The Application of Metal–Organic Frameworks in Water Treatment and Their Large-Scale Preparation: A Review
Abstract
:1. Introduction
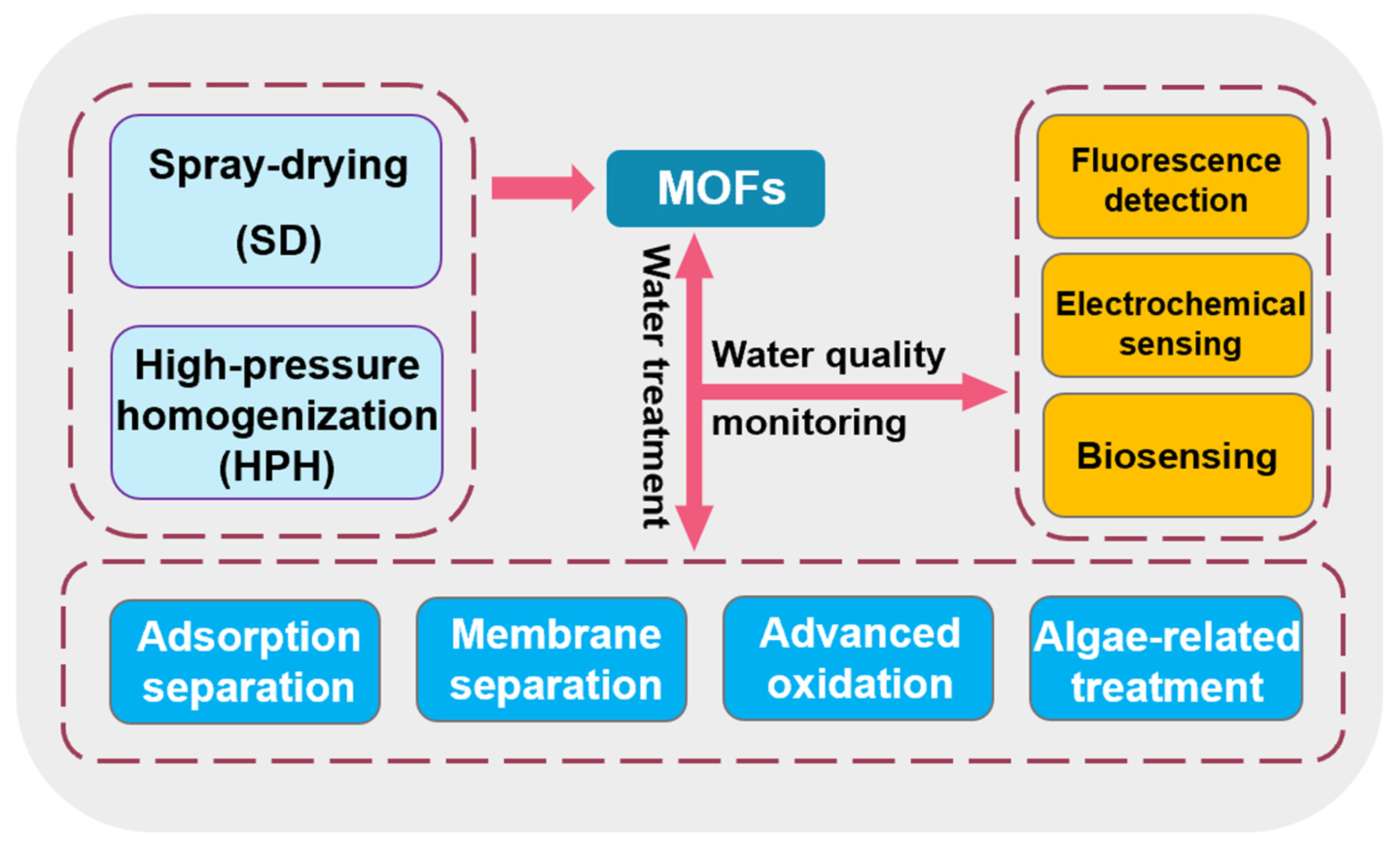
2. MOF-Based Water Treatment Strategy
2.1. Separation Method for the Treatment of Pollutants in Water
2.1.1. Adsorption Separation

2.1.2. Membrane Separation
| Membrane Type | Strategy | Unique Characteristics | References |
|---|---|---|---|
| TFN | Integrating MOF nanoparticles within the polyamide (PA) layer is deftly achieved using the interfacial polymerization (IP) process | PA layer can be tailored, affecting factors such as the degree of cross-linking and the layer’s thickness. These modifications have the potential to enhance the layer’s permeability to water and, in certain instances, can also improve its selectivity in terms of solute rejection. | [61] |
| Applying a layer of nanoMOFs onto the PA layer | Modifying a membrane’s surface can alter its hydrophilicity and charge, which can introduce or enhance antifouling and antibacterial properties. Such enhancements are integral to developing durable membranes that maintain high separation efficiency over extended periods. | [62] | |
| MMM | Embedding MOF nanoparticles into the matrix of a substrate or a single-layer membrane | Adjusting the fabrication parameters of a membrane offers the potential to enhance its porosity and reduce both its thickness and the complexity of its internal pathway, known as tortuosity. These modifications could lead to a reduced structural parameter (S-value) and an increased water flux, thereby optimizing the membrane’s filtration performance. | [63] |
| Others | Incorporating water unstable MOF nanoparticles as pore formers in the fabrication of membranes can create a network of pores | By calibrating the size of the nanoparticles used in membrane construction, it is feasible to enhance membrane porosity and precisely control the mean pore size. This approach can be undertaken without altering the inherent chemical properties of the membrane, such as hydrophilicity and surface charge, maintaining its functional integrity while optimizing its physical structure for improved performance. | [64] |
| Creating a selective layer of nanoMOFs on a substrate | Advancements in membrane technology offer the potential to augment the mechanical durability of membranes, precisely tailor surface physicochemical characteristics—including hydrophilicity, electrical charge, and surface roughness—and amplify selectivity, particularly in nanofiltration (NF) and membrane distillation (MD) processes. These enhancements can lead to more resilient and efficient filtration systems. | [65] |
| Membrane Components | Craft | Target Contaminants | References | |
|---|---|---|---|---|
| 1 | D6/TiO2/MoS2/NiCo-NC/PVDF | Negative pressure assisted method | Pesticides, pharmaceuticals, Personal physical items | [66] |
| 2 | MIL-101(Fe)/Cu-POM/IPN | In-situ deposition, pouring | Dye, Drugs | [67] |
| 3 | MOF-5/coal-based fiber | Electrospinning | Dye | [68] |
| 4 | FS-50/COF(MATPA)-MOFs(Zr)/PDA@PVDF | High pressure induction | Microplastics, dye, pesticides | [69] |
| 5 | CoFe-MOF/TiO2/PVDF | Non-solvent-induced phase separation | Antibiotic | [70] |
| 6 | PAN/Co-MOFs | In-situ growth, electrospinning | Dye | [71] |
| 7 | MOF/GO | Vacuum filtration | Dye | [72] |
| 8 | MOF/PCL | Solvent/non-solvent methods | Dye | [73] |
| 9 | PVA/GO/MOF | Chemical crosslinking and suction filtration | Greasy dirt | [74] |
| 10 | Co-CAT-1/PEI/GO | Coating process | Greasy dirt | [75] |
| 11 | Bio-MOF-2Me/MMM | Pouring | Cationic dyes | [76] |
| 12 | CF/PDA/UiO-66-NH2 | Grown in situ | Rhodamine B and Pb(II) metalions | [77] |
| 13 | Ag(I)-CP/PES | Scraper casting | Dye | [78] |
| 14 | MOF/PAN-MIM | In-situ deposition | Bisphenol A | [79] |
| 15 | MOF-1a/PVDF | Drip casting method | TNP | [80] |
2.2. Advanced Oxidation
| Catalyst | Organic Pollutant/Removal Efficiency (%) | Co-Existing Substance/Removal Efficiency (%) | Oxidant/ Irrigation | Dominant Mechanism | References |
|---|---|---|---|---|---|
| FeCo MOF | CR/95%, RhB/99%, MO/95%, BPA/100% | 3-NP/40%, p-BA/50% | PMS | 1O2 | [93] |
| Fe-MOF membrane | BPS/75.7% | BA/- | H2O2 | Size exclusion | [94] |
| MIL-53(Fe)@ anionicresin | MB/84% | SRB/11% | visible light | Electrostatic interaction | [95] |
| MIL-53(Fe)@ anionicresin | SRB/73% | MB/59% | visible light | Electrostatic interaction | [95] |
| Fe-BTC@resin | MB/71% | SRB/12% | visible light | Electrostatic interaction | [96] |
| Fe-MOF@MIP | SMX/97% | BA/- | PS | MIT | [97] |
| Fe-MOF-74@MIP | DMP/95% | DEP/80%, DBP/70%, DEHP/70% | PS | MIT | [98] |
| Fe-MOF- 74@SiO2@MIP | DMP/93% | DEP/68%, OG/18%, SMX/23% | PS | MIT | [99] |
| Zn4Co1-C | Phenol/100% | BA/1% | PMS | 1O2 | [100] |
| yolk-shell Co/C | BPA/100% | BA/- | PMS | Size exclusion | [101] |
| Cu/RGO | 2,4-DCP/95.8% | BA/0%, CBZ/10%, IBU/30% | PDS | Cu(III) | [102] |
| Fe/Fe3O4@rGO | BPA/90%, BPF/60%, MeP/12%, p-BA/13%, 2,4-DCP/100%, CP/30%, BPS/12%, phenol/12% | - | PDS | Hydrophobicity | [103] |
2.3. Other MOF-Based Water Treatment Strategies
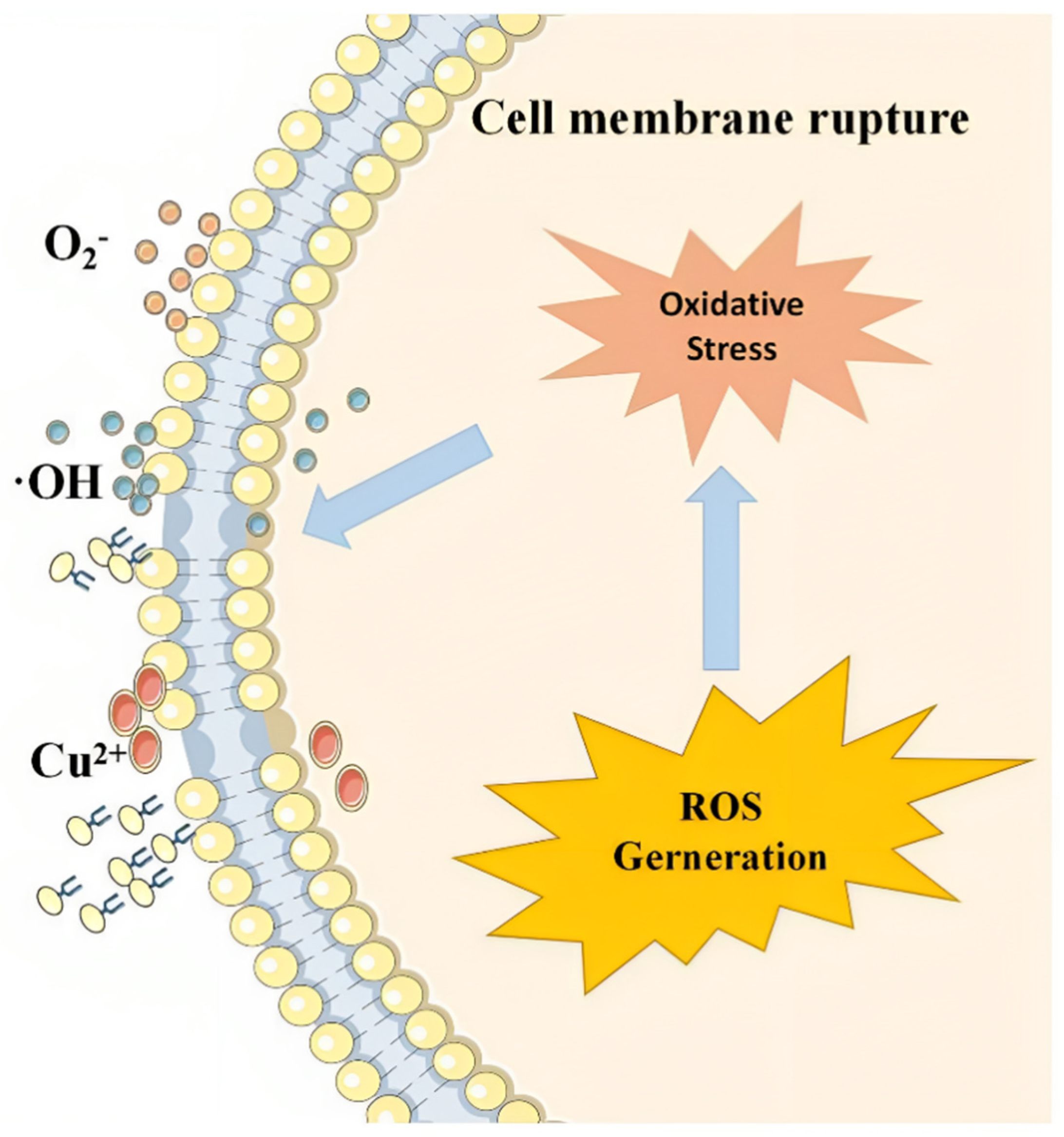
2.3.1. Acts as a Flocculant to Remove Harmful Algae
2.3.2. Inhibit the Growth of Algae
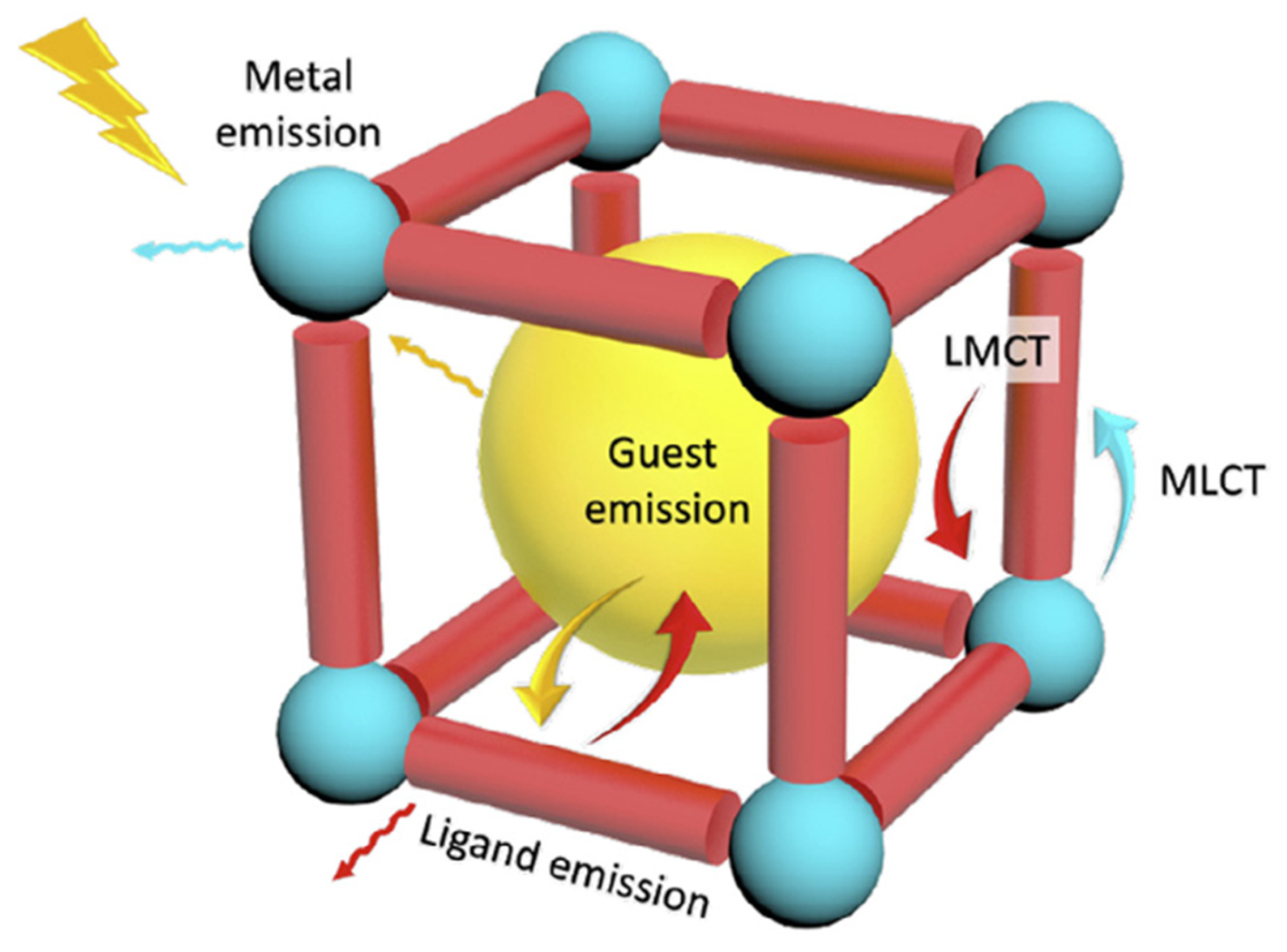
2.4. Sensing Monitoring and Detection
2.4.1. Fluorescence Detection
2.4.2. Electrochemical Sensing
2.4.3. Biosensing
3. Large-Scale Preparation of MOF
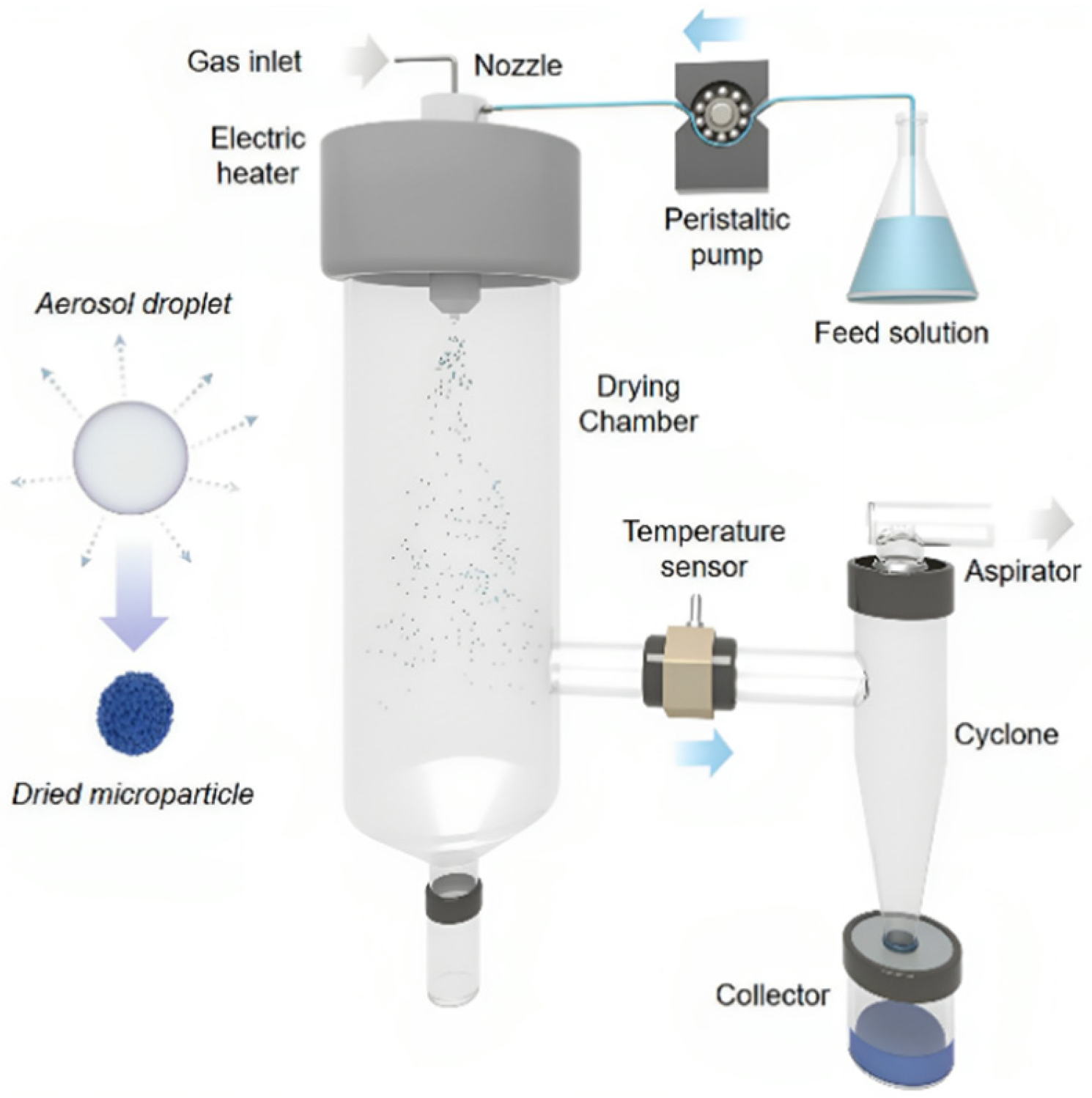
3.1. Spray-Drying (SD)
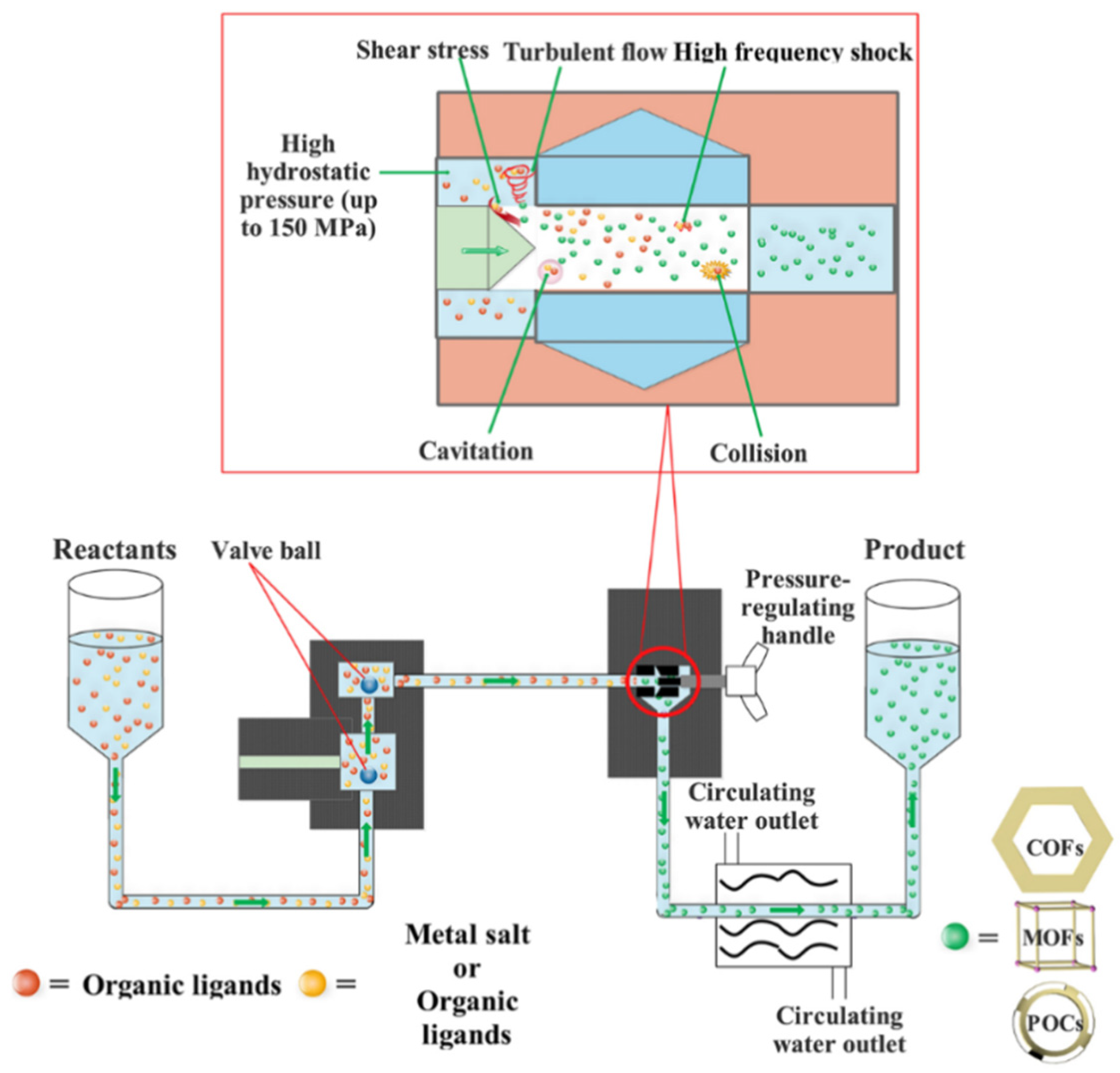
3.2. High-Pressure Homogenization (HPH)
4. Summary and Prospect
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, K.; Li, Q.; Andrady, A.L.; Wang, X.; He, Y.; Li, D. Underestimated activity-based microplastic intake under scenario-specific exposures. Environ. Sci. Ecotechnol. 2024, 18, 100316. [Google Scholar] [CrossRef] [PubMed]
- Kutil, Z.; Novotna, K.; Cermakova, L.; Pivokonsky, M. Tunnel vision in the drinking water research field-Time for non-targeted analysis implementation? Sci. Total Environ. 2024, 908, 168367. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Gu, H.; Yang, Y.; Lam, S.S.; Li, H.; Sonne, C.; Ouyang, H.; Chen, X. Recent progress in advanced covalent organic framework composites for environmental remediation. Adv. Compos. Hybrid Mater. 2023, 6, 199. [Google Scholar] [CrossRef]
- Guo, Z.; Yang, J.; Li, K.; Shi, J.; Peng, Y.; Sarkodie, E.K.; Miao, B.; Liu, H.; Liu, X.; Jiang, L. Leaching Behavior of As and Pb in Lead-Zinc Mining Waste Rock under Mine Drainage and Rainwater. Toxics 2023, 11, 943. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Xu, M.; Ahamad, L.; Chaudhary, A.; Kumar, G.; Adeleke, B.S.; Verma, K.K.; Hu, D.M.; Širić, I.; Kumar, P.; et al. Application of Synthetic Consortia for Improvement of Soil Fertility, Pollution Remediation, and Agricultural Productivity: A Review. Agronomy 2023, 13, 643. [Google Scholar] [CrossRef]
- Wang, B.; Lan, J.; Bo, C.; Gong, B.; Ou, J. Adsorption of heavy metal onto biomass-derived activated carbon: Review. RSC Adv. 2023, 13, 4275–4302. [Google Scholar] [CrossRef] [PubMed]
- Kulsum, P.; Khanam, R.; Das, S.; Nayak, A.K.; Tack, F.M.; Meers, E.; Vithanage, M.; Shahid, M.; Kumar, A.; Chakraborty, S.; et al. A state-of-the-art review on cadmium uptake, toxicity, and tolerance in rice: From physiological response to remediation process. Environ. Res. 2023, 220, 115098. [Google Scholar] [CrossRef]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. 2016, 23, 8244–8259. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shan, Y.; Zhang, S.; Kong, Q.; Pang, H. Application of metal organic framework in wastewater treatment. Green Energy Environ. 2023, 8, 698–721. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Yang, T.; Sridhar, D.; Algadi, H.; Xu, B.B.; El-Bahy, Z.M.; Li, H.; Ma, Y.; Li, T.; et al. An overview of metal-organic frameworks and their magnetic composites for the removal of pollutants. Sep. Purif. Technol. 2023, 320, 124144. [Google Scholar] [CrossRef]
