Advances in Synthesis and Applications of Single-Atom Catalysts for Metal Oxide-Based Gas Sensors
Abstract
:1. Introduction
2. Synthesis Methods of SAC-Functionalized Metal Oxides
2.1. Atomic Layer Deposition (ALD)
2.2. Impregnation
2.3. Coprecipitation and Hydrothermal

2.4. Photochemical Method
2.5. Space Limitation Strategy

3. Characterization of SAC-Functionalized Metal Oxides
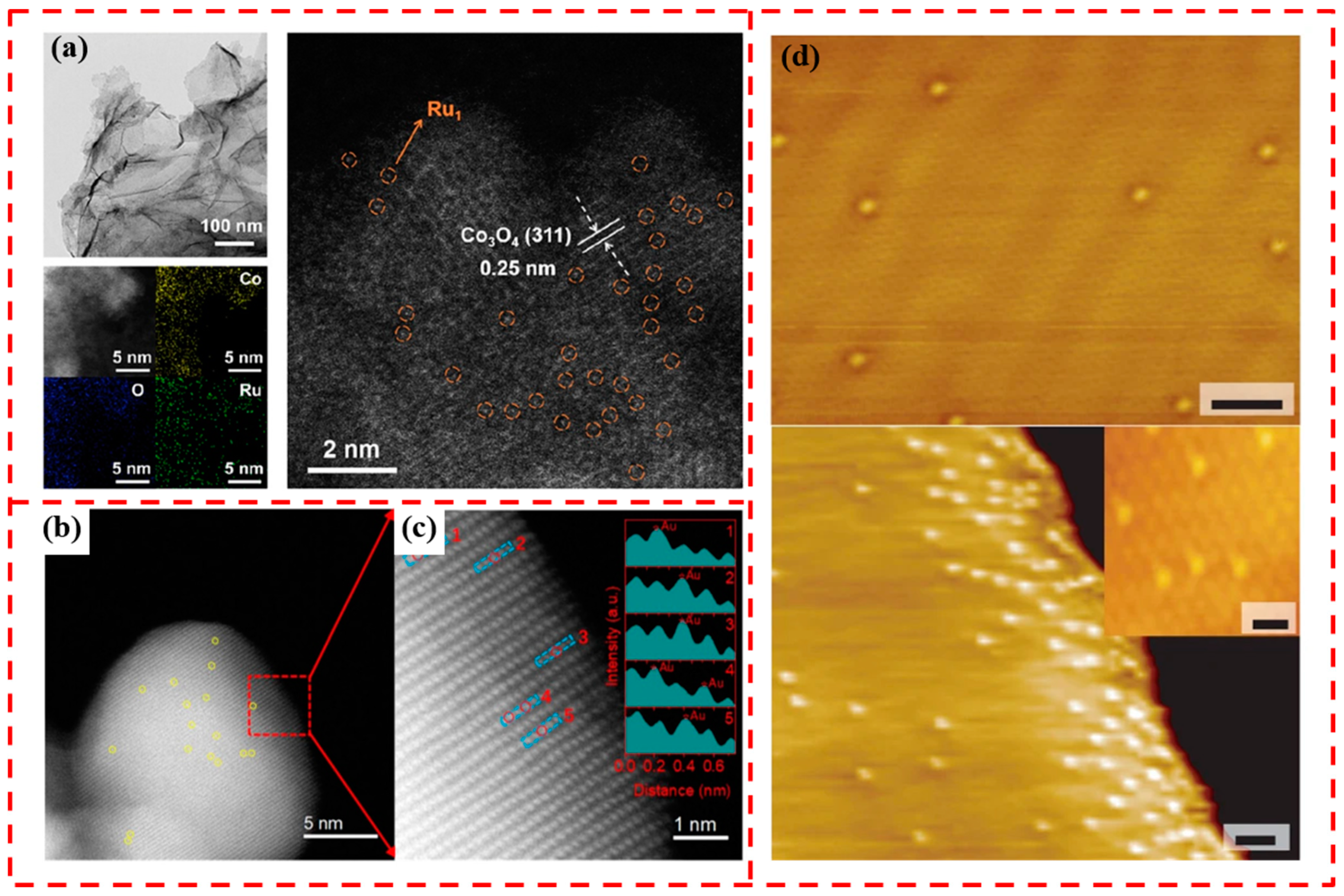
3.1. HAADF-STEM and STM
3.2. XANES and EXAFS
3.3. FT-IR
4. Stabilization Strategies of Single Atoms in Metal Oxides
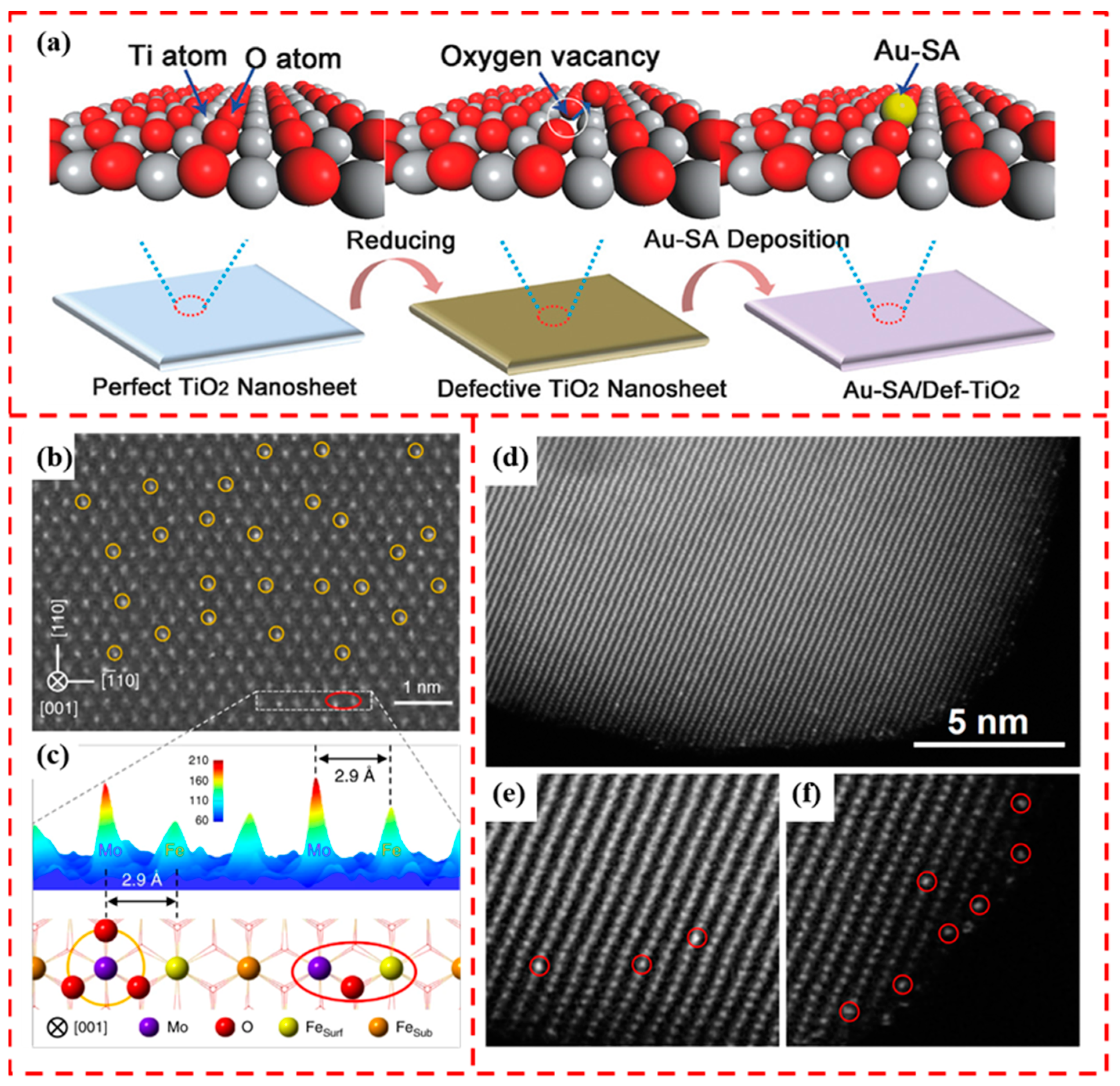
4.1. Stabilization through Defects
4.2. Stabilization through Surface-O(OH)x Species
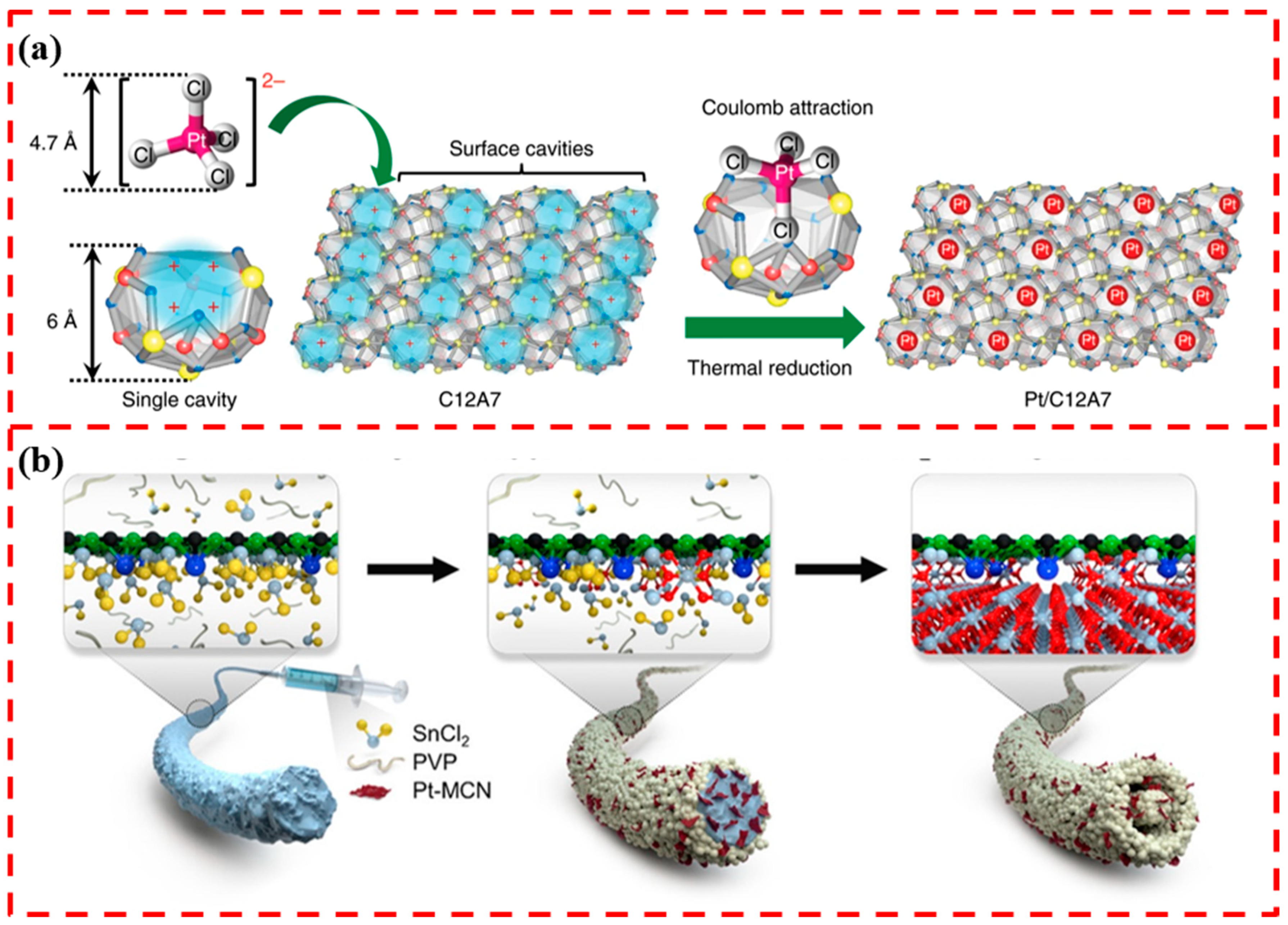
4.3. Stabilization through Spatial Constraints
5. Gas-Sensitive Properties of SAC-Functionalized Metal Oxides
5.1. SnO2-Based Support
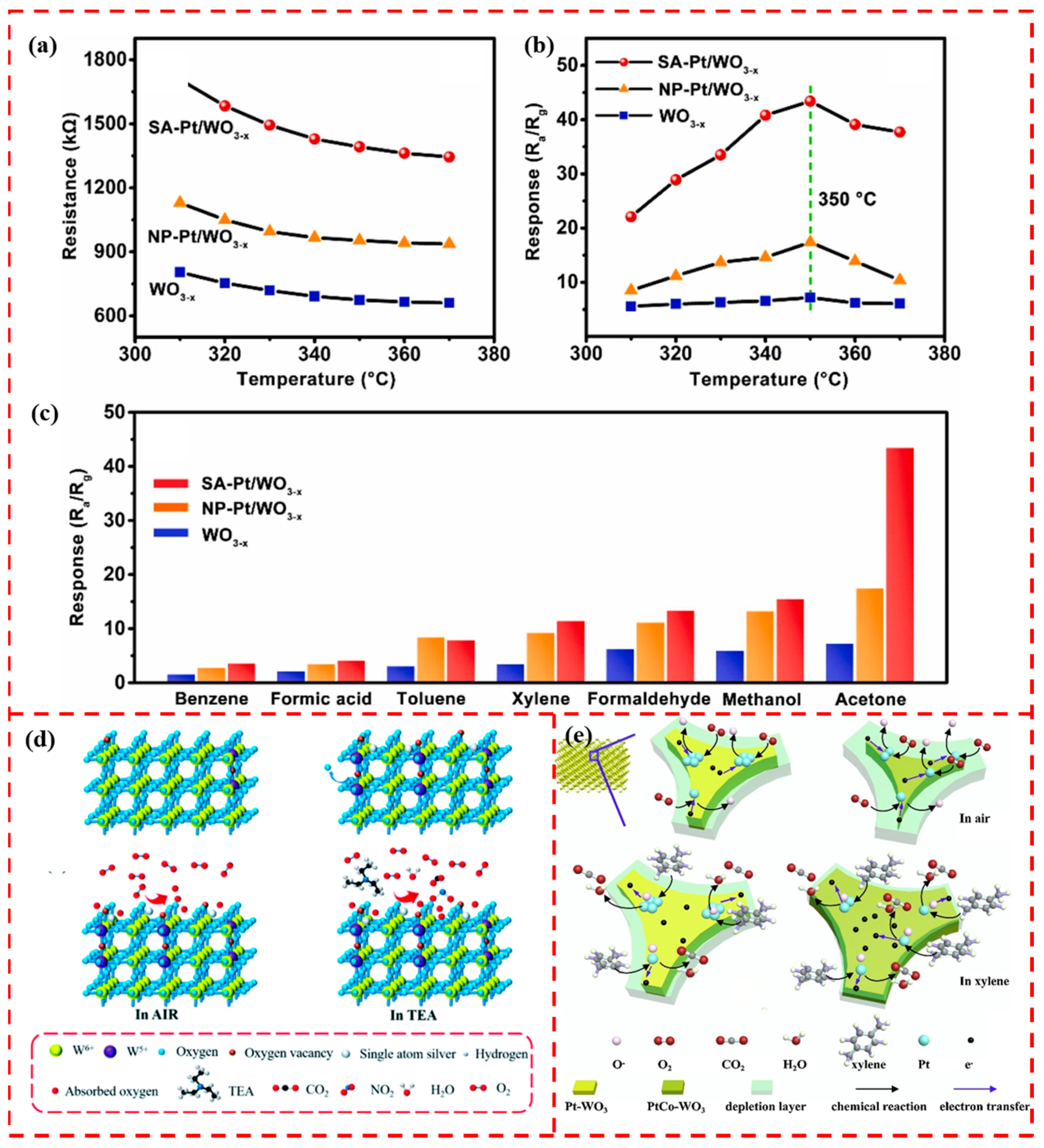
5.2. WOx-Based Support
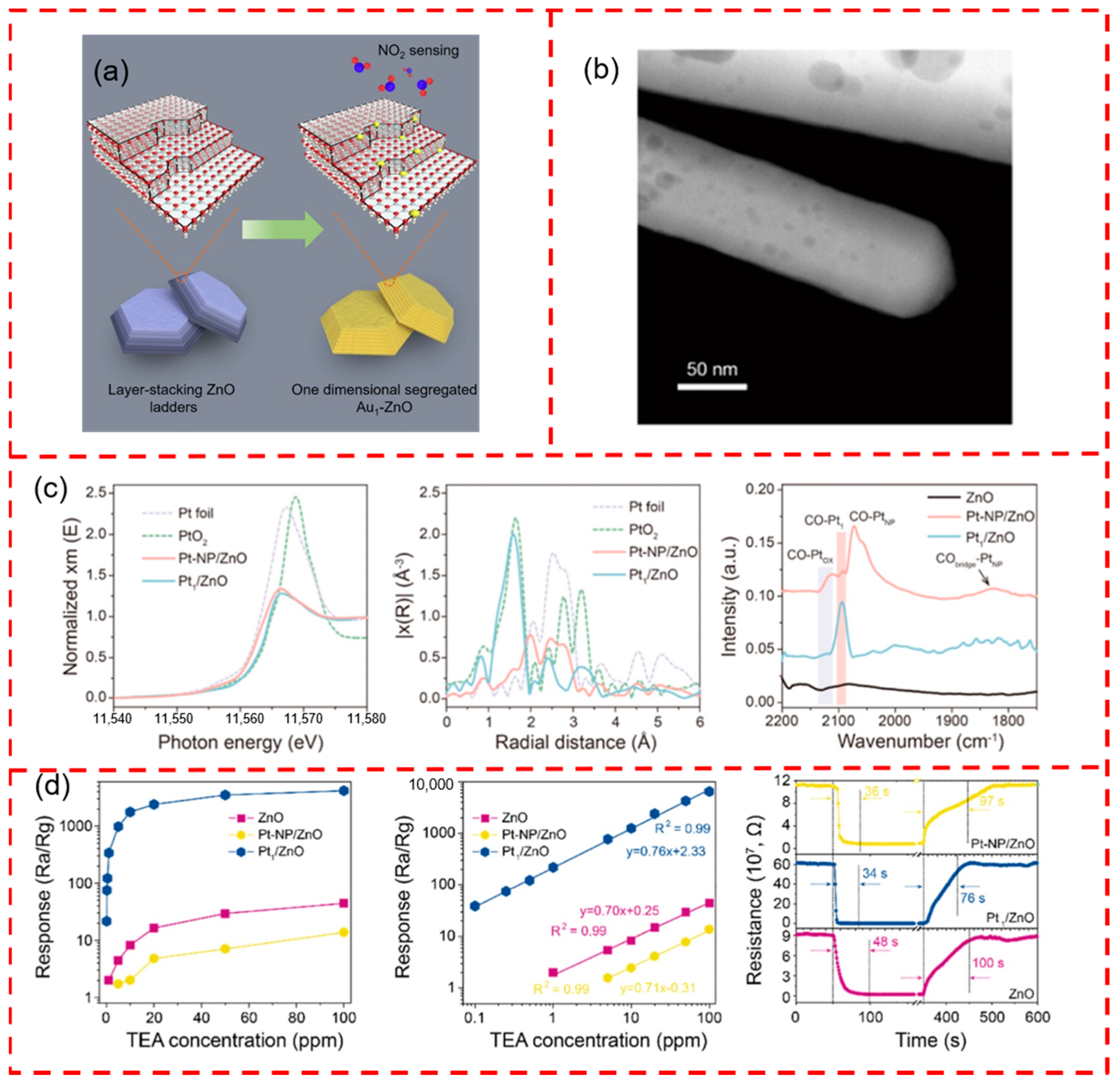
5.3. ZnO-Based Support
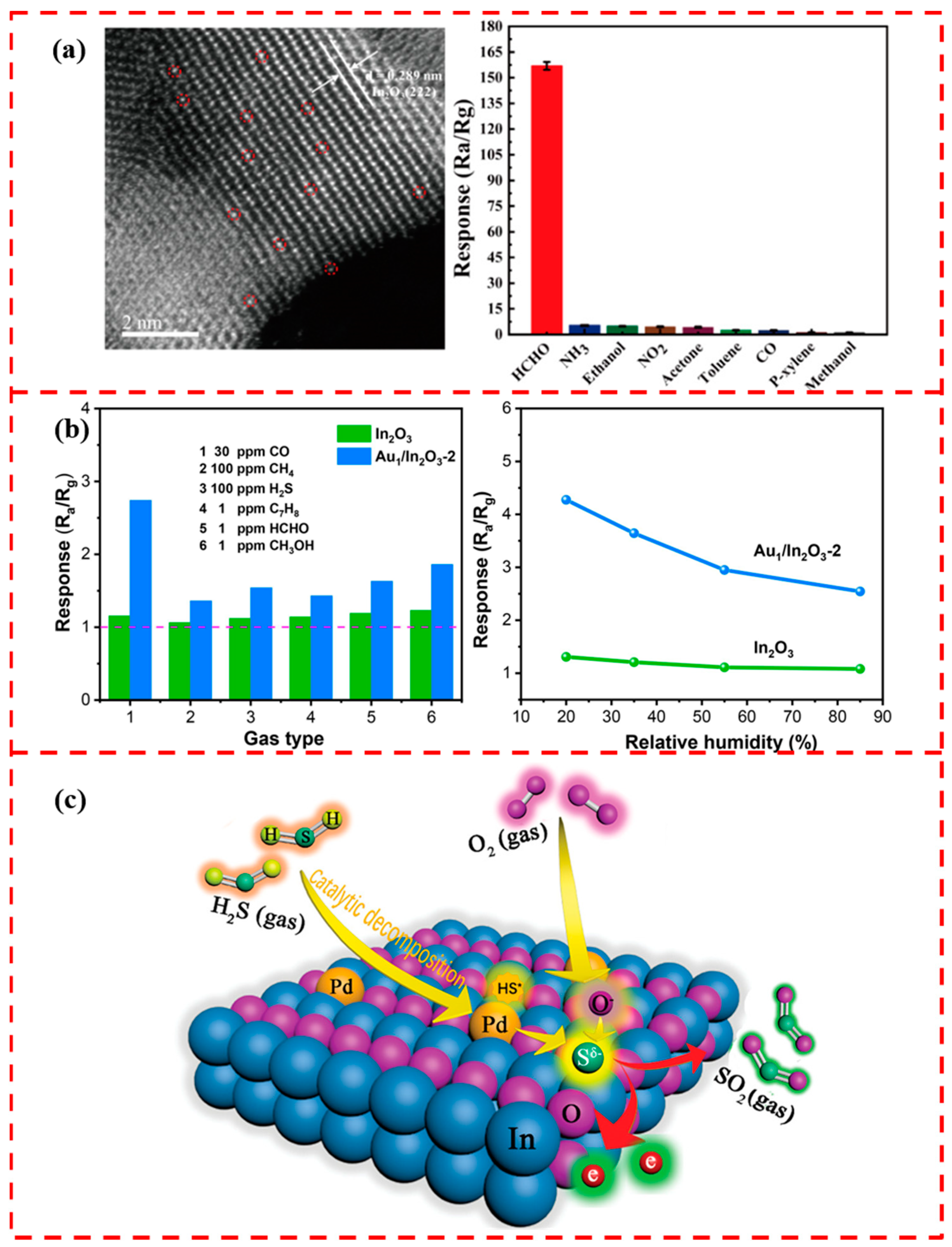
5.4. In2O3-Based Support
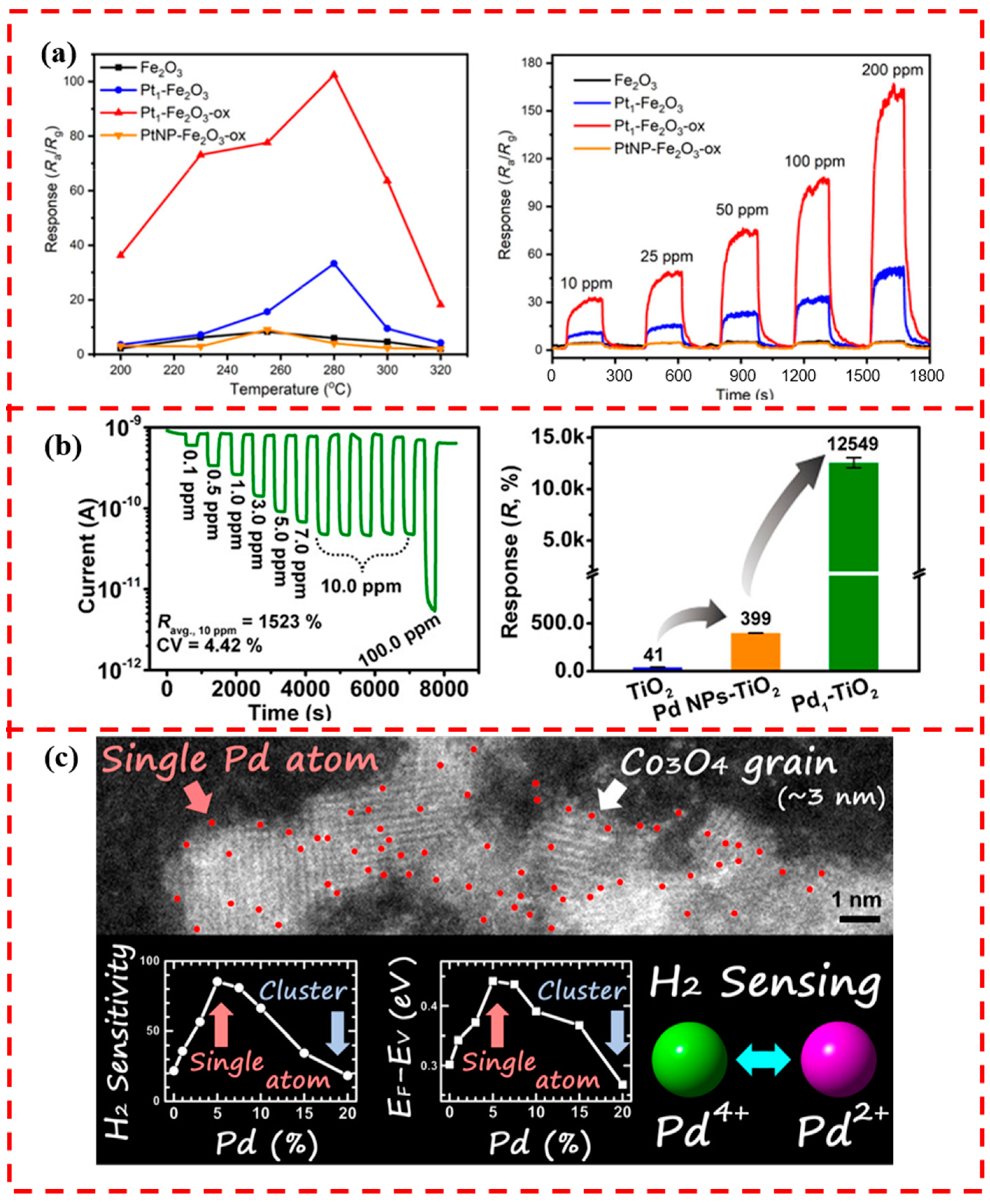
5.5. Other Metal Oxides-Based Supports
6. Summary and Perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Acosta, S.; Quintana, M. Chemically Functionalized 2D Transition Metal Dichalcogenides for Sensors. Sensors 2024, 24, 1817. [Google Scholar] [CrossRef]
- Otero Vélez, C.; Flores, S.Y.; Fonseca, L.F.; Piñero Cruz, D.M. Palladium Phthalocyanine Nanowire-Based Highly Sensitive Sensors for NO2(g) Detection. Sensors 2024, 24, 1819. [Google Scholar] [CrossRef]
- Flores, S.Y.; Gonzalez-Espiet, J.; Cintrón, J.; Villanueva, N.D.J.; Camino, F.E.; Kisslinger, K.; Cruz, D.M.P.; Rivera, R.D.; Fonseca, L.F. Fluorinated Iron and Cobalt Phthalocyanine Nanowire Chemiresistors for Environmental Gas Monitoring at Parts-per-Billion Levels. ACS Appl. Nano Mater. 2022, 5, 4688–4699. [Google Scholar] [CrossRef]
- Jian, Y.; Zhang, N.; Liu, T.; Zhu, Y.; Wang, D.; Dong, H.; Guo, L.; Qu, D.; Jiang, X.; Du, T.; et al. Artificially Intelligent Olfaction for Fast and Noninvasive Diagnosis of Bladder Cancer from Urine. ACS Sens. 2022, 7, 1720–1731. [Google Scholar] [CrossRef]
- Matsumoto, K.; Murakami, Y.; Shimizu, Y.; Hirayama, T.; Ishikawa, W.; Iwamura, M. Electronic Nose to Distinguish Bladder Cancer by Urinary Odour Feature: A Pilot Study. Cancer Biomark. 2020, 28, 33–39. [Google Scholar] [CrossRef]
- Bruderer, T.; Gaisl, T.; Gaugg, M.T.; Nowak, N.; Streckenbach, B.; Müller, S.; Moeller, A.; Kohler, M.; Zenobi, R. On-Line Analysis of Exhaled Breath: Focus Review. Chem. Rev. 2019, 119, 10803–10828. [Google Scholar] [CrossRef]
- Damdam, A.N.; Ozay, L.O.; Ozcan, C.K.; Alzahrani, A.; Helabi, R.; Salama, K.N. IoT-Enabled Electronic Nose System for Beef Quality Monitoring and Spoilage Detection. Foods 2023, 12, 2227. [Google Scholar] [CrossRef]
- Banerjee, M.B.; Roy, R.B.; Tudu, B.; Bandyopadhyay, R.; Bhattacharyya, N. Black Tea Classification Employing Feature Fusion of E-Nose and E-Tongue Responses. J. Food Eng. 2019, 244, 55–63. [Google Scholar] [CrossRef]
- Salinas, Y.; Ros-Lis, J.V.; Vivancos, J.-L.; Martínez-Máñez, R.; Marcos, M.D.; Aucejo, S.; Herranz, N.; Lorente, I.; Garcia, E. A Novel Colorimetric Sensor Array for Monitoring Fresh Pork Sausages Spoilage. Food Control 2014, 35, 166–176. [Google Scholar] [CrossRef]
- Guo, L.; Wang, T.; Wu, Z.; Wang, J.; Wang, M.; Cui, Z.; Ji, S.; Cai, J.; Xu, C.; Chen, X. Portable Food-Freshness Prediction Platform Based on Colorimetric Barcode Combinatorics and Deep Convolutional Neural Networks. Adv. Mater. 2020, 32, 2004805. [Google Scholar] [CrossRef]
- Kiani, S.; Minaei, S.; Ghasemi-Varnamkhasti, M. Application of Electronic Nose Systems for Assessing Quality of Medicinal and Aromatic Plant Products: A Review. J. Appl. Res. Med. Aromat. Plants 2016, 3, 1–9. [Google Scholar] [CrossRef]
- Ray, M.; Ray, A.; Dash, S.; Mishra, A.; Achary, K.G.; Nayak, S.; Singh, S. Fungal Disease Detection in Plants: Traditional Assays, Novel Diagnostic Techniques and Biosensors. Biosens. Bioelectron. 2017, 87, 708–723. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E. Sites of Synthesis, Biochemistry and Functional Role of Plant Volatiles. S. Afr. J. Bot. 2010, 76, 612–631. [Google Scholar] [CrossRef]
- Moseley, P.T. Progress in the Development of Semiconducting Metal Oxide Gas Sensors: A Review. Meas. Sci. Technol. 2017, 28, 082001. [Google Scholar] [CrossRef]
