Corrosion Study on Duplex Stainless Steel UNS S31803 Subjected to Solutions Containing Chloride Ions
Abstract
:1. Introduction
- Hydrogen reduction (in a deaerated acid medium)
- 2.
- Water reduction (in a neutral or basic deaerated medium)
- 3.
- Reduction of dissolved oxygen in an aerated acidic medium
- 4.
- Reduction of dissolved oxygen in an aerated neutral or basic medium
2. Materials and Methods
2.1. Material
2.2. Metallographic Characterization
2.3. Corrosion Test
2.4. Mechanical Properties
2.5. Determination of Uniform and Pitting Corrosion Rate
2.6. Polarization Test
3. Results
3.1. Microstructural Characterization in the As Received State
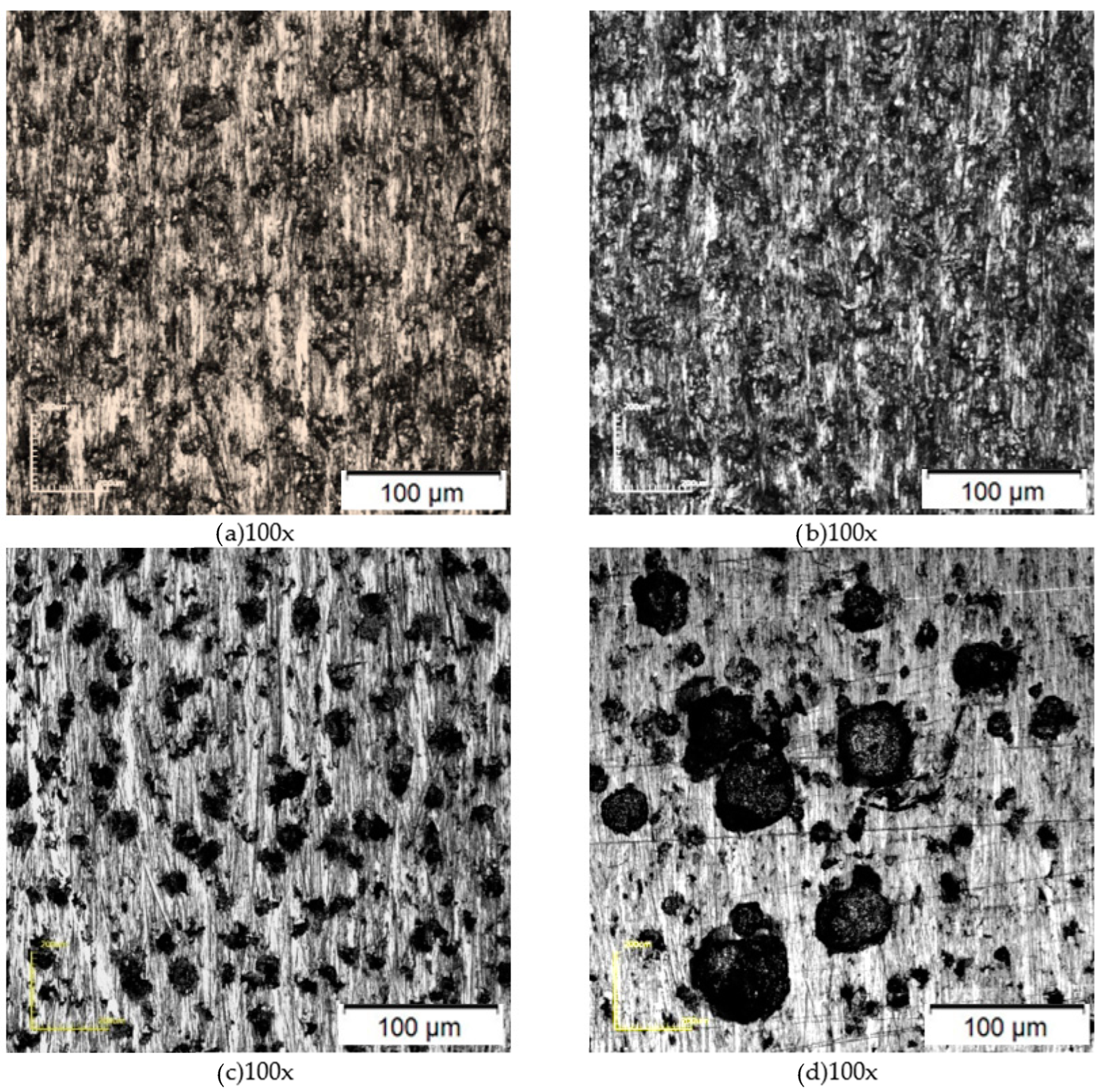