- Qi, M.; Lin, P.; Shi, Q.; Bai, H.; Zhang, H.; Zhu, W. A metal-organic framework (MOF) and graphene oxide (GO) based peroxymonosulfate (PMS) activator applied in pollutant removal. Process Saf. Environ. Prot. 2023, 171, 847–858. [Google Scholar] [CrossRef]
- Chang, Z.; Yang, D.H.; Xu, J.; Hu, T.L.; Bu, X.H. Flexible Metal–Organic Frameworks: Recent Advances and Potential Applications. Adv. Mater. 2015, 27, 5432–5441. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Peterson, G.W. Metal–Organic Frameworks for Air Purification of Toxic Chemicals. Chem. Rev. 2014, 114, 5695–5727. [Google Scholar] [CrossRef] [PubMed]
- Abney, C.; Gilhula, J.; Lu, K.; Lin, W. Metal-Organic Framework Templated Inorganic Sorbents for Rapid and Efficient Extraction of Heavy Metals. Adv. Mater. 2014, 26, 7993–7997. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Chen, J.; Xie, Q.; Mi, L. Functional metal/covalent organic framework materials for triboelectric nanogenerator. Coord. Chem. Rev. 2023, 486, 215118. [Google Scholar] [CrossRef]
- Xiao, J.D.; Li, R.; Jiang, H.L. Metal-Organic Framework-Based Photocatalysis for Solar Fuel Production. Small Methods 2023, 7, 2201258. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.X.; Zhang, Z.W.; Wang, F.; Li, Y.; Zhang, Z.C.; Wang, C.C.; Yu, B.; Du, X.; Wang, P.; Fu, H.; et al. Fe-Cu bimetal metal-organic framework for efficient decontamination via Fenton-like process: Synthesis, performance and mechanism. J. Colloid Interface Sci. 2023, 649, 384–393. [Google Scholar] [CrossRef]
- Rabeie, B.; Mahmoodi, N.M. Heterogeneous MIL-88A on MIL-88B hybrid: A promising eco-friendly hybrid from green synthesis to dual application (Adsorption and photocatalysis) in tetracycline and dyes removal. J. Colloid Interface Sci. 2024, 654, 495–522. [Google Scholar] [CrossRef] [PubMed]
- Channab, B.E.; El Ouardi, M.; Layachi, O.A.; Marrane, S.E.; El Idrissi, A.; Baqais, A.A.; Ahsaine, H.A. Recent trends on MIL-Fe metal-organic frameworks: Synthesis approaches, structural insights, and applications in organic pollutant adsorption and photocatalytic degradation. Environ. Sci. Nano 2023, 11, 2957–2988. [Google Scholar] [CrossRef]
- Zhu, W.; Han, M.; Kim, D.; Park, J.; Choi, H.; Kwon, G.; You, J.; Li, S.; Park, T.; Kim, J. Highly catalytic and durable nanocellulose fibers-based nanoporous membrane film for efficient organic pollutant degradation. J. Water Process Eng. 2023, 53, 103620. [Google Scholar] [CrossRef]
- Bétard, A.; Fischer, R.A. Metal–Organic Framework Thin Films: From Fundamentals to Applications. Chem. Rev. 2011, 112, 1055–1083. [Google Scholar] [CrossRef] [PubMed]
- Kadhom, M.; Deng, B. Metal-organic frameworks (MOFs) in water filtration membranes for desalination and other applications. Appl. Mater. Today 2018, 11, 219–230. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Yuan, X.; Zhang, J.; Chew, J.W. Metal-organic framework membranes for wastewater treatment and water regeneration. Coord. Chem. Rev. 2020, 404, 213116. [Google Scholar] [CrossRef]
- Mueller, U.; Schubert, M.; Teich, F.; Puetter, H.; Schierle-Arndt, K.; Pastre, J. Metal–organic frameworks—Prospective industrial applications. J. Mater. Chem. 2006, 16, 626–636. [Google Scholar] [CrossRef]
- Bilecka, I.; Niederberger, M. Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale 2010, 2, 1358–1374. [Google Scholar] [CrossRef]
- Kaupp, G. Solid-state molecular syntheses: Complete reactions without auxiliaries based on the new solid-state mechanism. CrystEngComm 2003, 5, 117–133. [Google Scholar] [CrossRef]
- Carne-Sanchez, A.; Imaz, I.; Cano-Sarabia, M.; Maspoch, D. A spray-drying strategy for synthesis of nanoscale metal-organic frameworks and their assembly into hollow superstructures. Nat. Chem. 2013, 5, 203–211. [Google Scholar] [CrossRef]
- Faustini, M.; Kim, J.; Jeong, G.Y.; Kim, J.Y.; Moon, H.R.; Ahn, W.S.; Kim, D.P. Microfluidic Approach toward Continuous and Ultrafast Synthesis of Metal–Organic Framework Crystals and Hetero Structures in Confined Microdroplets. J. Am. Chem. Soc. 2013, 135, 14619–14626. [Google Scholar] [CrossRef]
- Liu, X.; Wang, A.; Wang, C.; Li, J.; Zhang, Z.; Al-Enizi, A.M.; Nafady, A.; Shui, F.; You, Z.; Li, B. A general large-scale synthesis approach for crystalline porous materials. Nat. Commun. 2023, 14, 7022. [Google Scholar] [CrossRef]
- Hegde, V.; Uthappa, U.; Suneetha, M.; Altalhi, T.; Han, S.S.; Kurkuri, M.D. Functional porous Ce-UiO-66 MOF@Keratin composites for the efficient adsorption of trypan blue dye from wastewater: A step towards practical implementations. Chem. Eng. J. 2023, 461, 142103. [Google Scholar] [CrossRef]
- Chen, J.Q.; Sharifzadeh, Z.; Bigdeli, F.; Gholizadeh, S.; Li, Z.; Hu, M.L.; Morsali, A. MOF composites as high potential materials for hazardous organic contaminants removal in aqueous environments. J. Environ. Chem. Eng. 2023, 11, 109469. [Google Scholar] [CrossRef]
- Saglam, S.; Türk, F.N.; Arslanoglu, H. Use and applications of metal-organic frameworks (MOF) in dye adsorption: Review. J. Environ. Chem. Eng. 2023, 11, 110568. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2018, 17, 195–213. [Google Scholar] [CrossRef]
- Lv, Z.; Fan, Q.; Xie, Y.; Chen, Z.; Alsaedi, A.; Hayat, T.; Wang, X.; Chen, C. MOFs-derived magnetic chestnut shell-like hollow sphere NiO/Ni@C composites and their removal performance for arsenic(V). Chem. Eng. J. 2019, 362, 413–421. [Google Scholar] [CrossRef]
- Hasan, Z.; Jhung, S.H. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Zhao, J.; Ou, X.; Wan, J.; Cai, Y.; Lin, Z.; Dang, Z.; Xing, B. Enhanced Adsorption of p-Arsanilic Acid from Water by Amine-Modified UiO-67 as Examined Using Extended X-ray Absorption Fine Structure, X-ray Photoelectron Spectroscopy, and Density Functional Theory Calculations. Environ. Sci. Technol. 2018, 52, 3466–3475. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Tong, Y.; Xu, J.; Wang, S.; Wang, J.; Zeng, T.; He, Z.; Yang, W.; Song, S. Ni-based layered metal-organic frameworks with palladium for electrochemical dechlorination. Appl. Catal. B Environ. 2020, 264, 118505. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, H.; Zhao, X.; Qu, Y.; Liu, D. Synergistic effect of electrostatic and coordination interactions for adsorption removal of cephalexin from water using a zirconium-based metal-organic framework. J. Colloid Interface Sci. 2020, 580, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Dai, Q.Z.; Zhan, T.T.; Wang, L.; Bian, X.Z.; Fan, S.Q.; Xiong, P.; Xia, Y.; Chen, J.M. Adsorption removal of pharmaceutical and personal care products with functionalized metal-organic framework: Adsorptive selectivity and mechanism. Desalination Water Treat. 2020, 191, 231–238. [Google Scholar] [CrossRef]
- Ahmed, I.; Hasan, Z.; Lee, G.; Lee, H.J.; Jhung, S.H. Contribution of hydrogen bonding to liquid-phase adsorptive removal of hazardous organics with metal-organic framework-based materials. Chem. Eng. J. 2022, 430, 132596. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.H.; Rutledge, W.; Li, J.R.; Zhou, H.C. Zr-based metal–organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef] [PubMed]
- Lacalamita, D.; Hoyez, G.; Mongioví, C.; Ponchel, A.; Morin-Crini, N.; Rousseau, C.; Loup, C.; Rousseau, J.; Raschetti, M.; Monflier, E. Efficient removal of fluoride ions present in industrial effluents using metal-organic frameworks of UiO-66-NH2. J. Water Process Eng. 2023, 53, 103791. [Google Scholar] [CrossRef]
- Keshta, B.E.; Yu, H.; Wang, L. MIL series-based MOFs as effective adsorbents for removing hazardous organic pollutants from water. Sep. Purif. Technol. 2023, 322, 124301. [Google Scholar] [CrossRef]
- Batra, R.; Chen, C.; Evans, T.G.; Walton, K.S.; Ramprasad, R. Prediction of water stability of metal–organic frameworks using machine learning. Nat. Mach. Intell. 2020, 2, 704–710. [Google Scholar] [CrossRef]
