Spin-Coating Fabrication Method of PDMS/NdFeB Composites Using Chitosan/PCL Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- Sylgard 184 elastomer (Dow Corning, Midland, MI, USA);
- NdFeB microparticles (MQFP-14-12-20000-088, Magnequench, Singapore);
- Low molecular weight (LMW) chitosan, Mw~50,000 (Pol-Aura, Olsztyn, Poland);
- Ferulic acid (FA), Mw = 194.18 (Pol-Aura, Olsztyn, Poland);
- Polycaprolactone (PCL) with two different molecular weights: Mw~14,000; and Mw~80,000 (Sigma-Aldrich, Steinheim, Germany).
2.2. Composite Preparation
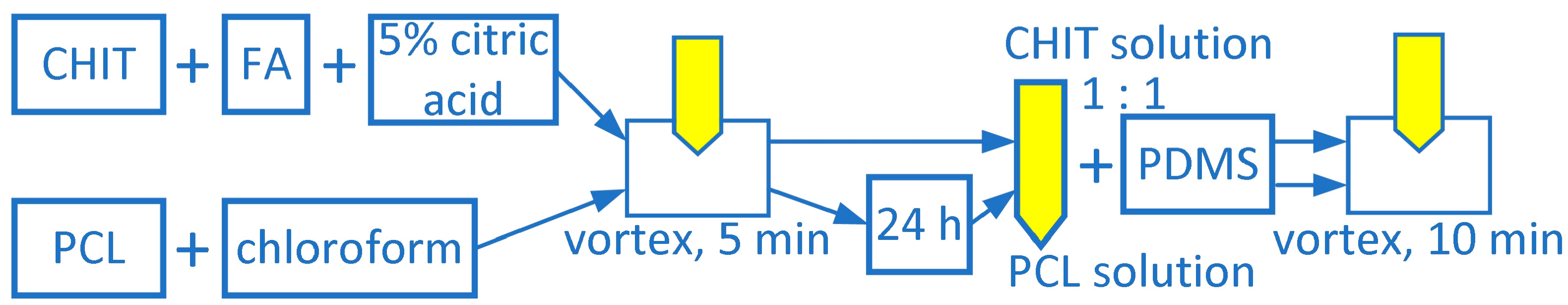
2.3. Coatings Preparation
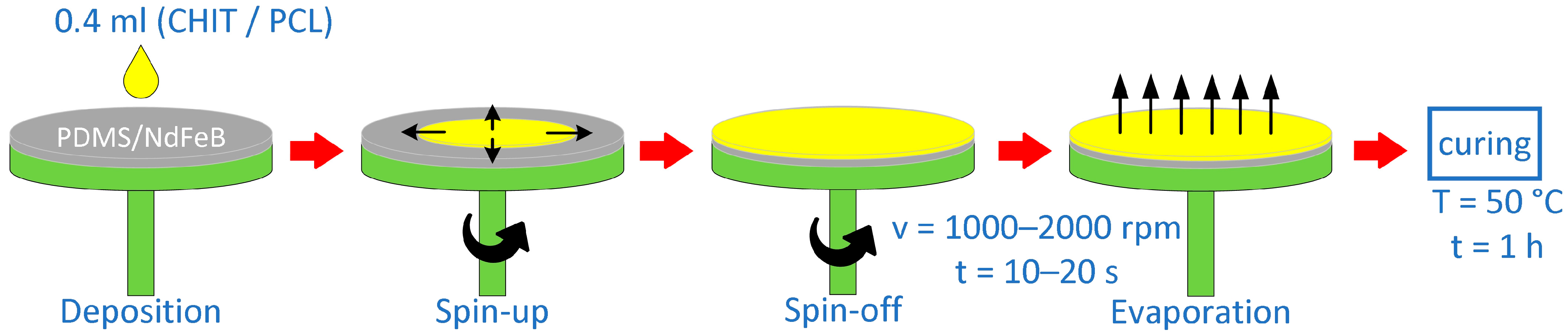
2.4. Preparation of Layered Composites Using Spin-Coating Method
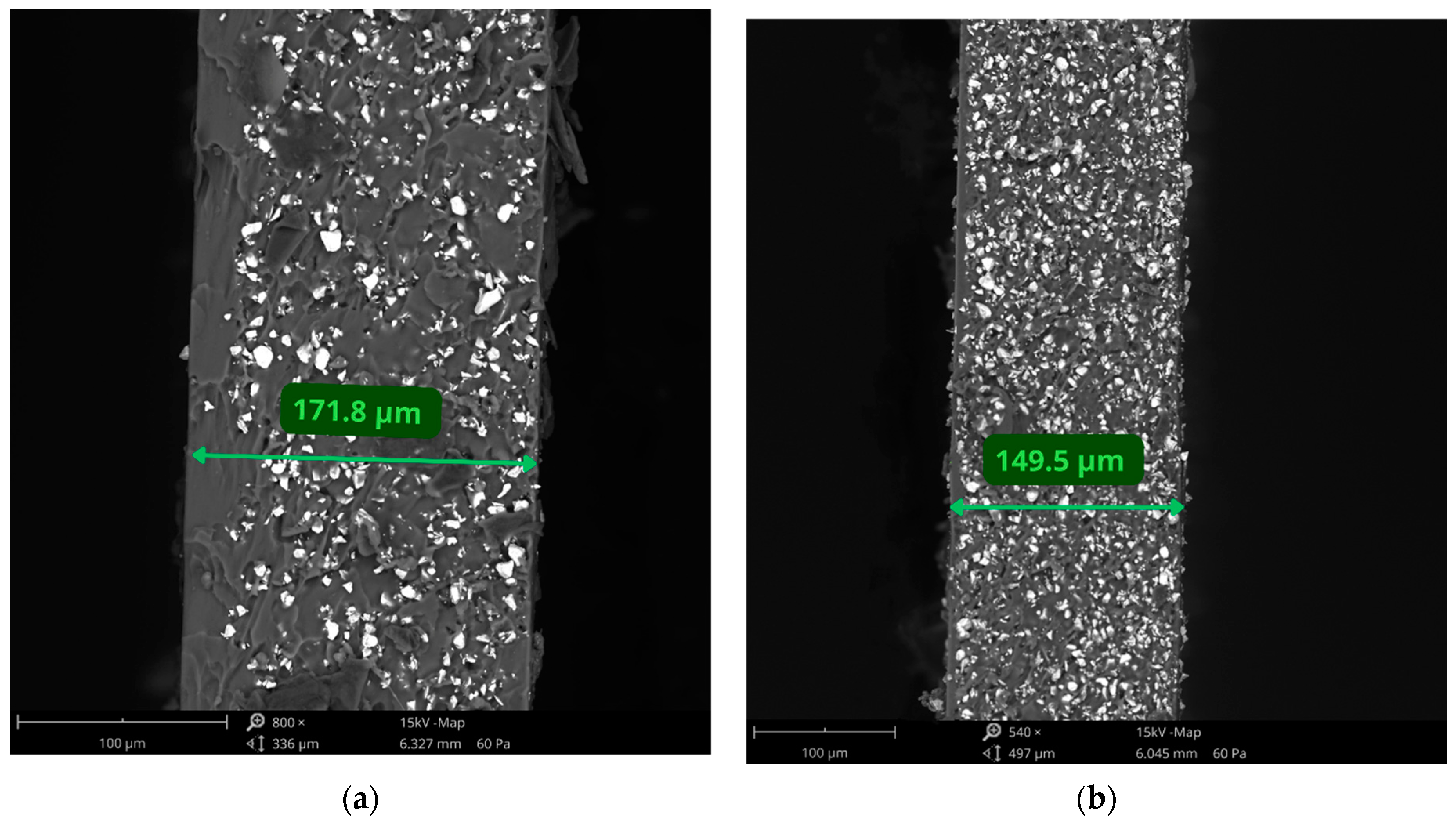
2.5. Thickness Measurements
2.6. Surface Roughness Measurements
2.7. Water Contact Angle Measurements
2.8. Density Measurements
2.9. Tensile Tests
2.10. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Layered Composites Using Spin-Coating Method
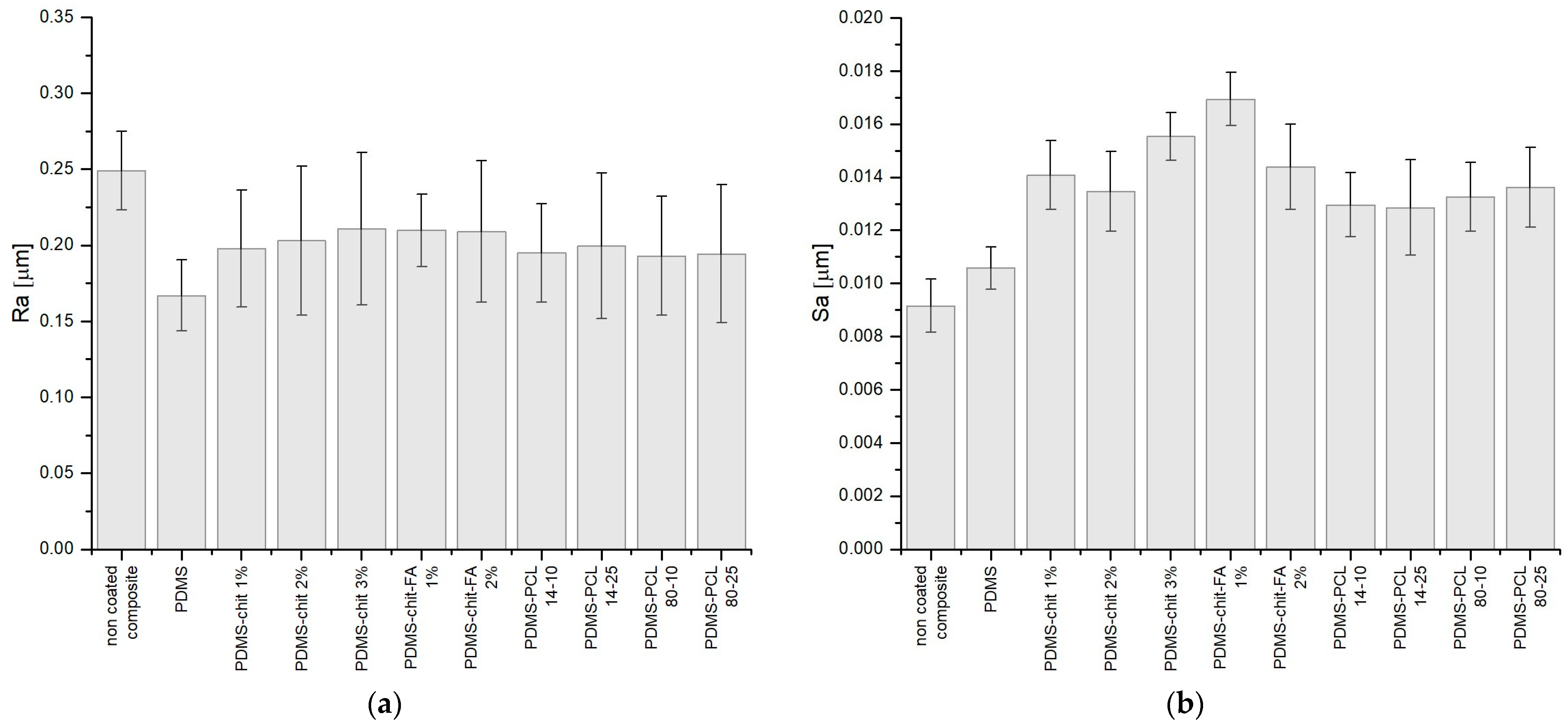
3.2. Linear and Surface Roughness
3.3. Contact Angle
3.4. Density of the Coatings
3.5. Tensile Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iacovacci, V.; Lucarini, G.; Innocenti, C.; Comisso, N.; Dario, P.; Ricotti, L.; Menciassi, A. Polydimethylsiloxane Films Doped with NdFeB Powder: Magnetic Characterization and Potential Applications in Biomedical Engineering and Microrobotics. Biomed. Microdevices 2015, 17, 112. [Google Scholar] [CrossRef] [PubMed]
- Powojska, A.; Niewęgłowska, J.; Suska, S.; Cavadas, A.; Mystkowska, J. Chemical Stability Assessment of Soft Magnetic Composites for Biomedical Applications. Eng. Biomater. 2022, 164, 2–8. [Google Scholar] [CrossRef]
- Dogru, S.; Aydemir, D.; Salman, N.; Ulusu, N.N.; Alaca, B.E. Impact of PDMS Surface Treatment in Cell-Mechanics Applications. J. Mech. Behav. Biomed. Mater. 2020, 103, 103538. [Google Scholar] [CrossRef] [PubMed]
- Trantidou, T.; Elani, Y.; Parsons, E.; Ces, O. Hydrophilic Surface Modification of PDMS for Droplet Microfluidics Using a Simple, Quick, and Robust Method via PVA Deposition. Microsyst. Nanoeng. 2017, 3, 16091. [Google Scholar] [CrossRef] [PubMed]
- Yirijor, J.; Danyuo, Y.; Salifu, A.A.; Ezenwafor, T.; McBagonluri, F. Surface Coating and Wettability Study of PDMS-Based Composites: Effect on Contact Angle and Cell-Surface Interaction. MRS Adv. 2022, 7, 656–662. [Google Scholar] [CrossRef]
- Mata, A.; Fleischman, A.J.; Roy, S. Characterization of Polydimethylsiloxane (PDMS) Properties for Biomedical Micro/Nanosystems. Biomed. Microdevices 2005, 7, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, X.; Ji, B.; Yang, X.; Zhou, B.; Lu, Z.; Gao, X. PLGA Nanofiber/PDMS Microporous Composite Membrane-Sandwiched Microchip for Drug Testing. Micromachines 2020, 11, 1054. [Google Scholar] [CrossRef] [PubMed]