3.2. Characterization of Corrosion after Tests
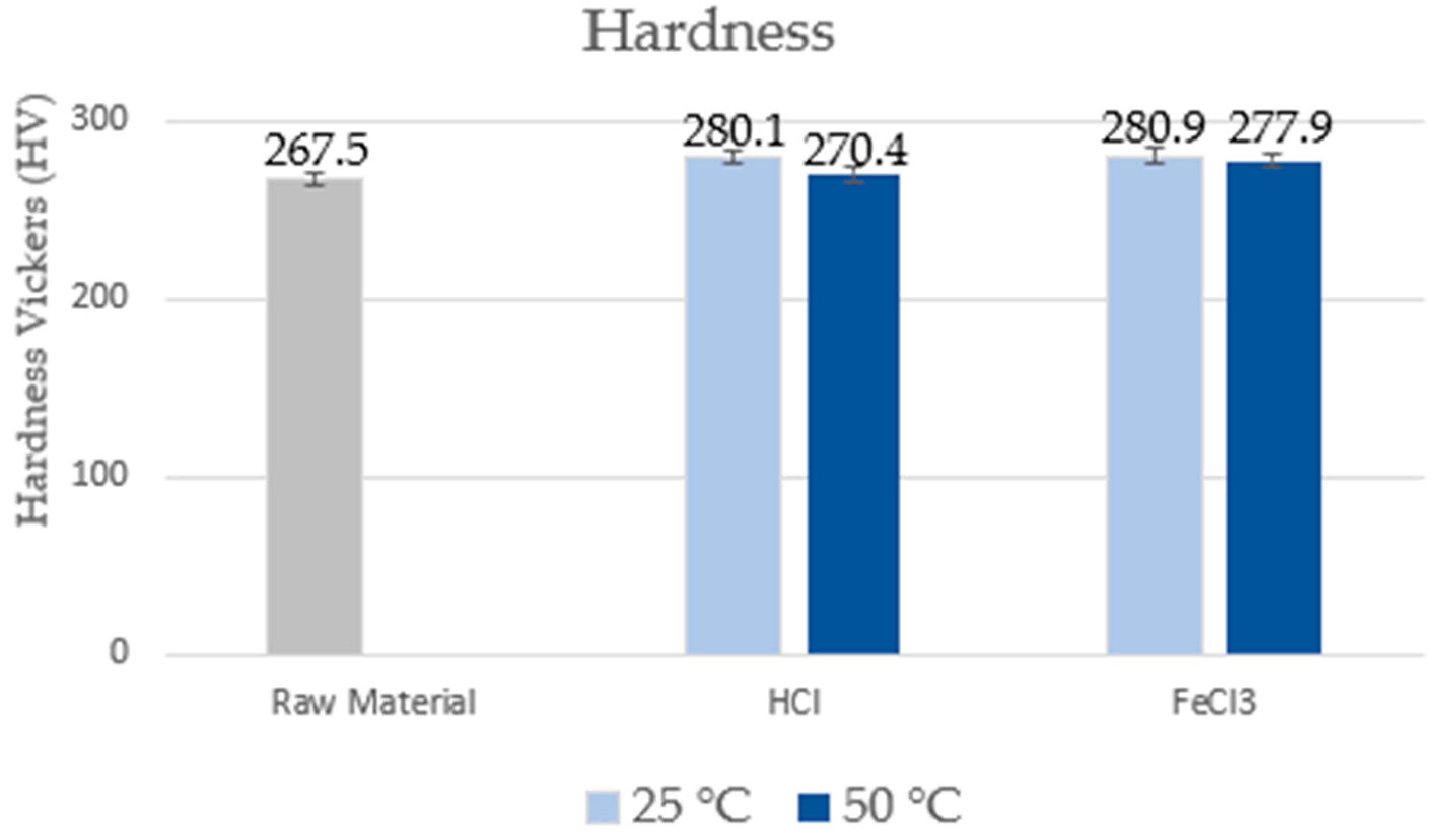
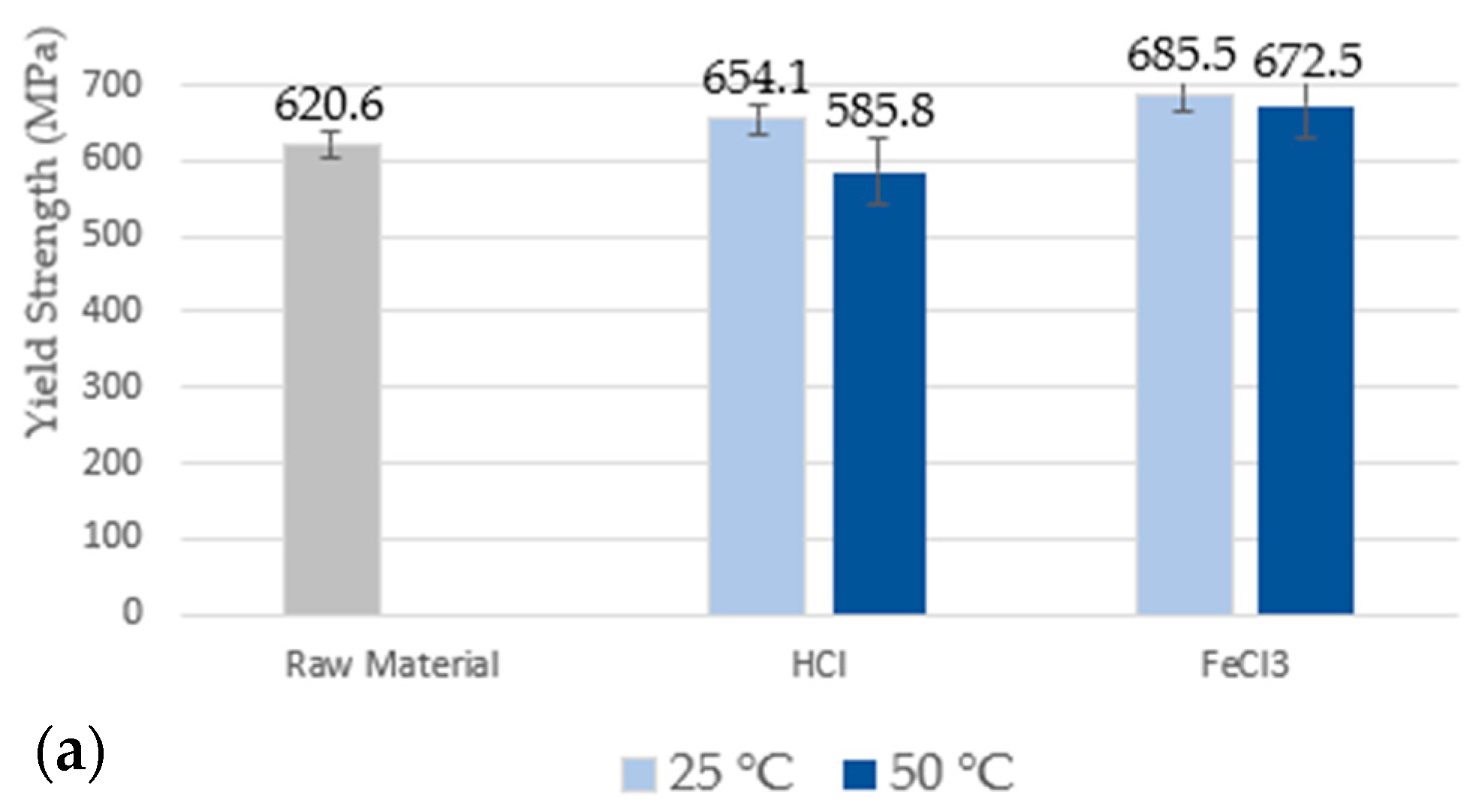
3.3. Mechanical Properties
3.4. Uniform Corrosion Rate
3.5. Pitting Corrosion Rate
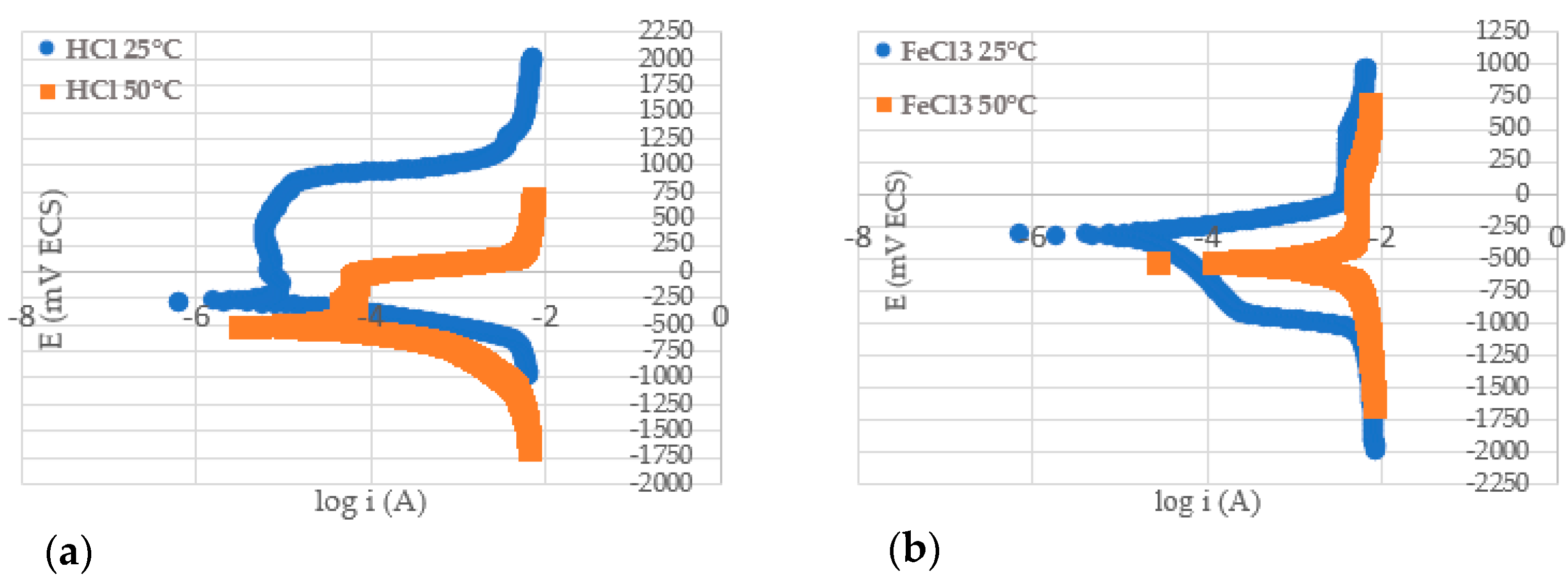
3.6. Polarization Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chiaverini, V. Steel and Cast Iron, 7th ed.; Associação Brasileira de Metals: Rio de Janeiro, Brazil, 2005; 599p. [Google Scholar]
- Gunn, R.N. Duplex Stainless Steels—Microstructure, Properties and Applications; Woodhead Publishing Ltd.: Cambridge, UK, 1997; 204p. [Google Scholar]
- Liu, H.; Xu, D.; Yang, K.; Liu, H.; Cheng, Y.F. Corrosion of antibacterial Cu-bearing 316L stainless steels in the presence of sulfate reducing bacteria. Corros. Sci. 2018, 132, 46–55. [Google Scholar] [CrossRef]