- Yan, C.; Jin, J.; Wang, J.; Zhang, F.; Tian, Y.; Liu, C.; Zhang, F.; Cao, L.; Zhou, Y.; Han, Q. Metal–organic frameworks (MOFs) for the efficient removal of contaminants from water: Underlying mechanisms, recent advances, challenges, and future prospects. Coord. Chem. Rev. 2022, 468, 214595. [Google Scholar] [CrossRef]
- Abdi, J.; Sisi, A.J.; Hadipoor, M.; Khataee, A. State of the art on the ultrasonic-assisted removal of environmental pollutants using metal-organic frameworks. J. Hazard. Mater. 2022, 424, 127558. [Google Scholar] [CrossRef]
- Yuan, N.; Gong, X.; Sun, W.; Yu, C. Advanced applications of Zr-based MOFs in the removal of water pollutants. Chemosphere 2021, 267, 128863. [Google Scholar] [CrossRef]
- Zango, Z.U.; Jumbri, K.; Sambudi, N.S.; Ramli, A.; Abu Bakar, N.H.H.; Saad, B.; Rozaini, M.N.H.; Isiyaka, H.A.; Jagaba, A.H.; Aldaghri, O.; et al. A Critical Review on Metal-Organic Frameworks and Their Composites as Advanced Materials for Adsorption and Photocatalytic Degradation of Emerging Organic Pollutants from Wastewater. Polymers 2020, 12, 2648. [Google Scholar] [CrossRef]
- Yoo, D.K.; Bhadra, B.N.; Jhung, S.H. Adsorptive removal of hazardous organics from water and fuel with functionalized metal-organic frameworks: Contribution of functional groups. J. Hazard. Mater. 2021, 403, 123655. [Google Scholar] [CrossRef]
- Ibrahim, A.O.; Adegoke, K.A.; Adegoke, R.O.; Abdulwahab, Y.A.; Oyelami, V.B.; Adesina, M.O. Adsorptive removal of different pollutants using metal-organic framework adsorbents. J. Mol. Liq. 2021, 333, 115593. [Google Scholar] [CrossRef]
- Nguyen, D.A.; Nguyen, D.V.; Jeong, G.; Asghar, N.; Jang, A. Critical evaluation of hybrid metal-organic framework composites for efficient treatment of arsenic-contaminated solutions by adsorption and membrane-separation process. Chem. Eng. J. 2023, 461, 141789. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Abdel-Gawad, H.; Elshahat, M.; Emam, H.E. Cu–BTC@cotton composite: Design and removal of ethion insecticide from water. RSC Adv. 2016, 6, 42324–42333. [Google Scholar] [CrossRef]
- Schelling, M.; Kim, M.; Otal, E.; Hinestroza, J. Decoration of Cotton Fibers with a Water-Stable Metal–Organic Framework (UiO-66) for the Decomposition and Enhanced Adsorption of Micropollutants in Water. Bioengineering 2018, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Dahlan, I.; Wan Mazlan, W.H.; Mulkan, A.; Zwain, H.M.; Hassan, S.R.; Aziz, H.A.; Hasan, H.Y.A.; Zekker, I. Modeling of Batch Organic Dye Adsorption Using Modified Metal-Organic Framework-5. Chem. Eng. Technol. 2022, 45, 2080–2087. [Google Scholar] [CrossRef]
- Tran, T.K.N.; Phan, C.P.K.; Ngo, T.C.Q.; Hoang, N.B.; Truong, L.D.; Nguyen, T.K.O. Synthesis and Characterization Bimetallic Organic Framework CoxFex(BDC) and Adsorption Cationic and Anionic Dyes. Processes 2022, 10, 1352. [Google Scholar] [CrossRef]
- Qin, Z.; Xiang, S.; Jing, Z.; Deng, M.; Jiang, W.; Yao, L.; Yang, L.; Deng, L.; Dai, Z. Thin film nanocomposite membranes fabricated via 2D ZIF-67 nanosheets and 1D nanofibers with ultrahigh water flux for dye removal from wastewater. Sep. Purif. Technol. 2024, 330, 125308. [Google Scholar] [CrossRef]
- Cheng, B.; Fu, X.; Song, Y.; Li, Z.; Weng, P.; Yin, X. A versatile MOF liquids-based Janus fibrous membrane towards complex oil/water separation and heavy metal ions removal. Sep. Purif. Technol. 2024, 331, 125701. [Google Scholar] [CrossRef]
- Valverde, A.; de Fernandez-de Luis, R.; Salazar, H.; Gonçalves, B.F.; King, S.; Almásy, L.; Kriechbaum, M.; Laza, J.M.; Vilas-Vilela, J.L.; Martins, P.M. On The Multiscale Structure and Morphology of Pvdf-Hfp@Mof Membranes in The Scope of Water Remediation Applications. Adv. Mater. Interfaces 2023, 10, 2300424. [Google Scholar] [CrossRef]
- Ali, Z.; Naz, A.; Haq, N.U.; Nazir, A.; Munawar, A.; Khan, A.L.; Elqahtani, Z.M.; Alwadai, N.; Younas, U.; Iqbal, M. Fabrication of novel Zn (II)-imidazole based mixed matrix membranes for heavy metal removals from drinking water. Z. Fur Phys. Chem. 2023, 237, 951–967. [Google Scholar] [CrossRef]
- Wang, L.; Huang, J.; Li, Z.; Han, Z.; Fan, J. Review of Synthesis and Separation Application of Metal-Organic Framework-Based Mixed-Matrix Membranes. Polymers 2023, 15, 1950. [Google Scholar] [CrossRef]
- Arjmandi, M.; Peyravi, M.; Chenar, M.P.; Jahanshahi, M. Channelization of water pathway and encapsulation of DS in the SL of the TFC FO membrane as a novel approach for controlling dilutive internal concentration polarization. Environ. Sci. Water Res. Technol. 2019, 5, 1436–1452. [Google Scholar] [CrossRef]
- Liu, L.; Luo, X.B.; Ding, L.; Luo, S.L. Application of nanotechnology in the removal of heavy metal from water. In Nanomaterials for the Removal of Pollutants and Resource Reutilization; Luo, X.B., Deng, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 83–147. [Google Scholar]
- Arjmandi, M.; Pourafshari Chenar, M.; Peyravi, M.; Jahanshahi, M. Physical modification of polymeric support layer for thin film composite forward osmosis membranes by metal–organic framework-based porous matrix membrane strategy. J. Appl. Polym. Sci. 2020, 137, 48672. [Google Scholar] [CrossRef]
- Ding, C.; Yin, J.; Deng, B. Effects of polysulfone (PSf) support layer on the performance of thin-film composite (TFC) membranes. J. Chem. Process Eng. 2014, 1, 1–8. [Google Scholar] [CrossRef]
- Li, Q.; Li, J.; Fang, X.; Liao, Z.; Wang, D.; Sun, X.; Shen, J.; Han, W.; Wang, L. Interfacial growth of metal–organic framework membranes on porous polymers via phase transformation. Chem. Commun. 2018, 54, 3590–3593. [Google Scholar] [CrossRef]
- Chen, X.; Han, R.; Guo, Z.; Ma, H.; Ji, X.; Wang, L.; Meng, J.; Fang, Y.; Pang, K.; Peng, S. Photocatalytic and Superhydrophobic Nanoporous Membranes for Emulsion Separation and Removal of Pesticides and Pharmaceutical Products. ACS Appl. Nano Mater. 2024, 7, 4288–4301. [Google Scholar] [CrossRef]
- Dutta, S.; Patel, B.M.; Singh, Y.; Hegde, G.; Bose, S. Photocatalytic driven ‘self-cleaning’ IPN membranes infused with a ‘host-guest’ pair consisting of metal-organic framework encapsulated anionic ‘nano-clusters’ for water remediation. J. Membr. Sci. 2024, 694, 122422. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, M.; Leng, C.; Ma, Q.; Dai, J.; Feng, S.; Wang, N.; Wei, J.; Wang, L. Bifunctional MOF-5@ coal-based fiber membrane for oil-water separation and dye adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2024, 683, 133021. [Google Scholar] [CrossRef]
- Peng, S.; Ma, H.; Hao, X.; Han, R.; Ji, X.; Wang, L.; Fang, Y.; Pang, K.; Il-Ho, K.; Chen, X. Constructing green superhydrophilic and superoleophobic COFs-MOFs hybrid-based membrane for efficiently emulsion separation and synchronous removal of microplastics, dyes, and pesticides. Environ. Res. 2024, 243, 117777. [Google Scholar] [CrossRef]
- Xu, H.; Chen, S.; Zhao, Y.F.; Wang, F.; Guo, F. MOF-Based Membranes for Remediated Application of Water Pollution. ChemPlusChem 2024, 18, e202400027. [Google Scholar] [CrossRef]
- Jia, F.; Yang, L.; Sun, L.; Yu, D.; Song, Y.; Wang, Y.; Kipper, M.J.; Tang, J.; Huang, L. Efficient separation of dyes using two-dimensional heterogeneous composite membranes. Water Res. 2023, 247, 120693. [Google Scholar] [CrossRef]
- Yang, S.; Zou, Q.; Wang, T.; Zhang, L. Effects of GO and MOF@ GO on the permeation and antifouling properties of cellulose acetate ultrafiltration membrane. J. Membr. Sci. 2019, 569, 48–59. [Google Scholar] [CrossRef]
- Hani, A.; Haikal, R.R.; El-Mehalmey, W.A.; Safwat, Y.; Alkordi, M.H. Durable and recyclable MOF@ polycaprolactone mixed-matrix membranes with hierarchical porosity for wastewater treatment. Nanoscale 2023, 15, 19617–19628. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Gong, J.; Sun, Y.; Li, J. Robust PVA/GO@MOF membrane with fast photothermal self-cleaning property for oily wastewater purification. J. Hazard. Mater. 2024, 462, 132803. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, X.; Liu, J.; Li, B.; Liu, H.; Tao, W.; Xu, X.; Li, Z. Self-assembled superhydrophilic MOF-decorated membrane for highly efficient treatment and separation mechanism of multi-component emulsions. Desalination 2024, 569, 117047. [Google Scholar] [CrossRef]
