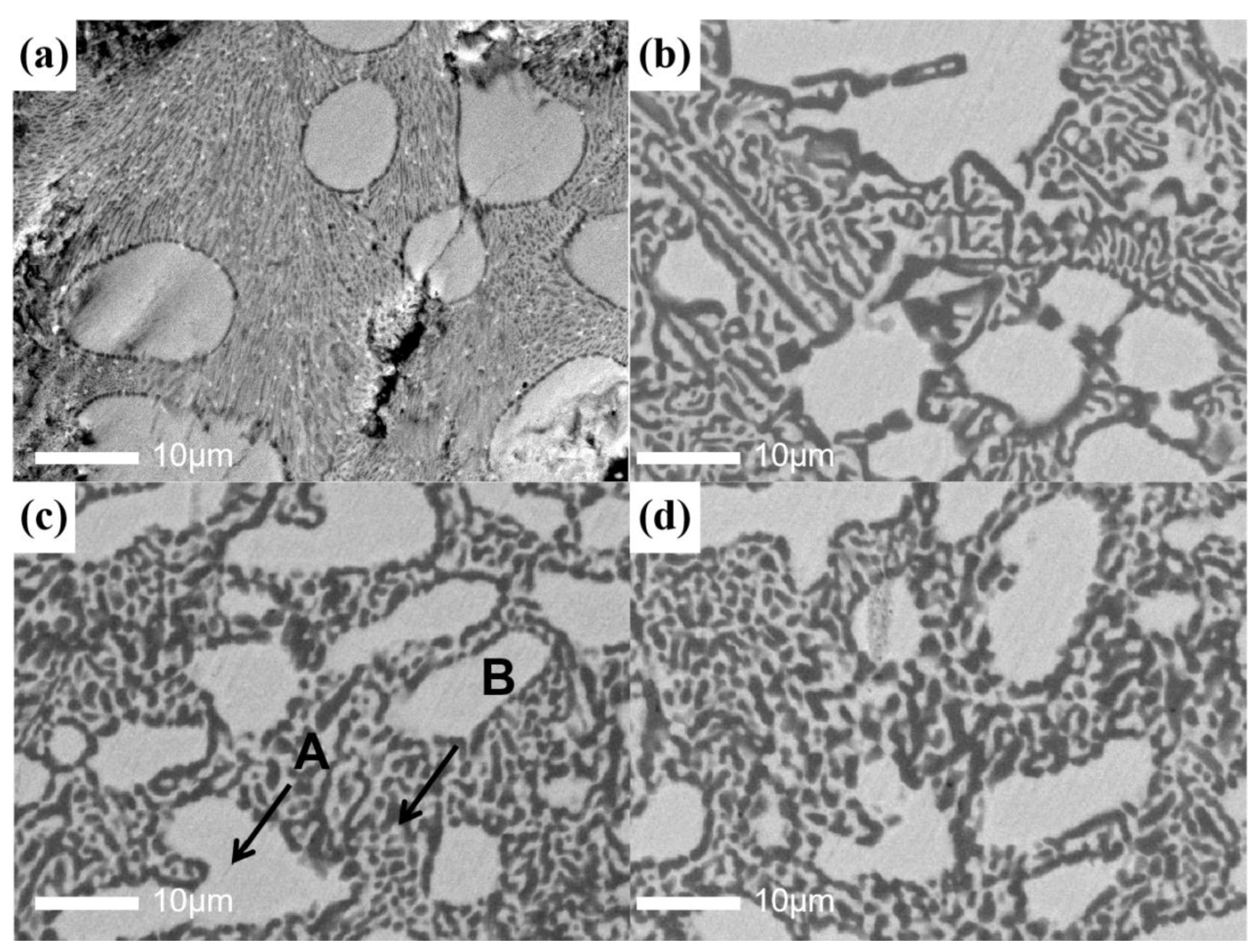

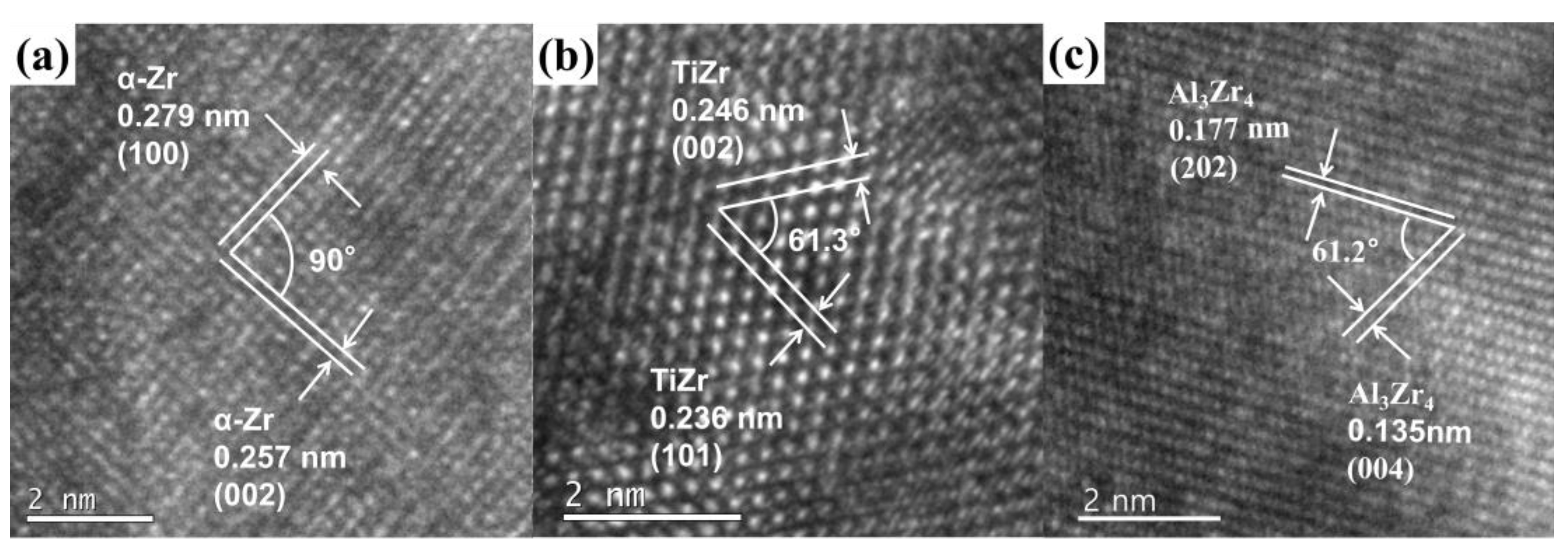
| Zr (at%) | Ti (at%) | V (at%) | Al (at%) | |
|---|---|---|---|---|
| TiZrV | 50 | 20 | 30 | - |
| TiZrVAl 1# | 50 | 20 | 30 | 3 |
| TiZrVAl 2# | 50 | 20 | 30 | 7 |
| TiZrVAl 3# | 50 | 20 | 30 | 11 |
| Sample | Name | Start BE | Peak BE | End BE | FWHM eV | Atomic % |
|---|---|---|---|---|---|---|
| TiZrVAl 3# | O1s | 537.50 | 531.85 | 523.00 | 2.97 | 31.18 |
| C1s | 292.50 | 284.79 | 277.00 | 2.63 | 58.44 | |
| Al2p | 79.50 | 74.14 | 64.50 | 2.72 | 7.25 | |
| Ca2p | 356.50 | 347.28 | 340.00 | 2.97 | 3.13 | |
| TiZrVAl 2# | O1s | 538.50 | 531.78 | 523.00 | 3.03 | 27.17 |
| C1s | 292.00 | 284.71 | 277.50 | 1.79 | 55.47 | |
| Si2p | 106.00 | 99.02 | 93.50 | 2.91 | 17.37 |
| Sample | Ca (W%) | Zn (W%) | Mg (W%) | Al (W%) |
|---|---|---|---|---|
| Zr | <0.2 | <0.002 | <0.002 | <0.0021 |
| Ti | <0.001 | <0.001 | <0.001 | / |
| V | <0.003 | <0.001 | <0.001 | / |
| Al | <0.001 | <0.001 | <0.001 | / |
| A (at%) | B (at%) | |
|---|---|---|
| Zr | 61.56 | 38.05 |
| Ti | 29.83 | 18.67 |
| V | 6.19 | 33.99 |
| Al | 2.29 | 9.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Li, Y.; Guo, D.; Jin, Q.; Zhang, Z.; Yang, Z. Effect of Adding Al on the Phase Structure and Gettering Performance of TiZrV Non-Evaporable Getter Materials. Materials 2024, 17, 1969. https://doi.org/10.3390/ma17091969
Wang L, Li Y, Guo D, Jin Q, Zhang Z, Yang Z. Effect of Adding Al on the Phase Structure and Gettering Performance of TiZrV Non-Evaporable Getter Materials. Materials. 2024; 17(9):1969. https://doi.org/10.3390/ma17091969
Chicago/Turabian StyleWang, Lulu, Yang Li, Deyu Guo, Qingxi Jin, Zhenbin Zhang, and Zhimin Yang. 2024. "Effect of Adding Al on the Phase Structure and Gettering Performance of TiZrV Non-Evaporable Getter Materials" Materials 17, no. 9: 1969. https://doi.org/10.3390/ma17091969