- Said, M.M.; Yunas, J.; Pawinanto, R.E.; Majlis, B.Y.; Bais, B. PDMS Based Electromagnetic Actuator Membrane with Embedded Magnetic Particles in Polymer Composite. Sens. Actuators A Phys. 2016, 245, 85–96. [Google Scholar] [CrossRef]
- Porrelli, D.; Mardirossian, M.; Musciacchio, L.; Pacor, M.; Berton, F.; Crosera, M.; Turco, G. Antibacterial Electrospun Polycaprolactone Membranes Coated with Polysaccharides and Silver Nanoparticles for Guided Bone and Tissue Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 17255–17267. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Dionísio, M.; López, C.R.; Grenha, A. Biocompatibility of Chitosan Carriers with Application in Drug Delivery. J. Funct. Biomater. 2012, 3, 615–641. [Google Scholar] [CrossRef] [PubMed]
- Vaz, J.M.; Pezzoli, D.; Chevallier, P.; Campelo, C.S.; Candiani, G.; Mantovani, D. Antibacterial Coatings Based on Chitosan for Pharmaceutical and Biomedical Applications. Curr. Pharm. Des. 2018, 24, 866–885. [Google Scholar] [CrossRef] [PubMed]
- Zduńska-Pęciak, K.; Kołodziejczak, A.; Rotsztejn, H. Two Superior Antioxidants: Ferulic Acid and Ascorbic Acid in Reducing Signs of Photoaging—A Split-face Comparative Study. Dermatol. Ther. 2022, 35, e15254. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Yang, J.-M.; Chang, Y.-H. Preparation and Characterization of Ferulic Acid-Modified Water Soluble Chitosan and Poly (γ-Glutamic Acid) Polyelectrolyte Films through Layer-by-Layer Assembly towards Protein Adsorption. Int. J. Biol. Macromol. 2021, 171, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Soares, Í.; Faria, J.; Marques, A.; Ribeiro, I.A.C.; Baleizão, C.; Bettencourt, A.; Ferreira, I.M.M.; Baptista, A.C. Drug Delivery from PCL/Chitosan Multilayer Coatings for Metallic Implants. ACS Omega 2022, 7, 23096–23106. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wang, D.; Liu, D.; Su, J.; Jin, Y.; Wang, D.; Han, B.; Jiang, Z.; Liu, B. Applications of Chitosan and Its Derivatives in Skin and Soft Tissue Diseases. Front. Bioeng. Biotechnol. 2022, 10, 894667. [Google Scholar] [CrossRef] [PubMed]
- Yanat, M.; Schroën, K. Preparation Methods and Applications of Chitosan Nanoparticles; with an Outlook toward Reinforcement of Biodegradable Packaging. React. Funct. Polym. 2021, 161, 104849. [Google Scholar] [CrossRef]
- Hedl, E.; Fabijanić, I.; Šrut Rakić, I.; Vadla, I.; Sancho-Parramon, J. Fabrication by Spin-Coating and Optical Characterization of Poly(Styrene-Co-Acrylonitrile) Thin Films. Coatings 2021, 11, 1015. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical Applications of Chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, J.; Tang, Q.; Qiu, P.; Gou, D.; Zhao, J. Chitosan-Based Materials: An Overview of Potential Applications in Food Packaging. Foods 2022, 11, 1490. [Google Scholar] [CrossRef] [PubMed]