- Xiang, W.; Wang, Q.; Li, Z.; Dong, J.; Liu, J.; Zhang, L.; Xia, T.; He, Y.; Zhao, D. Water-stable methyl-modified MOF and mixed matrix membrane for efficient adsorption and separation of cationic dyes. Sep. Purif. Technol. 2024, 330, 125268. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, P.; Zheng, Q.; Hameed, M.U.; Raza, S. Synthesis of cellulose cotton-based UiO-66 MOFs for the removal of rhodamine B and Pb (II) metal ions from contaminated wastewater. Int. J. Biol. Macromol. 2023, 253, 126986. [Google Scholar] [CrossRef] [PubMed]
- Daraei, P.; Rostami, E.; Nasirmanesh, F.; Nobakht, V. Preparation of pH-sensitive composite polyethersulfone membranes embedded by Ag (I) coordination polymer for the removal of cationic and anionic dyes. J. Environ. Manag. 2023, 347, 119083. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.H.; Tang, D.Y.; Wang, X.H.; Yan, L.L.; Deng, L.L.; Zhao, M.Q.; Deng, E.N.; Zhou, Q.H. Molecularly imprinted MOF/PAN hybrid nanofibrous membranes for selective bisphenol A adsorption and antibacterial fouling in water treatment. Sep. Purif. Technol. 2024, 328, 124984. [Google Scholar] [CrossRef]
- Mukherjee, D.; Pal, S.C.; Das, G.; Gore, K.R.; Das, M.C. Devising robust hydrophobic MOFs and its membrane for ultra-sensitive aqueous phase detection of antibiotics and toxic nitro-explosives and adsorption of TNP. J. Environ. Chem. Eng. 2023, 11, 110528. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Ozaki, N.; Karimidermani, B.; Razmi, E.; Kasmuri, N. Occurrence of per- and polyfluoroalkyl substances in aquatic environments and their removal by advanced oxidation processes. Chemosphere 2023, 330, 138666. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Bao, J.; Chen, D.; Shah, S.J.; Subhan, S.; Gong, W.; Li, W.; Luan, X.; Zhao, Z.; Zhao, Z. Accelerating the Fe(III)/Fe(II) cycle via enhanced electronic effect in NH2-MIL-88B(Fe)/TPB-DMTP-COF composite for boosting photo-Fenton degradation of sulfamerazine. J. Colloid Interface Sci. 2022, 624, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.X.; Wang, C.C.; Du, X.; Li, Y.; Wang, F.; Wang, P. Efficient removal of emerging organic contaminants via photo-Fenton process over micron-sized Fe-MOF sheet. Chem. Eng. J. 2022, 429, 132495. [Google Scholar] [CrossRef]
- Wenderich, K.; Mul, G. Methods, Mechanism, and Applications of Photodeposition in Photocatalysis: A Review. Chem. Rev. 2016, 116, 14587–14619. [Google Scholar] [CrossRef] [PubMed]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Du, X.; Oturan, M.A.; Zhou, M.; Belkessa, N.; Su, P.; Cai, J.; Trellu, C.; Mousset, E. Nanostructured electrodes for electrocatalytic advanced oxidation processes: From materials preparation to mechanisms understanding and wastewater treatment applications. Appl. Catal. B Environ. 2021, 296, 120332. [Google Scholar] [CrossRef]
- von Gunten, U. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton catalysts: A review of recent advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Liu, S.; Tan, X.; Dai, M.; Chen, Q.; Huang, X. Polydopamine-modified MOF-5-derived carbon as persulfate activator for aniline aerofloat degradation. Chemosphere 2023, 345, 140436. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, M.; Xia, Z.; Liu, J.; Chen, Y.; Lv, X.; Jia, Z.; Xie, Z. Highly dispersed Co on N-doped carbon derived from metal-organic framework composite for enhanced peroxymonosulfate activation toward tetracycline degradation. Diam. Relat. Mater. 2023, 140, 110544. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, L.; Geng, H.; Wang, B.; Tong, X.; Li, Y.; Chen, D.; Sun, P.; Yang, Y. Fe-MOF-derived carbon compounds as catalysts for trichloroethylene degradation via persulfate oxidation: Role of precursor template and pyrolysis temperature. J. Environ. Chem. Eng. 2023, 11, 110649. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, J.; Jiang, Y.; Yang, S.; Yang, Y.; Wang, Z. FeCo bimetallic metal organic framework nanosheets as peroxymonosulfate activator for selective oxidation of organic pollutants. Chem. Eng. J. 2022, 443, 136483. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, S.; Zhang, J.; Lu, J.; Shan, C.; Zhang, Y.; Dionysiou, D.D.; Lv, L.; Pan, B.; Zhang, W. Enhancing the performance of Fenton-like oxidation by a dual-layer membrane: A sequential interception-oxidation process. J. Hazard. Mater. 2021, 402, 123766. [Google Scholar] [CrossRef] [PubMed]
- Araya, T.; Jia, M.; Yang, J.; Zhao, P.; Cai, K.; Ma, W.; Huang, Y. Resin modified MIL-53 (Fe) MOF for improvement of photocatalytic performance. Appl. Catal. B Environ. 2017, 203, 768–777. [Google Scholar] [CrossRef]
- Araya, T.; Chen, C.C.; Jia, M.K.; Johnson, D.; Li, R.; Huang, Y.P. Selective degradation of organic dyes by a resin modified Fe-based metal-organic framework under visible light irradiation. Opt. Mater. 2017, 64, 512–523. [Google Scholar] [CrossRef]
- Tang, M.; Wan, J.; Wang, Y.; Yan, Z.; Ma, Y.; Sun, J.; Ding, S. Developing a molecularly imprinted channels catalyst based on template effect for targeted removal of organic micropollutants from wastewaters. Chem. Eng. J. 2022, 445, 136755. [Google Scholar] [CrossRef]
- Ding, S.; Wan, J.; Ma, Y.; Wang, Y.; Li, X.; Sun, J.; Pu, M. Targeted degradation of dimethyl phthalate by activating persulfate using molecularly imprinted Fe-MOF-74. Chemosphere 2021, 270, 128620. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Wan, J.; Wang, Y.; Yan, Z.; Ma, Y. Activation of persulfate by molecularly imprinted Fe-MOF-74@SiO2 for the targeted degradation of dimethyl phthalate: Effects of operating parameters and chlorine. Chem. Eng. J. 2021, 422, 130406. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, C.; Yan, X.; Zhang, H.; Xiao, C.; Qi, J.; Zhu, Z.; Zhou, Y.; Sun, X.; Duan, X.; et al. Rational Regulation of Co–N–C Coordination for High-Efficiency Generation of 1O2 toward Nearly 100% Selective Degradation of Organic Pollutants. Environ. Sci. Technol. 2022, 56, 8833–8843. [Google Scholar] [CrossRef]
- Liu, Y.; Miao, W.; Feng, Y.; Fang, X.; Li, Q.; Du, N.; Wang, D.; Mao, S. Enhanced peroxydisulfate oxidation via Cu(III) species with a Cu-MOF-derived Cu nanoparticle and 3D graphene network. J. Hazard. Mater. 2021, 403, 123691. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Qin, H.; Ye, Z.; Wei, X.; Miao, W.; Yang, D.; Mao, S. Selective Removal of Phenolic Compounds by Peroxydisulfate Activation: Inherent Role of Hydrophobicity and Interface ROS. Environ. Sci. Technol. 2022, 56, 2665–2676. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, C.; Schmidt, R. Physical Mechanisms of Generation and Deactivation of Singlet Oxygen. Chem. Rev. 2003, 103, 1685–1757. [Google Scholar] [CrossRef]
- Mazarji, M.; Minkina, T.; Sushkova, S.; Mandzhieva, S.; Bayero, M.T.; Fedorenko, A.; Mahmoodi, N.M.; Sillanpää, M.; Bauer, T.; Soldatov, A. Metal-organic frameworks (MIL-101) decorated biochar as a highly efficient bio-based composite for immobilization of polycyclic aromatic hydrocarbons and copper in real contaminated soil. J. Environ. Chem. Eng. 2022, 10, 108821. [Google Scholar] [CrossRef]
- Zhi, G.; Qi, X.; Li, Y.; Wang, J.; Wang, J. Efficient treatment of smelting wastewater: 3D nickel foam @MOF shatters the previous limitation, enabling high-throughput selective capture of arsenic to form non-homogeneous nuclei. Sep. Purif. Technol. 2024, 328, 124927. [Google Scholar] [CrossRef]