- Gu, F.; Li, C.; Han, D.; Wang, Z. Manipulating the Defect Structure (VO) of In2O3 Nanoparticles for Enhancement of Formaldehyde Detection. ACS Appl. Mater. Interfaces 2018, 10, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Al-Hashem, M.; Akbar, S.; Morris, P. Role of Oxygen Vacancies in Nanostructured Metal-Oxide Gas Sensors: A Review. Sens. Actuators B Chem. 2019, 301, 126845. [Google Scholar] [CrossRef]
- Tischner, A.; Maier, T.; Stepper, C.; Köck, A. Ultrathin SnO2 Gas Sensors Fabricated by Spray Pyrolysis for the Detection of Humidity and Carbon Monoxide. Sens. Actuators B Chem. 2008, 134, 796–802. [Google Scholar] [CrossRef]
- Karbalaei Akbari, M.; Hai, Z.; Wei, Z.; Detavernier, C.; Solano, E.; Verpoort, F.; Zhuiykov, S. ALD-Developed Plasmonic Two-Dimensional Au–WO3–TiO2 Heterojunction Architectonics for Design of Photovoltaic Devices. ACS Appl. Mater. Interfaces 2018, 10, 10304–10314. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Li, Q.; Ma, M.; Li, N.; Sheng, H.; Li, H.; Huang, Y.; Yun, F. Acidic Gas Determination Using Indium Tin Oxide-Based Gas Sensors. Sensors 2024, 24, 1286. [Google Scholar] [CrossRef]
- Santos-Betancourt, A.; Santos-Ceballos, J.C.; Alouani, M.A.; Malik, S.B.; Romero, A.; Ramírez, J.L.; Vilanova, X.; Llobet, E. ZnO Decorated Graphene-Based NFC Tag for Personal NO2 Exposure Monitoring during a Workday. Sensors 2024, 24, 1431. [Google Scholar] [CrossRef]
- Sun, G.; Chen, H.; Li, Y.; Chen, Z.; Zhang, S.; Ma, G.; Jia, T.; Cao, J.; Bala, H.; Wang, X.; et al. Synthesis and Improved Gas Sensing Properties of NiO-Decorated SnO2 Microflowers Assembled with Porous Nanorods. Sens. Actuators B Chem. 2016, 233, 180–192. [Google Scholar] [CrossRef]
- Navale, S.; Shahbaz, M.; Mirzaei, A.; Kim, S.S.; Kim, H.W. Effect of Ag Addition on the Gas-Sensing Properties of Nanostructured Resistive-Based Gas Sensors: An Overview. Sensors 2021, 21, 6454. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, Y.; Sung, S.-H.; Lee, G.; Kim, J.; Seong, J.; Shim, Y.-S.; Jun, S.C.; Jeon, S. High-Performance Gas Sensor Array for Indoor Air Quality Monitoring: The Role of Au Nanoparticles on WO3, SnO2, and NiO-Based Gas Sensors. J. Mater. Chem. A 2021, 9, 1159–1167. [Google Scholar] [CrossRef]
- Zhu, L.-Y.; Ou, L.-X.; Mao, L.-W.; Wu, X.-Y.; Liu, Y.-P.; Lu, H.-L. Advances in Noble Metal-Decorated Metal Oxide Nanomaterials for Chemiresistive Gas Sensors: Overview. Nano-Micro Lett. 2023, 15, 89. [Google Scholar] [CrossRef]
- Bahrami, B.; Khodadadi, A.; Kazemeini, M.; Mortazavi, Y. Enhanced CO Sensitivity and Selectivity of Gold Nanoparticles-Doped SnO2 Sensor in Presence of Propane and Methane. Sens. Actuators B Chem. 2008, 133, 352–356. [Google Scholar] [CrossRef]
- Y Nishchakova, A.D.; Bulusheva, L.G.; Bulushev, D.A. Supported Ni Single-Atom Catalysts: Synthesis, Structure, and Applications in Thermocatalytic Reactions. Catalysts 2023, 13, 845. [Google Scholar] [CrossRef]
- Flytzani-Stephanopoulos, M.; Gates, B.C. Atomically Dispersed Supported Metal Catalysts. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 545–574. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Xu, L.; Wang, D.; Li, Y. Metal Organic Frameworks Derived Single Atom Catalysts for Electrocatalytic Energy Conversion. Nano Res. 2019, 12, 2067–2080. [Google Scholar] [CrossRef]
- Cai, Y.; Bai, Z.; Chintalapati, S.; Zeng, Q.; Feng, Y.P. Transition Metal Atoms Pathways on Rutile TiO2 (110) Surface: Distribution of Ti3+ States and Evidence of Enhanced Peripheral Charge Accumulation. J. Chem. Phys. 2013, 138, 154711. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Guo, X.; Chen, C.; Dong, C.-L.; Liu, R.-S.; Han, C.-P.; Li, Y.; Gogotsi, Y.; Wang, G. Single Platinum Atoms Immobilized on an MXene as an Efficient Catalyst for the Hydrogen Evolution Reaction. Nat. Catal. 2018, 1, 985–992. [Google Scholar] [CrossRef]
- Wei, H.; Liu, X.; Wang, A.; Zhang, L.; Qiao, B.; Yang, X.; Huang, Y.; Miao, S.; Liu, J.; Zhang, T. FeOx-Supported Platinum Single-Atom and Pseudo-Single-Atom Catalysts for Chemoselective Hydrogenation of Functionalized Nitroarenes. Nat. Commun. 2014, 5, 5634. [Google Scholar] [CrossRef] [PubMed]