- Bajić, M.; Oberlintner, A.; Kõrge, K.; Likozar, B.; Novak, U. Formulation of Active Food Packaging by Design: Linking Composition of the Film-Forming Solution to Properties of the Chitosan-Based Film by Response Surface Methodology (RSM) Modelling. Int. J. Biol. Macromol. 2020, 160, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Bajić, M.; Ročnik, T.; Oberlintner, A.; Scognamiglio, F.; Novak, U.; Likozar, B. Natural Plant Extracts as Active Components in Chitosan-Based Films: A Comparative Study. Food Packag. Shelf Life 2019, 21, 100365. [Google Scholar] [CrossRef]
- Mohammadzadeh Pakdel, P.; Peighambardoust, S.J. Review on Recent Progress in Chitosan-Based Hydrogels for Wastewater Treatment Application. Carbohydr. Polym. 2018, 201, 264–279. [Google Scholar] [CrossRef]
- Woranuch, S.; Yoksan, R.; Akashi, M. Ferulic Acid-Coupled Chitosan: Thermal Stability and Utilization as an Antioxidant for Biodegradable Active Packaging Film. Carbohydr. Polym. 2015, 115, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-Based Materials in Biomedical Applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as Biomaterial for Bone Scaffolds: Review of Literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-K.; Jang, S.-J.; Cho, Y.-S.; Park, H.-H. Fabrication of Nanostructured Polycaprolactone (PCL) Film Using a Thermal Imprinting Technique and Assessment of Antibacterial Function for Its Application. Polymers 2022, 14, 5527. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, S.M.; Patil, H.I.; San Martin Martinez, E.; Casañas Pimentel, R.; Ige, P.P. Poly-ε-Caprolactone (PCL), a Promising Polymer for Pharmaceutical and Biomedical Applications: Focus on Nanomedicine in Cancer. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 85–126. [Google Scholar] [CrossRef]
- Chi, Y.; Zhu, S.; Wang, C.; Zhou, L.; Zhang, L.; Li, Z.; Dai, Y. Glioma Homing Peptide-Modified PEG-PCL Nanoparticles for Enhanced Anti-Glioma Therapy. J. Drug Target. 2016, 24, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Herrero, M.; Gómez-Tejedor, J.A.; Vallés-Lluch, A. PLA/PCL Electrospun Membranes of Tailored Fibres Diameter as Drug Delivery Systems. Eur. Polym. J. 2018, 99, 445–455. [Google Scholar] [CrossRef]
- Bahcecioglu, G.; Hasirci, N.; Bilgen, B.; Hasirci, V. A 3D Printed PCL/Hydrogel Construct with Zone-Specific Biochemical Composition Mimicking That of the Meniscus. Biofabrication 2019, 11, 025002. [Google Scholar] [CrossRef] [PubMed]
- Dethe, M.R.; Prabakaran, A.; Ahmed, H.; Agrawal, M.; Roy, U.; Alexander, A. PCL-PEG Copolymer Based Injectable Thermosensitive Hydrogels. J. Control. Release 2022, 343, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Asadi, N.; Alizadeh, E.; Rahmani Del Bakhshayesh, A.; Mostafavi, E.; Akbarzadeh, A.; Davaran, S. Fabrication and in Vitro Evaluation of Nanocomposite Hydrogel Scaffolds Based on Gelatin/PCL–PEG–PCL for Cartilage Tissue Engineering. ACS Omega 2019, 4, 449–457. [Google Scholar] [CrossRef]