- Li, X.; Zheng, S.; Li, Y.; Ding, J.; Qin, W. Effectively facilitating the degradation of chloramphenicol by the synergism of Shewanella oneidensis MR-1 and the metal-organic framework. J. Hazard. Mater. 2023, 454, 131545. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Wang, W.X. Effective flocculation of harmful algae Microcystis aeruginosa by nanoscale metal–organic framework NH2-MIL-101(Cr). Chem. Eng. J. 2022, 433, 134584. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Y.; Wang, C.; Yue, L.; Liu, T.; Lan, Q.; Cao, X.; Xing, B. Photocatalytic inactivation of harmful algae Microcystis aeruginosa and degradation of microcystin by g-C3N4/Cu-MOF nanocomposite under visible light. Sep. Purif. Technol. 2023, 313, 123515. [Google Scholar] [CrossRef]
- Kim, Y.; Kalimuthu, P.; Nam, G.; Jung, J. Cyanobacteria control using Cu-based metal organic frameworks derived from waste PET bottles. Environ. Res. 2023, 224, 115532. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, M.; Cao, Y.; Xu, M.; Zhang, H.; Zhao, W.; Wang, R.; Yang, Y.; Chen, J. Inhibition of growth for Microcystis aeruginosa by insertion of iron ion into biochar modified copper metal organic framework (Fe3O4-BC@Cu-MOF-74) under visible light. J. Environ. Chem. Eng. 2023, 11, 111130. [Google Scholar] [CrossRef]
- Fan, G.; Zhou, J.; Zheng, X.; Chen, W. Growth Inhibition of Microcystis aeruginosa by Copper-based MOFs: Performance and Physiological Effect on Algal Cells. Appl. Organomet. Chem. 2018, 32, e4600. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Khaneghah, A.M.; Khataee, A. New emerging techniques for detection and degradation of hazardous materials in environments: Challenges and perspectives. Chemosphere 2022, 286, 131589. [Google Scholar] [CrossRef] [PubMed]
- Diamantis, S.A.; Margariti, A.; Pournara, A.D.; Papaefstathiou, G.S.; Manos, M.J.; Lazarides, T. Luminescent metal–organic frameworks as chemical sensors: Common pitfalls and proposed best practices. Inorg. Chem. Front. 2018, 5, 1493–1511. [Google Scholar] [CrossRef]
- Cui, A.Q.; Wu, X.Y.; Ye, J.B.; Song, G.; Chen, D.Y.; Xu, J.; Liu, Y.; Lai, J.P.; Sun, H. Two-in-one? dual-function luminescent MOF hydrogel for onsite ultra-sensitive detection and efficient enrichment of radioactive uranium in water. J. Hazard. Mater. 2023, 448, 130864. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Lou, Y.; Yang, Y.; Chen, Z.; Cai, Y.; Guo, Z.; Zhan, H.; Chen, B. Dye-Modified Metal–Organic Framework as a Recyclable Luminescent Sensor for Nicotine Determination in Urine Solution and Living Cell. ACS Appl. Mater. Interfaces 2019, 11, 47253–47258. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Li, Z.; Jia, Q. Detection of Purine Metabolite Uric Acid with Picolinic-Acid-Functionalized Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2019, 11, 34196–34202. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Fan, M.; Liu, Q.; Su, Z.; Li, X.; Pan, Q.; Hu, X. Two Highly Water-Stable Imidazole-Based Ln-MOFs for Sensing Fe3+,Cr2O72−/CrO42− in a Water Environment. Inorg. Chem. 2020, 59, 2005–2010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Q.; Yue, D.; Zhang, J.; Wang, J.; Li, B.; Yang, Y.; Cui, Y.; Qian, G. Flexible Metal–Organic Framework-Based Mixed-Matrix Membranes: A New Platform for H2S Sensors. Small 2018, 14, 1801563. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.A.A.; Masoomi, M.Y.; Morsali, A. Morphology-dependent sensing performance of dihydro-tetrazine functionalized MOF toward Al(III). Ultrason. Sonochem. 2018, 41, 17–26. [Google Scholar] [CrossRef]
- Sousaraei, A.; Queirós, C.; Moscoso, F.G.; Lopes-Costa, T.; Pedrosa, J.M.; Silva, A.M.; Cunha-Silva, L.; Cabanillas-Gonzalez, J. Subppm Amine Detection via Absorption and Luminescence Turn-On Caused by Ligand Exchange in Metal Organic Frameworks. Anal. Chem. 2019, 91, 15853–15859. [Google Scholar] [CrossRef]
- Maka, V.K.; Mukhopadhyay, A.; Savitha, G.; Moorthy, J.N. Fluorescent 2D metal–organic framework nanosheets (MONs): Design, synthesis and sensing of explosive nitroaromatic compounds (NACs). Nanoscale 2018, 10, 22389–22399. [Google Scholar] [CrossRef]
- Han, L.J.; Zheng, D.; Chen, S.G.; Zheng, H.G.; Ma, J. A Highly Solvent-Stable Metal–Organic Framework Nanosheet: Morphology Control, Exfoliation, and Luminescent Property. Small 2018, 14, 1703873. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.Z.; Wang, X.L.; Sun, X.J.; Hou, Y.; Zhang, X.; Yang, D.D.; Dong, H.; Zhang, F.M. Rapid and Large-Scale Synthesis of IRMOF-3 by Electrochemistry Method with Enhanced Fluorescence Detection Performance for TNP. Inorg. Chem. 2018, 57, 3818–3824. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Song, Y.; Wang, L. Multi-emission metal–organic framework composites for multicomponent ratiometric fluorescence sensing: Recent developments and future challenges. J. Mater. Chem. B 2020, 8, 3292–3315. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Min, H.; Shi, W.; Cheng, P. Multicenter Metal–Organic Framework-Based Ratiometric Fluorescent Sensors. Adv. Mater. 2019, 32, 1805871. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, D.; Peng, J.; Du, Q.; He, H. Ratiometric fluorescence sensing of metal-organic frameworks: Tactics and perspectives. Coord. Chem. Rev. 2020, 404, 213113. [Google Scholar] [CrossRef]
- Li, C.; Hai, J.; Li, S.; Wang, B.; Yang, Z. Luminescent magnetic nanoparticles encapsulated in MOFs for highly selective and sensitive detection of ClO-/SCN- and anti-counterfeiting. Nanoscale 2018, 10, 8667–8676. [Google Scholar] [CrossRef]
- Das, P.; Mandal, S.K. Strategic Design and Functionalization of an Amine-Decorated Luminescent Metal Organic Framework for Selective Gas/Vapor Sorption and Nanomolar Sensing of 2,4,6-Trinitrophenol in Water. ACS Appl. Mater. Interfaces 2018, 10, 25360–25371. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Liang, M.; Wang, X.; Kong, R.; Chen, G.; Xia, L.; Qu, F. The role of l-histidine as molecular tongs: A strategy of grasping Tb3+ using ZIF-8 to design sensors for monitoring an anthrax biomarker on-the-spot. Chem. Sci. 2020, 11, 2407–2413. [Google Scholar] [CrossRef]
- Dalapati, R.; Biswas, S. A Pyrene-Functionalized Metal–Organic Framework for Nonenzymatic and Ratiometric Detection of Uric Acid in Biological Fluid via Conformational Change. Inorg. Chem. 2019, 58, 5654–5663. [Google Scholar] [CrossRef]
- Pan, H.; Wang, S.; Dao, X.; Ni, Y. Fluorescent Zn-PDC/Tb3+ Coordination Polymer Nanostructure: A Candidate for Highly Selective Detections of Cefixime Antibiotic and Acetone in Aqueous System. Inorg. Chem. 2018, 57, 1417–1425. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, H.; Zhao, X.; Li, Z.; Gao, Z.; Wang, Y.; Huang, H. Aqueous phase sensing of bismuth ion using fluorescent metal-organic framework. Sens. Actuators B Chem. 2018, 266, 323–328. [Google Scholar] [CrossRef]
- Aswathi, R.; Sandhya, K.Y. Ultrasensitive and selective electrochemical sensing of Hg(ii) ions in normal and sea water using solvent exfoliated MoS2: Affinity matters. J. Mater. Chem. A 2018, 6, 14602–14613. [Google Scholar] [CrossRef]
- Sheng, S.; Zhang, Z.; Wang, M.; He, X.; Jiang, C.; Wang, Y. Synthesis of MIL-125(Ti) derived TiO2 for selective photoelectrochemical sensing and photocatalytic degradation of tetracycline. Electrochim. Acta 2022, 420, 140441. [Google Scholar] [CrossRef]
- Chai, X.; Zhou, X.; Zhu, A.; Zhang, L.; Qin, Y.; Shi, G.; Tian, Y. A Two-Channel Ratiometric Electrochemical Biosensor for In Vivo Monitoring of Copper Ions in a Rat Brain Using Gold Truncated Octahedral Microcages. Angew. Chem. Int. Ed. 2013, 52, 8129–8133. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Xu, H.; Zhou, S.; Song, T.; Wang, H.; Li, S.; Gan, W.; Yuan, Q. Simultaneous detection of Cd(II) and Pb(II) by differential pulse anodic stripping voltammetry at a nitrogen-doped microporous carbon/Nafion/bismuth-film electrode. Electrochim. Acta 2014, 143, 143–151. [Google Scholar] [CrossRef]
- Guo, H.; Zheng, Z.; Zhang, Y.; Lin, H.; Xu, Q. Highly selective detection of Pb2+ by a nanoscale Ni-based metal–organic framework fabricated through one-pot hydrothermal reaction. Sens. Actuators B Chem. 2017, 248, 430–436. [Google Scholar] [CrossRef]