- Kyriakou, G.; Boucher, M.B.; Jewell, A.D.; Lewis, E.A.; Lawton, T.J.; Baber, A.E.; Tierney, H.L.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Isolated Metal Atom Geometries as a Strategy for Selective Heterogeneous Hydrogenations. Science 2012, 335, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.; Pan, H.; Mei, H.; Liu, X.; Lu, G.; Lou, C.; Li, Z.; Zhang, J. Emerging Single Atom Catalysts in Gas Sensors. Chem. Soc. Rev. 2022, 51, 7260–7280. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.; Rong, C.; Zhou, L.; Mao, X.; Zhang, B.; Xuan, F. Progress and Perspectives of Single-Atom Catalysts for Gas Sensing. Adv. Mater. 2023, 35, 2206783. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Elam, J.W.; Stair, P.C. Synthesis and Stabilization of Supported Metal Catalysts by Atomic Layer Deposition. Acc. Chem. Res. 2013, 46, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.; Lu, J. Single-Atom Catalysts Designed and Prepared by the Atomic Layer Deposition Technique. ACS Catal. 2021, 11, 7018–7059. [Google Scholar] [CrossRef]
- Lu, J.; Elam, J.W.; Stair, P.C. Atomic Layer Deposition—Sequential Self-Limiting Surface Reactions for Advanced Catalyst “Bottom-up” Synthesis. Surf. Sci. Rep. 2016, 71, 410–472. [Google Scholar] [CrossRef]
- Zhao, C.; Tang, Y.; Yu, C.; Tan, X.; Banis, M.N.; Li, S.; Wan, G.; Huang, H.; Zhang, L.; Yang, H.; et al. Insights into the Electronic Origin of Enhancing the Catalytic Activity of Co3O4 for Oxygen Evolution by Single Atom Ruthenium. Nano Today 2020, 34, 100955. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B.; Jin, Y.; Wu, T.; Ma, L.; Liang, X. Supported Single Fe Atoms Prepared via Atomic Layer Deposition for Catalytic Reactions. ACS Appl. Nano Mater. 2020, 3, 2867–2874. [Google Scholar] [CrossRef]
- Zhang, L.; Banis, M.N.; Sun, X. Single-Atom Catalysts by the Atomic Layer Deposition Technique. Natl. Sci. Rev. 2018, 5, 628–630. [Google Scholar] [CrossRef]
- Wang, L.; Huang, L.; Liang, F.; Liu, S.; Wang, Y.; Zhang, H. Preparation, Characterization and Catalytic Performance of Single-Atom Catalysts. Chin. J. Catal. 2017, 38, 1528–1539. [Google Scholar] [CrossRef]
- Wang, N.; Sun, Q.; Zhang, T.; Mayoral, A.; Li, L.; Zhou, X.; Xu, J.; Zhang, P.; Yu, J. Impregnating Subnanometer Metallic Nanocatalysts into Self-Pillared Zeolite Nanosheets. J. Am. Chem. Soc. 2021, 143, 6905–6914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, J.; Wang, J.; Wang, Q.; Wang, Y.; Wang, K.; Wang, Z.; Gu, M.; Tang, Z.; Lim, J.; et al. Single-Atom Catalyst for High-Performance Methanol Oxidation. Nat. Commun. 2021, 12, 5235. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Datye, A.K.; Wang, Y. Thermally Stable Single-Atom Heterogeneous Catalysts. Adv. Mater. 2021, 33, 2004319. [Google Scholar] [CrossRef]
- Hai, X.; Xi, S.; Mitchell, S.; Harrath, K.; Xu, H.; Akl, D.F.; Kong, D.; Li, J.; Li, Z.; Sun, T.; et al. Scalable Two-Step Annealing Method for Preparing Ultra-High-Density Single-Atom Catalyst Libraries. Nat. Nanotechnol. 2022, 17, 174–181. [Google Scholar] [CrossRef]
- Li, W.; Guo, Z.; Yang, J.; Li, Y.; Sun, X.; He, H.; Li, S.; Zhang, J. Advanced Strategies for Stabilizing Single-Atom Catalysts for Energy Storage and Conversion. Electrochem. Energy Rev. 2022, 5, 9. [Google Scholar] [CrossRef]
- Millet, M.-M.; Algara-Siller, G.; Wrabetz, S.; Mazheika, A.; Girgsdies, F.; Teschner, D.; Seitz, F.; Tarasov, A.; Levchenko, S.V.; Schlögl, R.; et al. Ni Single Atom Catalysts for CO2 Activation. J. Am. Chem. Soc. 2019, 141, 2451–2461. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Dai, R.; Mateen, M.; Hassan, Z.; Zhuang, Z.; Liu, C.; Israr, M.; Cheong, W.-C.; Hu, B.; Tu, R.; et al. Cobalt Single Atom Incorporated in Ruthenium Oxide Sphere: A Robust Bifunctional Electrocatalyst for HER and OER. Angew. Chem. Int. Ed. 2022, 61, e202114951. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Liu, X.; Liu, X.; Ling, C.; Wei, K.; Zhan, G.; Guo, Y.; Zhang, L. Adjacent single-atom irons boosting molecular oxygen activation on MnO. Nat. Commun. 2021, 12, 5422. [Google Scholar] [CrossRef]