- Conde, G.; De Carvalho, J.R.G.; Dias, P.D.P.; Moranza, H.G.; Montanhim, G.L.; Ribeiro, J.D.O.; Chinelatto, M.A.; Moraes, P.C.; Taboga, S.R.; Bertolo, P.H.L.; et al. In Vivo Biocompatibility and Biodegradability of Poly(Lactic Acid)/Poly(ε-Caprolactone) Blend Compatibilized with Poly(ε-Caprolactone-b-Tetrahydrofuran) in Wistar Rats. Biomed. Phys. Eng. Express 2021, 7, 035005. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Y.; Deng, L.; Jiang, H.; Yang, Z.; Yang, R.; Wu, D. Preparation and Research of PCL/Cellulose Composites: Cellulose Derived from Agricultural Wastes. Int. J. Biol. Macromol. 2023, 235, 123785. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Ying, L.; Li, K.; Chen, F.; Zhao, F.; Sun, Z.; Feng, L.; Liu, J. Biodegradable Mulching Films Based on Polycaprolactone and Its Porous Structure Construction. Polymers 2022, 14, 5340. [Google Scholar] [CrossRef] [PubMed]
- Vashisth, P.; Kumar, N.; Sharma, M.; Pruthi, V. Biomedical Applications of Ferulic Acid Encapsulated Electrospun Nanofibers. Biotechnol. Rep. 2015, 8, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Neto-Neves, E.M.; Da Silva Maia Bezerra Filho, C.; Dejani, N.N.; De Sousa, D.P. Ferulic Acid and Cardiovascular Health: Therapeutic and Preventive Potential. MRMC 2021, 21, 1625–1637. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.; Palma-Florez, S.; Espinosa, V.; Soleimani Rokni, F.; Lagunas, A.; Mir, M.; García-Celma, M.J.; Samitier, J.; Rodríguez-Abreu, C.; Grijalvo, S. Ferulic Acid-Loaded Polymeric Nanoparticles Prepared from Nano-Emulsion Templates Facilitate Internalisation across the Blood–Brain Barrier in Model Membranes. Nanoscale 2023, 15, 7929–7944. [Google Scholar] [CrossRef] [PubMed]
- Turkez, H.; Arslan, M.E.; Barboza, J.N.; Kahraman, C.Y.; De Sousa, D.P.; Mardinoğlu, A. Therapeutic Potential of Ferulic Acid in Alzheimer’s Disease. Curr. Drug Deliv. 2022, 19, 860–873. [Google Scholar] [CrossRef]
- Chaudhary, A.; Jaswal, V.S.; Choudhary, S.; Sonika; Sharma, A.; Beniwal, V.; Tuli, H.S.; Sharma, S. Ferulic Acid: A Promising Therapeutic Phytochemical and Recent Patents Advances. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, G.R.; Duarte, F.I.C.; Converti, A.; De Lima, Á.A.N. Ferulic Acid Activity in Topical Formulations: Technological and Scientific Prospecting. Curr. Pharm. Des. 2021, 27, 2289–2298. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, Q.; Huang, J.; Yue, Y.; Chen, D.; Shi, Y.; Su, B. The Soft NdFeB/Ecoflex Composites for Soft Robot with a Considerable Magnetostimulated Shrinkability. Compos. Sci. Technol. 2022, 217, 109129. [Google Scholar] [CrossRef]