- Wu, T.; Gao, X.J.; Ge, F.; Zheng, H.G. Metal–organic frameworks (MOFs) as fluorescence sensors: Principles, development and prospects. CrystEngComm 2022, 24, 7881–7901. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Huang, W.; Zhang, T.; Hu, X.; Perman, J.A.; Ma, S. A metal–organic framework and conducting polymer based electrochemical sensor for high performance cadmium ion detection. J. Mater. Chem. A 2017, 5, 8385–8393. [Google Scholar] [CrossRef]
- Chen, M.; Xu, W.-M. An Anionic Calcium Metal–Organic Framework Encapsulated with TbIII Ions as a Recyclable Luminescent Sensor for CrIII and FeIII Ions. Aust. J. Chem. 2019, 72, 910–915. [Google Scholar] [CrossRef]
- Zhan, Z.; Liang, X.; Zhang, X.; Jia, Y.; Hu, M. A water-stable europium-MOF as a multifunctional luminescent sensor for some trivalent metal ions (Fe3+, Cr3+, Al3+), PO4 3-ions, and nitroaromatic explosives. Dalton Trans. 2019, 48, 1786–1794. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, M.; Ma, Y.; Li, L. Multi-Responsive Luminescent Sensors Based on Two-Dimensional Lanthanide–Metal Organic Frameworks for Highly Selective and Sensitive Detection of Cr(III) and Cr(VI) Ions and Benzaldehyde. Cryst. Growth Des. 2017, 17, 4326–4335. [Google Scholar] [CrossRef]
- Shi, W.; He, M.; Li, W.; Wei, X.; Bui, B.; Chen, M.; Chen, W. Cu-Based Metal–Organic Framework Nanoparticles for Sensing Cr(VI) Ions. ACS Appl. Nano Mater. 2021, 4, 802–810. [Google Scholar] [CrossRef]
- Liu, Z.; Cui, T.; Pulletikurthi, G.; Lahiri, A.; Carstens, T.; Olschewski, M.; Endres, F. Dendrite-Free Nanocrystalline Zinc Electrodeposition from an Ionic Liquid Containing Nickel Triflate for Rechargeable Zn-Based Batteries. Angew. Chem. Int. Ed. Engl. 2016, 55, 2889–2893. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Gao, G.; Zheng, L.; Chi, Y.; Chen, G. Encapsulation of Strongly Fluorescent Carbon Quantum Dots in Metal–Organic Frameworks for Enhancing Chemical Sensing. Anal. Chem. 2013, 86, 1223–1228. [Google Scholar] [CrossRef]
- Liu, S.; Li, J.; Luo, F. The first transition-metal metal–organic framework showing cation exchange for highly selectively sensing of aqueous Cu(II) ions. Inorg. Chem. Commun. 2010, 13, 870–872. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Narayanan, R.P.; George, S.J.; Maji, T.K. Luminescent microporous metal-organic framework with functional lewis basic sites on the pore surface: Specific sensing and removal of metal ions. Inorg. Chem. 2012, 51, 10089–10091. [Google Scholar] [CrossRef]
- Zheng, T.T.; Zhao, J.; Fang, Z.W.; Li, M.T.; Sun, C.Y.; Li, X.; Wang, X.L.; Su, Z.M. A luminescent metal organic framework with high sensitivity for detecting and removing copper ions from simulated biological fluids. Dalton Trans. 2017, 46, 2456–2461. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, Y.; Li, Q.; Li, S.; Yi, Z.; Xu, Z.; Wang, Y. Highly sensitive and selective fluorescent probe for Fe3+ and hazardous phenol compounds based on a water-stable Zn-based metal–organic framework in aqueous media. RSC Adv. 2017, 7, 50035–50039. [Google Scholar] [CrossRef]
- Zhao, S.S.; Yang, J.; Liu, Y.Y.; Ma, J.F. Fluorescent Aromatic Tag-Functionalized MOFs for Highly Selective Sensing of Metal Ions and Small Organic Molecules. Inorg. Chem. 2016, 55, 2261–2273. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.; Luo, Z.; Wang, J.; Li, Y.; Han, Y.; Liu, J. Fluorescence detection of Mn2+, Cr2O72−and nitroexplosives and photocatalytic degradation of methyl violet and rhodamine B based on two stable metal–organic frameworks. RSC Adv. 2017, 7, 10415–10423. [Google Scholar] [CrossRef]
- Li, L.; Shen, S.; Lin, R.; Bai, Y.; Liu, H. Rapid and specific luminescence sensing of Cu(ii) ions with a porphyrinic metal-organic framework. Chem. Commun. 2017, 53, 9986–9989. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.L.; Tian, D.; Liu, J.; Dong, L.Z.; Li, S.L.; Li, D.S.; Lan, Y.Q. A Water-Stable Metal-Organic Framework for Highly Sensitive and Selective Sensing of Fe3+ Ion. Inorg. Chem. 2016, 55, 10580–10586. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shi, Z.; Qin, L.; Jia, H.; Zheng, H. Two New Luminescent Cd(II)-Metal–Organic Frameworks as Bifunctional Chemosensors for Detection of Cations Fe3+, Anions CrO42−, and Cr2O72− in Aqueous Solution. Cryst. Growth Des. 2016, 17, 67–72. [Google Scholar] [CrossRef]
- Gai, Y.L.; Guo, Q.; Zhao, X.Y.; Chen, Y.; Liu, S.; Zhang, Y.; Zhuo, C.X.; Yao, C.; Xiong, K.C. Extremely stable europium-organic framework for luminescent sensing of Cr2O72− and Fe3+ in aqueous systems. Dalton Trans. 2018, 47, 12051–12055. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, Q.; Guo, C.; Sun, Y.; Xie, L.H.; Li, J.R. Stable Zr(IV)-Based Metal-Organic Frameworks with Predesigned Functionalized Ligands for Highly Selective Detection of Fe(III) Ions in Water. ACS Appl. Mater. Interfaces 2017, 9, 10286–10295. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yan, B. A novel photofunctional hybrid material of pyrene functionalized metal-organic framework with conformation change for fluorescence sensing of Cu2+. Sens. Actuators B Chem. 2016, 235, 541–546. [Google Scholar] [CrossRef]
- Jin, J.; Yang, G.; Liu, Y.; Cheng, S.; Liu, J.; Wu, D.; Wang, Y.Y. Two Series of Microporous Lanthanide-Organic Frameworks with Different Secondary Building Units and Exposed Lewis Base Active Sites: Sensing, Dye Adsorption, and Magnetic Properties. Inorg. Chem. 2019, 58, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Xue, J.; Liu, Y.; Yang, G.; Wang, Y.Y. Recent progresses in luminescent metal-organic frameworks (LMOFs) as sensors for the detection of anions and cations in aqueous solution. Dalton Trans. 2021, 50, 1950–1972. [Google Scholar] [CrossRef] [PubMed]
- Rath, B.B.; Vittal, J.J. Water Stable Zn(II) Metal-Organic Framework as a Selective and Sensitive Luminescent Probe for Fe(III) and Chromate Ions. Inorg. Chem. 2020, 59, 8818–8826. [Google Scholar] [CrossRef]
- Xu, R.X.; Yu, X.Y.; Gao, C.; Jiang, Y.J.; Han, D.D.; Liu, J.H.; Huang, X.J. Non-conductive nanomaterial enhanced electrochemical response in stripping voltammetry: The use of nanostructured magnesium silicate hollow spheres for heavy metal ions detection. Anal. Chim. Acta 2013, 790, 31–38. [Google Scholar] [CrossRef]
- Lim, K.S.; Jeong, S.Y.; Kang, D.W.; Song, J.H.; Jo, H.; Lee, W.R.; Phang, W.J.; Moon, D.; Hong, C.S. Luminescent Metal-Organic Framework Sensor: Exceptional Cd2+ Turn-On Detection and First In Situ Visualization of Cd2+ Ion Diffusion into a Crystal. Chemistry 2017, 23, 4803–4809. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, C.; Tan, H.; Tang, G.; Gao, J.; Chen, C.H. A turn on fluorescent sensor based on lanthanide coordination polymer nanoparticles for the detection of mercury(ii) in biological fluids. RSC Adv. 2016, 6, 17811–17817. [Google Scholar] [CrossRef]
- Lin, X.; Luo, F.; Zheng, L.; Gao, G.; Chi, Y. Fast, sensitive, and selective ion-triggered disassembly and release based on tris(bipyridine)ruthenium(II)-functionalized metal-organic frameworks. Anal. Chem. 2015, 87, 4864–4870. [Google Scholar] [CrossRef]
- Wang, P.; Ma, J.P.; Dong, Y.B.; Huang, R.Q. Tunable luminescent lanthanide coordination polymers based on reversible solid-state ion-exchange monitored by ion-dependent photoinduced emission spectra. J. Am. Chem. Soc. 2007, 129, 10620–10621. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Hong, Y.; Zhang, C.; Huang, R.; Wang, C.; Lin, W. Pre-concentration and energy transfer enable the efficient luminescence sensing of transition metal ions by metal-organic frameworks. Chem. Commun. 2015, 51, 16996–16999. [Google Scholar] [CrossRef]
- Burtch, N.C.; Jasuja, H.; Walton, K.S. Water stability and adsorption in metal-organic frameworks. Chem. Rev. 2014, 114, 10575–10612. [Google Scholar] [CrossRef]
- He, W.; Ifraemov, R.; Raslin, A.; Hod, I. Room-Temperature Electrochemical Conversion of Metal–Organic Frameworks into Porous Amorphous Metal Sulfides with Tailored Composition and Hydrogen Evolution Activity. Adv. Funct. Mater. 2018, 28, 1707244. [Google Scholar] [CrossRef]