- Li, Z.; Wang, D.; Wu, Y.; Li, Y. Recent Advances in the Precise Control of Isolated Single-Site Catalysts by Chemical Methods. Natl. Sci. Rev. 2018, 5, 673–689. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, Y.; Qin, R.; Mo, S.; Chen, G.; Gu, L.; Chevrier, D.M.; Zhang, P.; Guo, Q.; Zang, D.; et al. Photochemical Route for Synthesizing Atomically Dispersed Palladium Catalysts. Science 2016, 352, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Huang, K.; Wang, D.; Zhang, R.; Ge, B.; Ma, J.; Wen, B.; Zhang, S.; Li, Q.; Lei, M.; et al. Iced Photochemical Reduction to Synthesize Atomically Dispersed Metals by Suppressing Nanocrystal Growth. Nat. Commun. 2017, 8, 1490. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Fu, S.; Zhu, C.; Song, J.; Du, D.; Lin, Y. Metal–Organic Frameworks-Based Catalysts for Electrochemical Oxygen Evolution. Mater. Horiz. 2019, 6, 684–702. [Google Scholar] [CrossRef]
- Yin, P.; Yao, T.; Wu, Y.; Zheng, L.; Lin, Y.; Liu, W.; Ju, H.; Zhu, J.; Hong, X.; Deng, Z.; et al. Single Cobalt Atoms with Precise N-Coordination as Superior Oxygen Reduction Reaction Catalysts. Angew. Chem. 2016, 128, 10958–10963. [Google Scholar] [CrossRef]
- Wang, J.; Han, G.; Wang, L.; Du, L.; Chen, G.; Gao, Y.; Ma, Y.; Du, C.; Cheng, X.; Zuo, P.; et al. ZIF-8 with Ferrocene Encapsulated: A Promising Precursor to Single-Atom Fe Embedded Nitrogen-Doped Carbon as Highly Efficient Catalyst for Oxygen Electroreduction. Small 2018, 14, 1704282. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, L.; Luo, Y.; Gao, L.; Duan, G. The Dehydrogenation of H-S Bond into Sulfur Species on Supported Pd Single Atoms Allows Highly Selective and Sensitive Hydrogen Sulfide Detection. Small 2021, 17, 2105643. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.B.; Koo, J.Y.; Disko, M.M.; Treacy, M.M.J. On the Imaging of Pt Atoms in Zeolite Frameworks. Ultramicroscopy 1990, 34, 108–118. [Google Scholar] [CrossRef]
- Besenbacher, F.; Brorson, M.; Clausen, B.S.; Helveg, S.; Hinnemann, B.; Kibsgaard, J.; Lauritsen, J.V.; Moses, P.G.; Nørskov, J.K.; Topsøe, H. Recent STM, DFT and HAADF-STEM Studies of Sulfide-Based Hydrotreating Catalysts: Insight into Mechanistic, Structural and Particle Size Effects. Catal. Today 2008, 130, 86–96. [Google Scholar] [CrossRef]
- Gu, W.; Pei, A.; Zhang, S.; Jiang, F.; Jia, Y.; Qin, Q.; Du, R.; Li, Z.; Liu, R.; Qiu, Y.; et al. Atomic-Interface Effect of Single-Atom Ru/CoOx for Selective Electrooxidation of 5-Hydroxymethylfurfural. ACS Appl. Mater. Interfaces 2023, 15, 28036–28043. [Google Scholar] [CrossRef]
- Wang, Y.; Hong, G.; Zhang, Y.; Liu, Y.; Cen, W.; Wang, L.; Wu, Z. Photocatalytic Oxidative Coupling of Methane over Au1Ag Single-Atom Alloy Modified ZnO with Oxygen and Water Vapor: Synergy of Gold and Silver. Angew. Chem. Int. Ed. 2023, 62, e202310525. [Google Scholar] [CrossRef]
- Marcinkowski, M.D.; Darby, M.T.; Liu, J.; Wimble, J.M.; Lucci, F.R.; Lee, S.; Michaelides, A.; Flytzani-Stephanopoulos, M.; Stamatakis, M.; Sykes, E.C.H. Pt/Cu Single-Atom Alloys as Coke-Resistant Catalysts for Efficient C–H Activation. Nat. Chem. 2018, 10, 325–332. [Google Scholar] [CrossRef]
- Barbara, S. Probing Active Sites of Heterogeneous Catalysts by X-ray Absorption Spectroscopy; University of California: Santa Barbara, CA, USA, 2021. [Google Scholar]
- Oikawa, T.; Ookoshi, T.; Tanaka, T.; Yamamoto, T.; Onaka, M. A New Heterogeneous Olefin Metathesis Catalyst Composed of Rhenium Oxide and Mesoporous Alumina. Microporous Mesoporous Mater. 2004, 74, 93–103. [Google Scholar] [CrossRef]
- Xu, T.; Zheng, H.; Zhang, P. Isolated Pt Single Atomic Sites Anchored on Nanoporous TiO2 Film for Highly Efficient Photocatalytic Degradation of Low Concentration Toluene. J. Hazard. Mater. 2020, 388, 121746. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, J.; Du, Y.; Zhang, Y.; Wu, X.; Jing, G. Self-assembly of Atomically Dispersed Ag Catalysts on Polyhedral Co3O4 at Elevated Temperatures: A Top-Down Nanofabrication of High-Loading Atomically Dispersed Catalysts. ChemCatChem 2020, 12, 561–568. [Google Scholar] [CrossRef]
- Che, M.; Védrine, J.C. (Eds.) Characterization of Solid Materials and Heterogeneous Catalysts: From Structure to Surface Reactivity, 1st ed.; Wiley: Hoboken, NJ, USA, 2012; ISBN 978-3-527-32687-7. [Google Scholar]
- Rodríguez, J.A.; Hanson, J.C.; Chupas, P.J. (Eds.) In-Situ Characterization of Heterogeneous Catalysts; Wiley: Hoboken, NJ, USA, 2013; ISBN 978-1-118-00016-8. [Google Scholar]
- Lamberti, C.; Zecchina, A.; Groppo, E.; Bordiga, S. Probing the Surfaces of Heterogeneous Catalysts by in Situ IR Spectroscopy. Chem. Soc. Rev. 2010, 39, 4951. [Google Scholar] [CrossRef] [PubMed]