- D’Almeida, M.; Attik, N.; Amalric, J.; Brunon, C.; Renaud, F.; Abouelleil, H.; Toury, B.; Grosgogeat, B. Chitosan Coating as an Antibacterial Surface for Biomedical Applications. PLoS ONE 2017, 12, e0189537. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Ahmed, M.F.; Li, Y.; Bejoy, J.; Zeng, C.; Li, Y. PCL-PDMS-PCL Copolymer-Based Microspheres Mediate Cardiovascular Differentiation from Embryonic Stem Cells. Tissue Eng. Part C Methods 2017, 23, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.S.; Lee, J.-S.; Han, J. Development of a Biodegradable Polycaprolactone Film Incorporated with an Antimicrobial Agent via an Extrusion Process. Sci. Rep. 2019, 9, 20236. [Google Scholar] [CrossRef] [PubMed]
- Visan, A.I.; Popescu-Pelin, G.; Gherasim, O.; Mihailescu, A.; Socol, M.; Zgura, I.; Chiritoiu, M.; Elena Sima, L.; Antohe, F.; Ivan, L.; et al. Long-Term Evaluation of Dip-Coated PCL-Blend-PEG Coatings in Simulated Conditions. Polymers 2020, 12, 717. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Xu, Q.; Li, X.; Chen, C.; Ma, L.; Li, S.; Che, Z.; Lin, H. Chitosan-Based Coating with Antimicrobial Agents: Preparation, Property, Mechanism, and Application Effectiveness on Fruits and Vegetables. Int. J. Polym. Sci. 2016, 2016, 4851730. [Google Scholar] [CrossRef]
- Frank, L.A.; Onzi, G.R.; Morawski, A.S.; Pohlmann, A.R.; Guterres, S.S.; Contri, R.V. Chitosan as a Coating Material for Nanoparticles Intended for Biomedical Applications. React. Funct. Polym. 2020, 147, 104459. [Google Scholar] [CrossRef]
- Powojska, A.; Mystkowska, J.; Mystkowski, A. Method of Obtaining a Protective Coating on the Surface of a Flexible Composite with Magnetic Powder (Chitosan). Patent Submission No. P.445106, 2 June 2023. [Google Scholar]
- Powojska, A.; Mystkowska, J.; Mystkowski, A. Method of Obtaining a Protective Coating on the Surface of a Flexible Composite with Magnetic Powder (PCL). Patent Submission No. P.445105, 2 June 2023. [Google Scholar]
- ISO 9220:2022; Metallic Coatings Measurement of Coating Thickness Scanning Electron Microscope Method. International Organization for Standardization: Geneva, Switzerland, 2022.
- ISO 25178-607:2019; Geometrical Product Specifications (GPS) Surface Texture: Areal Part 607: Nominal Characteristics of Non-Contact (Confocal Microscopy) Instruments. International Organization for Standardization: Geneva, Switzerland, 2019.
- Wrede, A.H.; Al-Masri, F.; Montazami, R.; Hashemi, N.N. Investigation of Cavitation-Induced Damage on PDMS Films. Anal. Methods 2019, 11, 5038–5043. [Google Scholar] [CrossRef]
- ISO 19403-2:2017; Paints and Varnishes Wettability Part 2: Determination of the Surface Free Energy of Solid Surfaces by Measuring the Contact Angle. International Organization for Standardization: Geneva, Switzerland, 2017.