- Hendon, C.H.; Tiana, D.; Walsh, A. Conductive metal-organic frameworks and networks: Fact or fantasy? Phys. Chem. Chem. Phys. 2012, 14, 13120–13132. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Qin, J.S.; Lollar, C.T.; Zhou, H.C. Stable Metal-Organic Frameworks with Group 4 Metals: Current Status and Trends. ACS Cent. Sci. 2018, 4, 440–450. [Google Scholar] [CrossRef]
- Gkaniatsou, E.; Sicard, C.E.M.; Ricoux, R.E.M.; Mahy, J.P.; Steunou, N.; Serre, C. Metal–organic frameworks: A novel host platform for enzymatic catalysis and detection. Mater. Horiz. 2017, 4, 55–63. [Google Scholar] [CrossRef]
- Gu, C.; Guo, C.; Li, Z.; Wang, M.; Zhou, N.; He, L.; Zhang, Z.; Du, M. Bimetallic ZrHf-based metal-organic framework embedded with carbon dots: Ultra-sensitive platform for early diagnosis of HER2 and HER2-overexpressed living cancer cells. Biosens. Bioelectron. 2019, 134, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Li, X.; Lyu, Z.; Pan, J.; Ding, S.; Ruan, X.; Zhu, W.; Du, D.; Lin, Y. Metal-organic framework based nanozymes: Promising materials for biochemical analysis. Chem. Commun. 2020, 56, 11338–11353. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Shi, Q.; Zhu, W.; Liu, D.; Tian, H.; Fu, S.; Cheng, N.; Li, S.; Smith, J.N.; Du, D.; et al. Unprecedented peroxidase-mimicking activity of single-atom nanozyme with atomically dispersed Fe-Nx moieties hosted by MOF derived porous carbon. Biosens. Bioelectron. 2019, 142, 111495. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Yan, H.; Wu, Y.; Gu, W.; Zhu, C.; Du, D.; Lin, Y. When Nanozymes Meet Single-Atom Catalysis. Angew. Chem. Int. Ed. 2020, 59, 2565–2576. [Google Scholar] [CrossRef]
- Lin, T.; Qin, Y.; Huang, Y.; Yang, R.; Hou, L.; Ye, F.; Zhao, S. A label-free fluorescence assay for hydrogen peroxide and glucose based on the bifunctional MIL-53(Fe) nanozyme. Chem. Commun. 2018, 54, 1762–1765. [Google Scholar] [CrossRef]
- Ye, K.; Wang, L.; Song, H.; Li, X.; Niu, X. Bifunctional MIL-53(Fe) with pyrophosphate-mediated peroxidase-like activity and oxidation-stimulated fluorescence switching for alkaline phosphatase detection. J. Mater. Chem. B 2019, 7, 4794–4800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Han, J.; Li, H.; Li, Z.; Zou, P.; Li, J.; Zhao, T.; Che, J.; Yang, Y.; Yang, M.; et al. Lymphocyte Membrane- and 12p1-Dual-Functionalized Nanoparticles for Free HIV-1 Trapping and Precise siRNA Delivery into HIV-1-Infected Cells. Adv. Sci. 2023, 10, 2300282. [Google Scholar] [CrossRef] [PubMed]
- Chandio, I.; Ai, Y.; Wu, L.; Liang, Q. Recent progress in MOFs-based nanozymes for biosensing. Nano Res. 2023, 17, 39–64. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Du, D.; Ni, L.; Pan, J.; Niu, X. Emerging applications of nanozymes in environmental analysis: Opportunities and trends. Trac-Trends Anal. Chem. 2019, 120, 115653. [Google Scholar] [CrossRef]
- Giunchedi, P.; Conte, U. Spray-drying as a preparation method of microparticulate drug-delivery systems—An overview. STP Pharma Sci. 1995, 5, 276–290. [Google Scholar]
- Okuyama, K.; Abdullah, M.; Lenggoro, I.W.; Iskandar, F. Preparation of functional nanostructured particles by spray drying. Adv. Powder Technol. 2006, 17, 587–611. [Google Scholar] [CrossRef]
- Thiele, J.; Windbergs, M.; Abate, A.R.; Trebbin, M.; Shum, H.C.; Forster, S.; Weitz, D.A. Early development drug formulation on a chip: Fabrication of nanoparticles using a microfluidic spray dryer. Lab A Chip 2011, 11, 2362–2368. [Google Scholar] [CrossRef]
- Rivas-Murias, B.; Fagnard, J.F.; Vanderbemden, P.; Traianidis, M.; Henrist, C.; Cloots, R.; Vertruyen, B. Spray drying: An alternative synthesis method for polycationic oxide compounds. J. Phys. Chem. Solids 2011, 72, 158–163. [Google Scholar] [CrossRef]
- Troyano, J.; Camur, C.; Garzon-Tovar, L.; Carné-Sánchez, A.; Imaz, I.; Maspoch, D. Spray-Drying Synthesis of MOFs, COFs, and Related Composites. Acc. Chem. Res. 2020, 53, 1206–1217. [Google Scholar] [CrossRef]
- Chaemchuen, S.; Zhou, K.; Mousavi, B.; Ghadamyari, M.; Heynderickx, P.M.; Zhuiykov, S.; Yusubov, M.S.; Verpoort, F. Spray drying of zeolitic imidazolate frameworks: Investigation of crystal formation and properties. CrystEngComm 2018, 20, 3601–3608. [Google Scholar] [CrossRef]
- Daro, N.; Moulet, L.; Penin, N.; Paradis, N.; Létard, J.F.; Lebraud, E.; Buffière, S.; Chastanet, G.; Guionneau, P. Spray-Drying to Get Spin-Crossover Materials. Materials 2017, 10, 60. [Google Scholar] [CrossRef]
- Wang, Z.; Ananias, D.; Carné-Sánchez, A.; Brites, C.D.; Imaz, I.; Maspoch, D.; Rocha, J.; Carlos, L.D. Lanthanide-Organic Framework Nanothermometers Prepared by Spray-Drying. Adv. Funct. Mater. 2015, 25, 2824–2830. [Google Scholar] [CrossRef]
- Guillerm, V.; Garzon-Tovar, L.; Yazdi, A.; Imaz, I.; Juanhuix, J.; Maspoch, D. Continuous One-Step Synthesis of Porous M-XF6-Based Metal-Organic and Hydrogen-Bonded Frameworks. Chemistry 2017, 23, 6829–6835. [Google Scholar] [CrossRef] [PubMed]
- Garzón-Tovar, L.; Cano-Sarabia, M.; Carné-Sánchez, A.; Carbonell, C.; Imaz, I.; Maspoch, D. A spray-drying continuous-flow method for simultaneous synthesis and shaping of microspherical high nuclearity MOF beads. React. Chem. Eng. 2016, 1, 533–539. [Google Scholar] [CrossRef]
- Avci-Camur, C.; Troyano, J.; Pérez-Carvajal, J.; Legrand, A.; Farrusseng, D.; Imaz, I.; Maspoch, D. Aqueous production of spherical Zr-MOF beads via continuous-flow spray-drying. Green Chem. 2018, 20, 873–878. [Google Scholar] [CrossRef]
- Bayliss, P.A.; Ibarra, I.A.; Pérez, E.; Yang, S.; Tang, C.C.; Poliakoff, M.; Schröder, M. Synthesis of metal–organic frameworks by continuous flow. Green Chem. 2014, 16, 3796–3802. [Google Scholar] [CrossRef]
- Tse, J.Y.; Kadota, K.; Nakajima, T.; Uchiyama, H.; Tanaka, S.; Tozuka, Y. Crystalline Rearranged CD-MOF Particles Obtained via Spray-Drying Synthesis Applied to Inhalable Formulations with High Drug Loading. Cryst. Growth Des. 2022, 22, 1143–1154. [Google Scholar] [CrossRef]
- Boix, G.; Han, X.; Imaz, I.; Maspoch, D. Millimeter-Shaped Metal-Organic Framework/Inorganic Nanoparticle Composite as a New Adsorbent for Home Water-Purification Filters. ACS Appl. Mater. Interfaces 2021, 13, 17835–17843. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xing, Y.; Liu, X.; Zhao, L. Numerical investigation of the effect of the injection angle on the spray structures of an air-blast atomizer. Eng. Comput. 2020, 38, 2048–2077. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, Z.W.; Li, W.F.; Xu, J.L.; Liu, H.F. Nonmonotonic Effects of Aerodynamic Force on Droplet Size of Prefilming Air-Blast Atomization. Ind. Eng. Chem. Res. 2018, 57, 1726–1732. [Google Scholar] [CrossRef]
- Diels, A.M.J.; Michiels, C.W. High-Pressure Homogenization as a Non-Thermal Technique for the Inactivation of Microorganisms. Crit. Rev. Microbiol. 2006, 32, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yin, H.; Wu, C.; He, J.; Wang, C.; Ren, B.; Wang, H.; Geng, D.; Zhang, Y.; Zhao, L. Preparation and in vivo evaluation of an intravenous emulsion loaded with an aprepitant-phospholipid complex. Drug Deliv. 2023, 30, 2183834. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhang, W.; Lan, X.; Gong, S.; Wu, J.; Wang, Z. Effects of high hydrostatic pressure and high pressure homogenization processing on characteristics of potato peel waste pectin. Carbohydr. Polym. 2018, 196, 474–482. [Google Scholar] [CrossRef]
- Sun, Y.; Su, S.; Han, L.; Jiang, S. Development of a combining sterilization equipment of high pressure carbon dioxide treatment and ultra-high pressure homogenization treatment. Food Mach. 2017, 33, 84–86. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Xu, X.; Song, L.; Bi, A.; Wu, C.; Ma, Y.; Du, M. Semisolid medium internal phase emulsions stabilized by dendritic-like mushroom cellulose nanofibrils: Concentration effect and stabilization mechanism. Food Chem. 2024, 436, 137693. [Google Scholar] [CrossRef]