- Zaera, F. New Advances in the Use of Infrared Absorption Spectroscopy for the Characterization of Heterogeneous Catalytic Reactions. Chem. Soc. Rev. 2014, 43, 7624–7663. [Google Scholar] [CrossRef]
- Lang, R.; Du, X.; Huang, Y.; Jiang, X.; Zhang, Q.; Guo, Y.; Liu, K.; Qiao, B.; Wang, A.; Zhang, T. Single-Atom Catalysts Based on the Metal–Oxide Interaction. Chem. Rev. 2020, 120, 11986–12043. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-Atom Catalysis of CO Oxidation Using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Chen, J.; Jia, H. Temperature-Induced Structure Reconstruction to Prepare a Thermally Stable Single-Atom Platinum Catalyst. Angew. Chem. Int. Ed. 2020, 59, 13562–13567. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Matsubu, J.C.; Zhang, S.; DeRita, L.; Marinkovic, N.S.; Chen, J.G.; Graham, G.W.; Pan, X.; Christopher, P. Adsorbate-Mediated Strong Metal–Support Interactions in Oxide-Supported Rh Catalysts. Nat. Chem. 2017, 9, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Tauster, S.J.; Fung, S.C.; Garten, R.L. Strong Metal-Support Interactions. Group 8 Noble Metals Supported on Titanium Dioxide. J. Am. Chem. Soc. 1978, 100, 170–175. [Google Scholar] [CrossRef]
- Han, B.; Guo, Y.; Huang, Y.; Xi, W.; Xu, J.; Luo, J.; Qi, H.; Ren, Y.; Liu, X.; Qiao, B.; et al. Strong Metal–Support Interactions between Pt Single Atoms and TiO. Angew. Chem. Int. Ed. 2020, 59, 11824–11829. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Cui, X.; Zeng, L.; Su, L.; Song, Y.; Shi, J. Oxygen Vacancy-Assisted Hydrogen Evolution Reaction of the Pt/WO3 Electrocatalyst. J. Mater. Chem. A 2019, 7, 6285–6293. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Y.; Lu, L.; Zhang, D.; Wang, X. Single-Atom Au Catalyst Loaded on CeO2: A Novel Single-Atom Nanozyme Electrochemical H2O2 Sensor. Talanta Open 2021, 4, 100075. [Google Scholar] [CrossRef]
- Duan, S.; Wang, R.; Liu, J. Stability Investigation of a High Number Density Pt1/Fe2O3 Single-Atom Catalyst under Different Gas Environments by HAADF-STEM. Nanotechnology 2018, 29, 204002. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.-K.; Qiao, B.; Huang, C.-Q.; Ding, W.-C.; Sun, K.; Zhan, E.; Zhang, T.; Liu, J.; Li, W.-X. Supported Single Pt1/Au1 Atoms for Methanol Steam Reforming. ACS Catal. 2014, 4, 3886–3890. [Google Scholar] [CrossRef]
- Sanchez, M. Oxygen Vacancy Model in Strong Metal-Support Interaction. J. Catal. 1987, 104, 120–135. [Google Scholar] [CrossRef]
- Wan, J.; Chen, W.; Jia, C.; Zheng, L.; Dong, J.; Zheng, X.; Wang, Y.; Yan, W.; Chen, C.; Peng, Q.; et al. Defect Effects on TiO2 Nanosheets: Stabilizing Single Atomic Site Au and Promoting Catalytic Properties. Adv. Mater. 2018, 30, 1705369. [Google Scholar] [CrossRef]
- Zhang, S.; Nguyen, L.; Liang, J.-X.; Shan, J.; Liu, J.; Frenkel, A.I.; Patlolla, A.; Huang, W.; Li, J.; Tao, F. Catalysis on Singly Dispersed Bimetallic Sites. Nat. Commun. 2015, 6, 7938. [Google Scholar] [CrossRef]
- Chang, T.-Y.; Tanaka, Y.; Ishikawa, R.; Toyoura, K.; Matsunaga, K.; Ikuhara, Y.; Shibata, N. Direct Imaging of Pt Single Atoms Adsorbed on TiO2 (110) Surfaces. Nano Lett. 2014, 14, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Liu, X.; Chen, J.; Dong, Y.; Tang, X.; Chen, Y. Single-Atom Catalysts Reveal the Dinuclear Characteristic of Active Sites in NO Selective Reduction with NH. Nat. Commun. 2020, 11, 1532. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.H.; Hu, J.; Mei, D.; Yi, C.-W.; Kim, D.H.; Peden, C.H.F.; Allard, L.F.; Szanyi, J. Coordinatively Unsaturated Al3+ Centers as Binding Sites for Active Catalyst Phases of Platinum on γ-Al2O. Science 2009, 325, 1670–1673. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Cui, X.; Gu, L.; Yu, S.; Fan, X.; Luo, M.; Xu, S.; Li, N.; Zheng, L.; Zhang, Q.; et al. Single-Atom Cobalt Array Bound to Distorted 1T MoS2 with Ensemble Effect for Hydrogen Evolution Catalysis. Nat. Commun. 2019, 10, 5231. [Google Scholar] [CrossRef] [PubMed]
- Heemeier, M.; Frank, M.; Libuda, J.; Wolter, K.; Kuhlenbeck, H.; Baumer, M. The Influence of OH Groups on the Growth of Rhodium on Alumina: A Model Study. Catal. Lett. 2000, 68, 19–24. [Google Scholar] [CrossRef]