- Zisman, W.A. Influence of constitution on adhesion. Ind. Eng. Chem. 1963, 55, 18–38. [Google Scholar] [CrossRef]
- Mystkowska, J.; Powojska, A.; Łysik, D.; Niewęgłowska, J.; Bermúdez, G.S.C.; Mystkowski, A.; Makarov, D. The Effect of Physiological Incubation on the Properties of Elastic Magnetic Composites for Soft Biomedical Sensors. Sensors 2021, 21, 7122. [Google Scholar] [CrossRef] [PubMed]
- Narbon, J.J.; Moreno-Díaz, C.; Arenas, J.M. Influence of Surface Treatment on the Surface Energy of an Aluminium Substrate. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 323–329. [Google Scholar] [CrossRef]
- Velásquez, P.; Skurtys, O.; Enrione, J.; Osorio, F. Evaluation of Surface Free Energy of Various Fruit Epicarps Using Acid–Base and Zisman Approaches. Food Biophys. 2011, 6, 349–358. [Google Scholar] [CrossRef]
- Vazquez, G.; Alvarez, E.; Navaza, J.M. Surface Tension of Alcohol Water + Water from 20 to 50 °C. J. Chem. Eng. Data 1995, 40, 611–614. [Google Scholar] [CrossRef]
- Howard, K.S.; McAllister, R.A. Surface Tension of Acetone-water Solutions up to Their Normal Boiling Points. AIChE J. 1957, 3, 325–329. [Google Scholar] [CrossRef]
- ISO 1183-1:2019; Plastics Methods for Determining the Density of Non-Cellular Plastics Part 1: Immersion Method, Liquid Pycnometer Method and Titration Method. International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 527-3:2018; Plastics—Determination of Tensile Properties—Part 3: Test Conditions for Films and Sheets. International Organization for Standardization: Geneva, Switzerland, 2018.
- Buschle-Diller, G.; Cooper, J.; Xie, Z.; Wu, Y.; Waldrup, J.; Ren, X. Release of Antibiotics from Electrospun Bicomponent Fibers. Cellulose 2007, 14, 553–562. [Google Scholar] [CrossRef]
- Lee, U.G.; Kim, W.-B.; Han, D.H.; Chung, H.S. A Modified Equation for Thickness of the Film Fabricated by Spin Coating. Symmetry 2019, 11, 1183. [Google Scholar] [CrossRef]
- Mendhe, A.C. Spin Coating: Easy Technique for Thin Films. In Simple Chemical Methods for Thin Film Deposition; Sankapal, B.R., Ennaoui, A., Gupta, R.B., Lokhande, C.D., Eds.; Springer Nature: Singapore, 2023; pp. 387–424. ISBN 978-981-9909-60-5. [Google Scholar]
- Fadzil, A.F.B.A.; Pramanik, A.; Basak, A.K.; Prakash, C.; Shankar, S. Role of Surface Quality on Biocompatibility of Implants—A Review. Ann. 3D Print. Med. 2022, 8, 100082. [Google Scholar] [CrossRef]
- Yeniyol, S.; Bölükbaşi, N.; Çakir, A.F.; Bilir, A.; Özdemir, T. Effects of Surface Modifications with Oxalic Acid Etching and Sandblasting on Surface Topography and Biocompatibility of cpTi Surfaces. Biotechnol. Biotechnol. Equip. 2013, 27, 3995–4001. [Google Scholar] [CrossRef]
- Quirynen, M.; Bollen, C.M.L. The Influence of Surface Roughness and Surface-free Energy on Supra- and Subgingival Plaque Formation in Man: A Review of the Literature. J. Clin. Periodontol. 1995, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-C.; Jung, G.-Y.; Kim, D.-J.; Han, J.-S. Initial Bacterial Adhesion on Resin, Titanium and Zirconia In Vitro. J. Adv. Prosthodont. 2011, 3, 81. [Google Scholar] [CrossRef] [PubMed]
- Santoso, A.; Van Den Berg, B.J.; Saedy, S.; Goodwin, E.; Van Steijn, V.; Van Ommen, J.R. Robust Surface Functionalization of PDMS through Atmospheric Pressure Atomic Layer Deposition. At. Layer Depos. 2023, 1, 1–13. [Google Scholar] [CrossRef]
- Paterlini, T.T.; Nogueira, L.F.B.; Tovani, C.B.; Cruz, M.A.E.; Derradi, R.; Ramos, A.P. The Role Played by Modified Bioinspired Surfaces in Interfacial Properties of Biomaterials. Biophys. Rev. 2017, 9, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Drelich, J.; Chibowski, E.; Meng, D.D.; Terpilowski, K. Hydrophilic and Superhydrophilic Surfaces and Materials. Soft Matter 2011, 7, 9804. [Google Scholar] [CrossRef]