- Yu, Y.; Saleh, A.S.; Sun, X.; Wang, Z.; Lu, Y.; Zhang, D.; Zhang, C. Exploring the interaction between myofibrillar proteins and pyrazine compounds: Based on molecular docking, molecular dynamics simulation, and multi-spectroscopy techniques. Int. J. Biol. Macromol. 2023, 253, 126844. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Xu, H.N. Ejected microcrystals probe jammed states of droplets in cyclodextrin-based emulsions. Carbohydr. Polym. 2024, 324, 121455. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.P.; Mota, M.J.; Gomes, A.M.; Delgadillo, I.; Saraiva, J.A. Application of High Pressure with Homogenization, Temperature, Carbon Dioxide, and Cold Plasma for the Inactivation of Bacterial Spores: A Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 532–555. [Google Scholar] [CrossRef] [PubMed]
- Chevalier-Lucia, D.; Picart-Palmade, L.; Dumay, E. Current trends in research and applications of dynamic high-pressure. J. Phys. Conf. Ser. 2017, 950, 032007. [Google Scholar] [CrossRef]
- Biswal, B.P.; Chandra, S.; Kandambeth, S.; Lukose, B.; Heine, T.; Banerjee, R. Mechanochemical Synthesis of Chemically Stable Isoreticular Covalent Organic Frameworks. J. Am. Chem. Soc. 2013, 135, 5328–5331. [Google Scholar] [CrossRef] [PubMed]
- Karak, S.; Kandambeth, S.; Biswal, B.P.; Sasmal, H.S.; Kumar, S.; Pachfule, P.; Banerjee, R. Constructing Ultraporous Covalent Organic Frameworks in Seconds via an Organic Terracotta Process. J. Am. Chem. Soc. 2017, 139, 1856–1862. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]

| Transition Metal Ion | MOF-Based Sensor | Detection Mechanism | References |
|---|---|---|---|
| Cr+3 | 3D Ca-MOF | Competitive absorption mechanism | [140] |
| Cr+3 | Eu-MOF | Cation exchange quenching mechanism | [141] |
| Cr+3 | Eu-MOF | Cation exchange quenching mechanism | [142] |
| Cr+3 | Tb-MOF | Cation exchange quenching mechanism | [142] |
| Cr+6 | MOF-199 | Oxidoreductase | [143] |
| Cr+3 | MIL53-L | LLCT mechanism | [144] |
| Cr+3 | BPEI-CQDs/ZIF-8-MOFs | Cation exchange quenching effect | [145] |
| Cr+3 | [NH4]2 [ZnL]·6H2O | Host-guest Interaction | [146] |
| Cr+3 | (Lin et al.)n | Cation exchange quenching mechanism | [147] |
| Cr+3 | Cd-MOF-74 | PET/FRE mechanism | [148] |
| Cr+3 | Zn-based HPU-1 | Zn-based HPU-1 | [149] |
| Cr+3 | 2D[Zn2(TPC4A)(DMF)(H2O4)]3H2O | Cation exchange quenching mechanism | [150] |
| Mn+2 | MOFs [Zn (dbp)]n | Competitive absorption | [151] |
| Mn+2 | [Cd (dbp) (H2O)]2H2O·CH3CN]n | Competitive absorption | [152] |
| Mn+2 | [NH4]2 [ZnL]·6H2O | Host-guest Interaction | [146] |
| Mn+2 | MOF-525 | Cation exchange quenching mechanism | [153] |
| Mn+2 | PCN-222-Pd | Cation exchange quenching effect | [144] |
| Fe+3 | 3D [[(CH3)2NH2]2 [Zn-(TNC4A)][[(CH3)2NH2]2[Zn-(TNC4A)][[(CH3)2NH2]2 [Zn-(TNC4A)]·4H2O | Cation exchange quenching mechanism | [150] |
| Fe+3 | NNU-1 | Cation exchange quenching effect | [154] |
| Fe+3 | [Zn2(OBA)2(BPTP)] | Competitive absorption quenching effect | [155] |
| Fe+3 | [Ni(OBA)2(BPTP)2(H2O)2] | Competitive absorption quenching effect | [155] |
| Fe+3 | [Cd2(OBA)2(BPTP) (H2O)] | Competitive absorption quenching effect | [155] |
| Fe+3 | [Cd(L) (BPDC)](H2O)2 | Competitive absorption quenching effect | [155] |
| Fe+3 | [Cd(L)(SDBA)(H2O)](H2O)0.5 | Competitive absorption quenching effect | [155] |
| Fe+3 | Cd-MOF | FRET/PET mechanism | [156] |
| Fe+3 | BUT-14 | RET/FRET mechanism | [156] |
| Fe+3 | BUT-15 | RET/FRET mechanism | [156] |
| Fe+3 | UMCM-1-NH2 | Fluorescence quenching mechanism | [156] |
| Fe+3 | [Cd (5-asbaz (bimb)]n | FRET/PET mechanism | [156] |
| Fe+3 | 3D Tb-MOF | FRET/PET mechanism | [157] |
| Fe+3 | Zirconium MOF | FRET/PET mechanism | [158] |
| Fe+3 | Zn-MOF | LLCT mechanism | [159] |
| Zn+2 | ([Ln (PDA)3Mn1.5(H2O)3]·3.25H2O | PET/FRET mechanism | [160] |
| Pd+2 | NH2-MIL-53(Cr) | PET/FRET mechanism | [160] |
| Cd+2 | Zn-MOF | LMCT mechanism | [161] |
| Hg+2 | ZnMOF | Cation exchange quenching effect | [162] |
| Hg+2 | EuMOF | PET/FRET mechanism | [163] |
| Hg+2 | RuMOF | PET/FRET mechanism | [164] |
| Hg+2 | Eu/IPA CPNPs | PET/FRET mechanism | [162] |
| Lanthanides | {[GdIII2L64)Mn(H2O)6]·XH2O}n | Cation exchange quenching effect | [165] |
| Lanthanides | CdMOFs | PET/FRET mechanism | [165] |
| Lanthanides | MgMOF | PET/FRET mechanism | [165] |
| Miscellaneous Transition Metal Ions | Eu3+@MIL-121 | PET/FRET mechanism | [166] |
| Miscellaneous Transition Metal Ions | UiO-bpydc | PET/FRET mechanism | [166] |
| Miscellaneous Transition Metal Ions | Eu-bpydc | PET/FRET mechanism | [166] |
| Catalysts | Precursors | Solvent (s) | Tinlet (Tcoil) [°C] | Yield [%] | SBET [m2/g] | References |
|---|---|---|---|---|---|---|
| HKUST-1 | Cu(NO3)2, BTC | DMF/EtOH/H2O | 180 | 70 | 1260 | [27] |
| Cu-BDC | Cu(NO3)2, BDC | DMF | 180 | 70 | 543 | [27] |
| NOTT-100 | Cu(NO3)2, BPTC | DMF/H2O | 180 | 54 | 1140 | [27] |
| MOF-14 | Cu(NO3)2, BTB | DMF/EtOH/H2O | 180 | 30 | - | [27] |
| Zn-MOF-74 | Zn(NO3)2, DHBDC | DMF/H2O | 180 | 50 | - | [27] |
| Mg-MOF-74 | Mg(NO3)2, DHBDC | DMF/EtOH/H2O | 180 | 35 | - | [27] |
| Ni-MOF-74 | Ni(NO3)2, DHBDC | DMF/EtOH/H2O | 180 | 40 | - | [27] |
| MIL-88B | FeCl3,NH2-BDC | DMF/MeOH/H2O | 180 | 27 | - | [27] |
| ZIF-8a | Zn(OAc)2, 2-MIM | H2O | 180 | - | 1634 | [186] |
| ZIF-67a | Co(OAc)2, 2-MIM | H2O | 180 | - | 1861 | [186] |
| Zn/Co-ZIFa | Zn(OAc)2, Co(OAc)2,2-MIM | H2O | 180 | - | 1746 | [186] |
| [Fe(NH2trz)3]Br2·nH2O | FeBr2, NH2-TRZ | 90 | - | - | [187] | |
| [Fe(NH2trz)3](BF4)n | FeBr2, NH2-TRZ | EtOH; H2O | 90 | - | - | [187] |
| [Fe(Htrz)2(trz)](BF4)n | Fe(BF4)2, HTRZ | EtOH; H2O | 90 | - | - | [187] |
| Tb0.914Eu0.086-PDA | Tb(NO3)3, Eu(NO3)3, PDA | DMF/H2O | 180 | 55 | [188] | |
| MIL-88A | FeCl3, FUM | DMF/MeOH/H2O | 180 | 40 | - | [27] |
| MOF-5 | Zn(OAc)2, BDC | DMF | 180 | 60 | 1215 | [27] |
| IRMOF-3 | Zn(OAc)2, NH2-BDC | DMF | 180 | 70 | [27] | |
| ZIF-8 | Zn(OAc)2, 2-MIM | H2O | 180 | 10 | 941 | [27] |
| Cu-PB | Cu(NO3)2, K3Co(CN)6 | H2O | 180 | 20 | 617 | [27] |
| SIFSIX-3-Co | CoSiF6, PYZ | MeOH | 85 | 44 | - | [189] |
| SIFSIX-3-Ni | NiSiF6, PYZ | MeOH | 85 | - | - | [189] |
| SIFSIX-3-Cu | CuSiF6,PYZ | MeOH | 85 | 55 | - | [189] |
| SIFSIX-3-Zn | ZnSiF6, PYZ | MeOH | 85 | 57 | - | [189] |
| SIFSIX-1-Zn | ZnSiF6, BPY | MeOH | 85 | 40 | - | [189] |
| TIFSIX-1-Cu | Cu(NO3)2, BPY | MeOH | 130 | 79 | - | [189] |
| UiO-66 | ZrCl4, BDC | DMF/H2O | 180 (115) | 70 | 1106 | [190] |
| UiO-66-NH2 | ZrCl4, NH2-BDC | DMF/H2O | 180 (115) | 67 | 752 | [190] |
| UiO-66-NO2 | ZrCl4, NO2-BDC | acetic acid/H2O | 180 (115) | 62 | 679 | [190] |
| UiO-66-Br | ZrCl4, Br-BDC | DMF/H2O | 180 (115) | 68 | 527 | [190] |
| UiO-66-(OH)2 | ZrCl4, (OH)2-BDC | DMF/H2O | 180 (115) | 81 | 401 | [190] |
| UiO-66-acetamido | ZrCl4, acetamido-BDC | DMF/H2O | 180 (115) | 51 | 586 | [190] |
| UiO-66-1,4-NDC | ZrCl4, 1,4-NDC | DMF/H2O | 180 (115) | 45 | 431 | [190] |
| UiO-66-2,6-NDC | ZrCl4, 2,6-NDC | DMF/H2O | 180 (115) | 49 | 557 | [190] |
| Fe-BTC/MIL-100 | Fe(NO3)3, BTC | DMF | 180 (135) | 78 | 1039 | [190] |
| Ni8(OH)4(H2O)2(L)6 | Ni(OAc)2, PCA | DMF/H2O | 180 (100) | 60 | 377 | [190] |
| UiO-66-NH2 | ZrOCl2, NH2-BDC | acetic acid/H2O | 150 (90) | 64 | 1261 | [191] |
| Zr-fumarate | ZrOCl2, FUM | acetic acid/H2O | 140 (90) | 58 | 664 | [191] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Zhang, T.; Wang, B.; Wang, W. The Application of Metal–Organic Frameworks in Water Treatment and Their Large-Scale Preparation: A Review. Materials 2024, 17, 1972. https://doi.org/10.3390/ma17091972
Xie Y, Zhang T, Wang B, Wang W. The Application of Metal–Organic Frameworks in Water Treatment and Their Large-Scale Preparation: A Review. Materials. 2024; 17(9):1972. https://doi.org/10.3390/ma17091972
Chicago/Turabian StyleXie, Yuhang, Teng Zhang, Bo Wang, and Wenju Wang. 2024. "The Application of Metal–Organic Frameworks in Water Treatment and Their Large-Scale Preparation: A Review" Materials 17, no. 9: 1972. https://doi.org/10.3390/ma17091972