- Wang, F.; Ma, J.; Xin, S.; Wang, Q.; Xu, J.; Zhang, C.; He, H.; Cheng Zeng, X. Resolving the Puzzle of Single-Atom Silver Dispersion on Nanosized γ-Al2O3 Surface for High Catalytic Performance. Nat. Commun. 2020, 11, 529. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, S.; Wang, Y.; Herron, J.A.; Xu, Y.; Allard, L.F.; Lee, S.; Huang, J.; Mavrikakis, M.; Flytzani-Stephanopoulos, M. Catalytically Active Au-O(OH)x—Species Stabilized by Alkali Ions on Zeolites and Mesoporous Oxides. Science 2014, 346, 1498–1501. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, J.; Lee, S.; Zugic, B.; Huang, J.; Allard, L.F.; Flytzani-Stephanopoulos, M. A Common Single-Site Pt(II)–O(OH)x—Species Stabilized by Sodium on “Active” and “Inert” Supports Catalyzes the Water-Gas Shift Reaction. J. Am. Chem. Soc. 2015, 137, 3470–3473. [Google Scholar] [CrossRef]
- Kuai, L.; Chen, Z.; Liu, S.; Kan, E.; Yu, N.; Ren, Y.; Fang, C.; Li, X.; Li, Y.; Geng, B. Titania Supported Synergistic Palladium Single Atoms and Nanoparticles for Room Temperature Ketone and Aldehydes Hydrogenation. Nat. Commun. 2020, 11, 48. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Asakura, H.; Zhang, B.; Zhang, J.; Zhou, M.; Han, Y.; Tanaka, T.; Wang, A.; Zhang, T.; et al. Thermally Stable Single Atom Pt/m-Al2O3 for Selective Hydrogenation and CO Oxidation. Nat. Commun. 2017, 8, 16100. [Google Scholar] [CrossRef]
- Yang, K.; Liu, Y.; Deng, J.; Zhao, X.; Yang, J.; Han, Z.; Hou, Z.; Dai, H. Three-Dimensionally Ordered Mesoporous Iron Oxide-Supported Single-Atom Platinum: Highly Active Catalysts for Benzene Combustion. Appl. Catal. B Environ. 2019, 244, 650–659. [Google Scholar] [CrossRef]
- Azofra, L.M.; Morlanés, N.; Poater, A.; Samantaray, M.K.; Vidjayacoumar, B.; Albahily, K.; Cavallo, L.; Basset, J. Single-Site Molybdenum on Solid Support Materials for Catalytic Hydrogenation of N2-into-NH. Angew. Chem. Int. Ed. 2018, 57, 15812–15816. [Google Scholar] [CrossRef]
- De, S.; Babak, M.V.; Hülsey, M.J.; Ang, W.H.; Yan, N. Designed Precursor for the Controlled Synthesis of Highly Active Atomic and Sub-nanometric Platinum Catalysts on Mesoporous Silica. Chem. Asian J. 2018, 13, 1053–1059. [Google Scholar] [CrossRef]
- Kosinov, N.; Liu, C.; Hensen, E.J.M.; Pidko, E.A. Engineering of Transition Metal Catalysts Confined in Zeolites. Chem. Mater. 2018, 30, 3177–3198. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Evolution of Isolated Atoms and Clusters in Catalysis. Trends Chem. 2020, 2, 383–400. [Google Scholar] [CrossRef]
- Ye, T.-N.; Xiao, Z.; Li, J.; Gong, Y.; Abe, H.; Niwa, Y.; Sasase, M.; Kitano, M.; Hosono, H. Stable Single Platinum Atoms Trapped in Sub-Nanometer Cavities in 12CaO·7Al2O3 for Chemoselective Hydrogenation of Nitroarenes. Nat. Commun. 2020, 11, 1020. [Google Scholar] [CrossRef]
- Shin, H.; Jung, W.-G.; Kim, D.-H.; Jang, J.-S.; Kim, Y.H.; Koo, W.-T.; Bae, J.; Park, C.; Cho, S.-H.; Kim, B.J.; et al. Single-Atom Pt Stabilized on One-Dimensional Nanostructure Support via Carbon Nitride/SnO2 Heterojunction Trapping. ACS Nano 2020, 14, 11394–11405. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, M. Single-Atom Catalysts Boosted Ultrathin Film Sensors. Rare Met. 2020, 39, 1110–1112. [Google Scholar] [CrossRef]
- Zhou, L.; Chang, X.; Zheng, W.; Liu, X.; Zhang, J. Single Atom Rh-Sensitized SnO2 via Atomic Layer Deposition for Efficient Formaldehyde Detection. Chem. Eng. J. 2023, 475, 146300. [Google Scholar] [CrossRef]
- Sun, L.; Wang, B.; Wang, Y. High-Temperature Gas Sensor Based on Novel Pt Single Atoms@SnO2 Nanorods@SiC Nanosheets Multi-Heterojunctions. ACS Appl. Mater. Interfaces 2020, 12, 21808–21817. [Google Scholar] [CrossRef]
- Lu, Z.; Meng, S.; Ma, Z.; Yang, M.; Ma, D.; Yang, Z.; Talib, S.H. Electronic and Catalytic Properties of Ti Single atoms@SnO2 and Its Implications on Sensing Mechanism for CO. Appl. Surf. Sci. 2022, 594, 153500. [Google Scholar] [CrossRef]
- Degler, D.; Müller, S.A.; Doronkin, D.E.; Wang, D.; Grunwaldt, J.-D.; Weimar, U.; Barsan, N. Platinum Loaded Tin Dioxide: A Model System for Unravelling the Interplay between Heterogeneous Catalysis and Gas Sensing. J. Mater. Chem. A 2018, 6, 2034–2046. [Google Scholar] [CrossRef]
- Degler, D.; Pereira De Carvalho, H.W.; Kvashnina, K.; Grunwaldt, J.-D.; Weimar, U.; Barsan, N. Structure and Chemistry of Surface-Doped Pt:SnO2 Gas Sensing Materials. RSC Adv. 2016, 6, 28149–28155. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, W.; Liu, X.; Zhang, L.; Zheng, L.; Yang, C.; Pinna, N.; Zhang, J. Platinum Single Atoms on Tin Oxide Ultrathin Films for Extremely Sensitive Gas Detection. Mater. Horiz. 2020, 7, 1519–1527. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, P.; Su, X.; Liu, Y.; Sun, Y.; Yang, H.; Fu, H.; Qu, X.; Liu, S.; Zheng, S. Synergistic Ni Single Atoms and Oxygen Vacancies on SnO2 Nanorods toward Promoting SO2 Gas Sensing. Sens. Actuators B Chem. 2022, 351, 130983. [Google Scholar] [CrossRef]