- Sowers, T.W.; Sarkar, R.; Eswarappa Prameela, S.; Izadi, E.; Rajagopalan, J. Capillary Driven Flow of Polydimethylsiloxane in Open Rectangular Microchannels. Soft Matter 2016, 12, 5818–5823. [Google Scholar] [CrossRef] [PubMed]
- Jothi Prakash, C.G.; Prasanth, R. Approaches to Design a Surface with Tunable Wettability: A Review on Surface Properties. J. Mater. Sci. 2021, 56, 108–135. [Google Scholar] [CrossRef]
- Menzies, K.L.; Jones, L. The Impact of Contact Angle on the Biocompatibility of Biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Datta, A.; Berg, J.M.; Gangopadhyay, S. Studies on Surface Wettability of Poly(Dimethyl) Siloxane (PDMS) and Glass under Oxygen-Plasma Treatment and Correlation with Bond Strength. J. Microelectromech. Syst. 2005, 14, 590–597. [Google Scholar] [CrossRef]
- Goldfain, A.M.; Lemaillet, P.; Allen, D.W.; Briggman, K.A.; Hwang, J. Polydimethylsiloxane Tissue-Mimicking Phantoms with Tunable Optical Properties. J. Biomed. Opt. 2021, 27, 074706. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, M.S.; Popov, A.P.; Bykov, A.V.; Tuchin, V.V.; Jędrzejewska-Szczerska, M. Nanoparticle-Free Tissue-Mimicking Phantoms with Intrinsic Scattering. Biomed. Opt. Express 2016, 7, 2088. [Google Scholar] [CrossRef]
- Sieryi, O.; Popov, A.P.; Kalchenko, V.; Bykov, A.V.; Meglinski, I. Tissue-Mimicking Phantoms for Biomedical Applications. In Proceedings of the Tissue Optics and Photonics; Zalevsky, Z., Tuchin, V.V., Blondel, W.C., Eds.; SPIE: Online Only, France, 12 May 2020; p. 39. [Google Scholar]
- Joseph, M.; Van Hileghem, L.; Postelmans, A.; Lammertyn, J.; Saeys, W. Fabrication and Characterization of Porous Tissue-mimicking Optical Phantoms as a Tool for Optical Sensor Validation. J. Biophotonics 2023, 16, e202200338. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, I.; Castro, I.; Carvalho, V.; Rodrigues, C.; Souza, A.; Lima, R.; Teixeira, S.; Ribeiro, J. Polydimethylsiloxane Mechanical Properties: A Systematic Review. Aimsmates 2021, 8, 952–973. [Google Scholar] [CrossRef]
- Zhukova, P.A.; Senatov, F.S.; Zadorozhnyy, M.Y.; Chmelyuk, N.S.; Zaharova, V.A. Polymer Composite Materials Based on Polylactide with a Shape Memory Effect for “Self-Fitting” Bone Implants. Polymers 2021, 13, 2367. [Google Scholar] [CrossRef] [PubMed]
- Ariati, R.; Sales, F.; Souza, A.; Lima, R.A.; Ribeiro, J. Polydimethylsiloxane Composites Characterization and Its Applications: A Review. Polymers 2021, 13, 4258. [Google Scholar] [CrossRef] [PubMed]
- Johnston, I.D.; McCluskey, D.K.; Tan, C.K.L.; Tracey, M.C. Mechanical Characterization of Bulk Sylgard 184 for Microfluidics and Microengineering. J. Micromech. Microeng. 2014, 24, 035017. [Google Scholar] [CrossRef]
- Arnold, F.J.; Maran, F.S. Young’s Modulus Determination of Elastomeric Materials Using Capacitance Measurement. Eur. J. Phys. 2019, 40, 055002. [Google Scholar] [CrossRef]
- Jang, S.-H.; Park, Y.-L.; Yin, H. Influence of Coalescence on the Anisotropic Mechanical and Electrical Properties of Nickel Powder/Polydimethylsiloxane Composites. Materials 2016, 9, 239. [Google Scholar] [CrossRef] [PubMed]





| Sample | Coating Solution |
|---|---|
| PDMS | PDMS |
| PDMS-CHIT1 | PDMS + 1 wt.% CHIT |
| PDMS-CHIT2 | PDMS + 2 wt.% CHIT |
| PDMS-CHIT3 | PDMS + 3 wt.% CHIT |
| PDMS-CHIT-FA1 | PDMS + 1 wt.% CHIT + 1 wt.% FA |
| PDMS-CHIT-FA2 | PDMS + 2 wt.% CHIT + 1 wt.% FA |
| PDMS-PCL14-10 | PDMS + 10 wt.% PCL (MwPCL~14,000) |