- Shin, H.; Ko, J.; Park, C.; Kim, D.; Ahn, J.; Jang, J.; Kim, Y.H.; Cho, S.; Baik, H.; Kim, I. Sacrificial Template-Assisted Synthesis of Inorganic Nanosheets with High-Loading Single-Atom Catalysts: A General Approach. Adv. Funct. Mater. 2022, 32, 2110485. [Google Scholar] [CrossRef]
- Wang, P.; Guo, S.; Hu, Z.; Zhou, L.; Li, T.; Pu, S.; Mao, H.; Cai, H.; Zhu, Z.; Chen, B.; et al. Single-Atom Cu Stabilized on Ultrathin WO2.72 Nanowire for Highly Selective and Ultrasensitive ppb-Level Toluene Detection. Adv. Sci. 2023, 10, 2302778. [Google Scholar] [CrossRef]
- Gu, F.; Cui, Y.; Han, D.; Hong, S.; Flytzani-Stephanopoulos, M.; Wang, Z. Atomically Dispersed Pt (II) on WO3 for Highly Selective Sensing and Catalytic Oxidation of Triethylamine. Appl. Catal. B Environ. 2019, 256, 117809. [Google Scholar] [CrossRef]
- Yuan, T.; Xue, Z.; Chen, Y.; Xu, J. Single Pt Atom-Based Gas Sensor: Break the Detection Limit and Selectivity of Acetone. Sens. Actuators B Chem. 2023, 397, 134139. [Google Scholar] [CrossRef]
- Zeng, J.; Rong, Q.; Xiao, B.; Yu, R.; Zi, B.; Kuang, X.; Deng, X.; Ma, Y.; Zhang, J.; Wu, J.; et al. Single-Atom Silver Loaded on Tungsten Oxide with Oxygen Vacancies for High Performance Triethylamine Gas Sensors. J. Mater. Chem. A 2021, 9, 8704–8710. [Google Scholar] [CrossRef]
- Gu, F.; Luo, C.; Han, D.; Hong, S.; Wang, Z. Atomically Dispersed Pt on 3DOM WO3 Promoted with Cobalt and Nickel Oxides for Highly Selective and Highly Sensitive Detection of Xylene. Sens. Actuators B Chem. 2019, 297, 126772. [Google Scholar] [CrossRef]
- Wang, C.; Xie, J.; Chang, X.; Zheng, W.; Zhang, J.; Liu, X. ZnO Single Nanowire Gas Sensor: A Platform to Investigate the Sensitization of Pt. Chem. Eng. J. 2023, 473, 145481. [Google Scholar] [CrossRef]
- Xue, Z.; Yan, M.; Yu, X.; Tong, Y.; Zhou, H.; Zhao, Y.; Wang, Z.; Zhang, Y.; Xiong, C.; Yang, J.; et al. One-Dimensional Segregated Single Au Sites on Step-Rich ZnO Ladder for Ultrasensitive NO2 Sensors. Chem 2020, 6, 3364–3373. [Google Scholar] [CrossRef]
- Liu, L.; Mao, C.; Fu, H.; Qu, X.; Zheng, S. ZnO Nanorod-Immobilized Pt Single-Atoms as an Ultrasensitive Sensor for Triethylamine Detection. ACS Appl. Mater. Interfaces 2023, 15, 16654–16663. [Google Scholar] [CrossRef]
- Rong, Q.; Xiao, B.; Zeng, J.; Yu, R.; Zi, B.; Zhang, G.; Zhu, Z.; Zhang, J.; Wu, J.; Liu, Q. Pt Single Atom-Induced Activation Energy and Adsorption Enhancement for an Ultrasensitive Ppb-Level Methanol Gas Sensor. ACS Sens. 2022, 7, 199–206. [Google Scholar] [CrossRef]
- Gu, F.; Di, M.; Han, D.; Hong, S.; Wang, Z. Atomically Dispersed Au on In2O3 Nanosheets for Highly Sensitive and Selective Detection of Formaldehyde. ACS Sens. 2020, 5, 2611–2619. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Wang, X.; Sun, G.; Cao, J.; Wang, Y. Surface Modification of In2O3 Porous Nanospheres with Au Single Atoms for Ultrafast and Highly Sensitive Detection of CO. Appl. Surf. Sci. 2023, 613, 155987. [Google Scholar] [CrossRef]
- Gu, F.; Su, Y.; Hong, S.; Wang, J.; Wang, P.; Han, D.; Wang, Z.; Qiao, Z.; Hu, Y. Effects of Hydrogen Treatment on the Triethylamine-Sensing Properties of the Platinum-Loaded In2O3 Nanosheets. Sens. Actuators B Chem. 2022, 372, 132632. [Google Scholar] [CrossRef]
- Li, Q.; Li, Z.; Zhang, Q.; Zheng, L.; Yan, W.; Liang, X.; Gu, L.; Chen, C.; Wang, D.; Peng, Q.; et al. Porous γ-Fe2O3 Nanoparticle Decorated with Atomically Dispersed Platinum: Study on Atomic Site Structural Change and Gas Sensor Activity Evolution. Nano Res. 2021, 14, 1435–1442. [Google Scholar] [CrossRef]
- Ye, X.-L.; Lin, S.-J.; Zhang, J.-W.; Jiang, H.-J.; Cao, L.-A.; Wen, Y.-Y.; Yao, M.-S.; Li, W.-H.; Wang, G.-E.; Xu, G. Boosting Room Temperature Sensing Performances by Atomically Dispersed Pd Stabilized via Surface Coordination. ACS Sens. 2021, 6, 1103–1110. [Google Scholar] [CrossRef]