| PDMS-PCL14-25 | PDMS + 25 wt.% PCL (MwPCL~14,000) |
| PDMS-PCL80-10 | PDMS + 10 wt.% PCL (MwPCL~80,000) |
| PDMS-PCL80-25 | PDMS + 25 wt.% PCL (MwPCL~80,000) |
| v (rpm) | t (s) | h (µm) |
|---|---|---|
| 500 | 10 | 317.9 ± 6.1 |
| 15 | 263.9 ± 2.8 | |
| 20 | 226.9 ± 6.2 | |
| 750 | 10 | 257.5 ± 2.5 |
| 15 | 213.7 ± 5.3 | |
| 20 | 185.9 ± 4.4 | |
| 1000 | 10 | 216.3 ± 2.9 |
| 15 | 181.7 ± 5.0 | |
| 20 | 147.2 ± 6.5 | |
| 1250 | 10 | 175.2 ± 3.8 |
| 15 | 141.9 ± 3.6 | |
| 20 | 122.0 ± 2.9 | |
| 1500 | 10 | 148.2 ± 4.2 |
| 15 | 110.7 ± 4.3 | |
| 20 | 94.1 ± 4.1 | |
| 1750 | 10 | 123.3 ± 5.2 |
| 15 | 104.8 ± 5.6 | |
| 20 | 86.0 ± 2.4 | |
| 2000 | 10 | 97.4 ± 2.6 |
| 15 | 82.8 ± 3.5 | |
| 20 | 72.9 ± 3.1 |
| Sample | v (rpm) | t (s) | h (µm) |
|---|---|---|---|
| PDMS | 1000 | 10 | 95.5 ± 3.8 |
| PDMS-CHIT1 | 1500 | 10 | 101.6 ± 5.1 |
| PDMS-CHIT2 | 2000 | 10 | 102.1 ± 3.7 |
| PDMS-CHIT3 | 2000 | 15 | 98.0 ± 4.2 |
| PDMS-CHIT-FA1 | 1500 | 20 | 96.2 ± 5.0 |
| PDMS-CHIT-FA2 | 2000 | 20 | 102.3 ± 3.9 |
| PDMS-PCL14-10 | 1000 | 15 | 100.9 ± 4.5 |
| PDMS-PCL14-25 | 1000 | 15 | 97.8 ± 4.8 |
| PDMS-PCL80-10 | 1500 | 10 | 96.5 ± 3.2 |
| PDMS-PCL80-25 | 1500 | 10 | 101.3 ± 4.4 |
| v (rpm) | t (s) | PDMS-CHIT2 h (µm) | PDMS-PCL 14-10 h (µm) |
|---|---|---|---|
| 500 | 10 | 343.4 * ± 4.3 | 159.0 ± 4.0 |
| 15 | 275.4 * ± 1.9 | 131.9 ± 2.0 | |
| 20 | 252.9 ± 4.3 | 113.5 ± 2.5 | |
| 750 | 10 | 268.0 * ± 1.8 | 128.7 ± 4.0 |
| 15 | 235.7 * ± 3.7 | 106.9 ± 3.9 | |
| 20 | 204.4 ± 3.1 | 93.0 ± 6.5 | |
| 1000 | 10 | 228.3 ± 2.0 | 108.1 ± 5.3 |
| 15 | 202.7 ± 3.5 | 100.9 ± 4.5 | |
| 20 | 174.2 ± 4.5 | 90.8 ± 4.8 | |
| 1250 | 10 | 191.2 ± 2.7 | 87.6 ± 6.0 |
| 15 | 156.9 ± 2.5 | 71.0 ± 4.5 | |
| 20 | 134.0 ± 2.0 | 61.0 ± 5.9 | |
| 1500 | 10 | 157.7 ± 2.9 | 70.1 ± 3.6 |
| 15 | 128.7 ± 3.0 | 55.4 ± 3.9 | |
| 20 | 111.1 ± 2.8 | 47.1 ± 2.8 | |
| 1750 | 10 | 149.1 ± 3.6 | 61.7 ± 2.8 |
| 15 | 133.0 ± 3.9 | 52.4 ± 2.3 | |
| 20 | 110.0 ± 3.3 | 43.0 * ± 2.8 | |
| 2000 | 10 | 102.1 ± 3.7 | 48.7 * ± 6.1 |
| 15 | 97.3 ± 2.4 | 41.4 * ± 5.8 | |
| 20 | 88.5 ± 4.2 | 36.4 * ± 3.8 |
| Sample | γ [mN/m] |
|---|---|
| PDMS | 20.12 |
| PDMS-CHIT1 | 28.28 |
| PDMS-CHIT2 | 32.41 |
| PDMS-CHIT3 | 31.75 |
| PDMS-CHIT-FA1 | 34.12 |
| PDMS-CHIT-FA2 | 33.61 |
| PDMS-PCL14-10 | 28.50 |
| PDMS-PCL14-25 | 32.69 |
| PDMS-PCL80-10 | 28.16 |
| PDMS-PCL80-25 | 30.41 |
| Sample | v (rpm) | t (s) | σ [MPa] |
|---|---|---|---|
| non coated composite | 1500 | 10 | 0.768 ± 0.176 |
| PDMS | 1000 | 10 | 1.609 ± 0.345 |
| PDMS-CHIT1 | 1500 | 10 | 3.204 ± 0.266 |
| PDMS-CHIT2 | 2000 | 10 | 4.083 ± 0.308 |
| PDMS-CHIT3 | 2000 | 15 | 4.742 ± 0.197 |
| PDMS-CHIT-FA1 | 1500 | 20 | 3.367 ± 0.241 |
| PDMS-CHIT-FA2 | 2000 | 20 | 3.608 ± 0.248 |
| PDMS-PCL14-10 | 1000 | 15 | 4.639 ± 0.232 |
| PDMS-PCL14-25 | 1000 | 15 | 6.104 ± 0.387 |
| PDMS-PCL80-10 | 1500 | 10 | 3.508 ± 0.288 |
| PDMS-PCL80-25 | 1500 | 10 | 4.926 ± 0.265 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Powojska, A.; Mystkowski, A.; Gundabattini, E.; Mystkowska, J. Spin-Coating Fabrication Method of PDMS/NdFeB Composites Using Chitosan/PCL Coating. Materials 2024, 17, 1973. https://doi.org/10.3390/ma17091973
Powojska A, Mystkowski A, Gundabattini E, Mystkowska J. Spin-Coating Fabrication Method of PDMS/NdFeB Composites Using Chitosan/PCL Coating. Materials. 2024; 17(9):1973. https://doi.org/10.3390/ma17091973
Chicago/Turabian StylePowojska, Anna, Arkadiusz Mystkowski, Edison Gundabattini, and Joanna Mystkowska. 2024. "Spin-Coating Fabrication Method of PDMS/NdFeB Composites Using Chitosan/PCL Coating" Materials 17, no. 9: 1973. https://doi.org/10.3390/ma17091973