- Koga, K. Electronic and Catalytic Effects of Single-Atom Pd Additives on the Hydrogen Sensing Properties of Co 3O4 Nanoparticle Films. ACS Appl. Mater. Interfaces 2020, 12, 20806–20823. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ma, H.; Zhou, Y.; Ma, L.; Zhao, S.; Shi, S.; Li, J.; Chang, Y. Gas-Sensing Properties and Mechanisms of 3D Networks Composed of ZnO Tetrapod Micro-Nano Structures at Room Temperature. Materials 2024, 17, 203. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Shao, J.; Sun, C.; Pan, G.; Yang, X. Preparation of Pt/WO3@ZnO hollow spheres for low-temperature and high-efficiency detection of triethylamine. Dalton Trans. 2024, 53, 3224–3235. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Lu, J.; Liu, Y. Carbon monoxide gas sensing properties of SnO2 modified metal-organic skeleton derived NiO. Sens. Actuators A Phys. 2024, 367, 115038. [Google Scholar] [CrossRef]
- Liu, M.; Song, P.; Zhao, B.; Ding, Y.; Yan, M. Construction of CuO nanoparticles decorated In2O3 hierarchical structure for ultrasensitive and rapid trace detection formaldehyde at low temperature. Sens. Actuators B Chem. 2024, 404, 135276. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z.; Mu, Y.; Yue, C.; Liu, Z.; Dastan, D.; Yin, X.; Ma, X. Improvement of gas sensitivity to ethanol by hydrothermal preparation of Dy-doped In2O. Sens. Actuators B Chem. 2024, 405, 135386. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, M.; Guo, Y. Highly Sensitive Ethanol Gas Sensor Based on Ag Nanoparticles Decorated In2O3. Chem. Res. Chin. Univ. 2024, 40, 1–8. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Q.; Song, P. In situ synthesis of Zn-doped In2S3/In2O3composites for the monitoring of trace ethanol at low temperature. Vacuum 2024, 222, 112956. [Google Scholar] [CrossRef]
- Bu, W.; Liu, N.; Zhang, Y.; Han, W.; Chuai, X.; Zhou, Z.; Hu, C.; Lu, G. Atomically dispersed Pt on MOF-derived In2O3 for chemiresistive formaldehyde gas sensing. Sens. Actuators B Chem. 2024, 404, 135260. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Z.; Zhou, Y.; Zhao, L.; Kou, X.; Wang, T.; Wang, Y.; Sun, P.; Lu, G. Imparting Chemiresistor with Humidity-Independent Sensitivity toward Trace-Level Formaldehyde via Substitutional Doping Platinum Single Atom. Small 2024, 1–10, 2310465. [Google Scholar] [CrossRef]




| Material | Strategy | Response | Conc. [ppm] | Temp. [°C] | Response/Recovery Time [s] | Analytes | Ref. |
|---|---|---|---|---|---|---|---|
| 3D Networks ZnO | Micro-Nanostructures | 3.338 | 1600 | 25 | 20/>60 | ethanol | [125] |
| WO3/ZnO | Heterostructured | 96 | 100 | 200 | 45/1350 | Triethylamine | [126] |
| MOF-NiO/SnO2 | MOF-Derived | 5.48 | 100 | 25 | 56/4 | CO | [127] |
| In2O3/SnO2 | Heterostructured | 1.8 | 10 | 240 | 80/60 | SO2 | [19] |
| CuO/In2O3 | Nanoparticles Decoration | 231.2 | 100 | 140 | 9/17 | HCHO | [128] |
| Pt NCs@SnO2@SiC | Noble Metal Decoration | 56 | 500 | 300 | / | ethanol | [103] |
| Dy doping In2O3 | Doping | 85 | 100 | 250 | 5/248 | ethanol | [129] |
| Ag NCs In2O3 | Noble Metal decoration | 35.6 | 100 | 270 | 25/43 | ethanol | [130] |
| Zn doping In2S3/In2O3 | Doping | 45 | 100 | 100 | 11/24 | ethanol | [131] |
| Pt SAs@SnO2@SiC | Single-Atom Catalysts | 119 | 500 | 350 | 15/20 | ethanol | [103] |
| Pt-In2O3 | Single-Atom Catalysts | 750 | 100 | 200 | 2/373 | HCHO | [132] |
| Pt SA-SnO2 | Single-Atom Catalysts | 25 | 5 | 175 | / | HCHO | [133] |
| Ni SA/SnO2 | Single-Atom Catalysts | 50 | 40 | 250 | 52/45 | SO2 | [108] |
| Ag SA-WO3 | Single-Atom Catalysts | 4700 | 50 | 175 | 189/1354 | Triethylamine | [113] |
| Pt SA-ZnO | Single-Atom Catalysts | 4170 | 100 | 175 | 34/76 | Triethylamine | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Tan, Y.; Niu, W.; Zhao, S.; Hao, J.; Shi, Y.; Dong, Y.; Liu, H.; Huang, C.; Gao, C.; et al. Advances in Synthesis and Applications of Single-Atom Catalysts for Metal Oxide-Based Gas Sensors. Materials 2024, 17, 1970. https://doi.org/10.3390/ma17091970
Yu Y, Tan Y, Niu W, Zhao S, Hao J, Shi Y, Dong Y, Liu H, Huang C, Gao C, et al. Advances in Synthesis and Applications of Single-Atom Catalysts for Metal Oxide-Based Gas Sensors. Materials. 2024; 17(9):1970. https://doi.org/10.3390/ma17091970
Chicago/Turabian StyleYu, Yuanting, Yiling Tan, Wen Niu, Shili Zhao, Jiongyue Hao, Yijie Shi, Yingchun Dong, Hangyu Liu, Chun Huang, Chao Gao, and et al. 2024. "Advances in Synthesis and Applications of Single-Atom Catalysts for Metal Oxide-Based Gas Sensors" Materials 17, no. 9: 1970. https://doi.org/10.3390/ma17091970