- Outokumpu. Handbook of Stainless Steel; Outokumpu: Helsinki, Finland, 2013. [Google Scholar]
- Morais, J.M. Oil in Deep Waters: A Technological History of Petrobras in Offshore Exploration and Production, 1st ed.; IPEA, Petrobras: Brasilia, Brazil, 2013; 424p. [Google Scholar]
- Sobolev, A.; Musin, A.; Whyman, G.; Borodianskiy, K.; Krichevski, O.; Kalashnikov, A.; Zinigrad, M. Stabilization of cubic phase in scandium-doped zirconia nanocrystals synthesized with sol-gel method. J. Am. Ceram. Soc. 2019, 102, 3236–3243. [Google Scholar] [CrossRef]
- Santos, F.P. Evaluation of the Effects of CO2 Partial Pressure on the Corrosion-Fatigue Process in Reinforcement and Tensile Strength of Flexible Pipes. Master’s Dissertation, Federal Center for Technological Education—Celso Suckow da Fonseca CEFET-RJ, Rio de Janeiro, RJ, Brazil, 2011; 121p. [Google Scholar]
- Li, D.G.; Wang, J.D.; Chen, D.R.; Liang, P. Influences of pH value, temperature, chloride ions and sulfide ions on the corrosion behaviors of 316L stainless steel in the simulated cathodic environment of proton exchange membrane fuel cell. J. Power Sources 2014, 272, 448–456. [Google Scholar] [CrossRef]
- Ribeiro, R.F. Evaluation of the Corrosion Resistance of a Duplex Stainless Steel Joint UNS S31803 Friction Welded with a Consumable Pin. Master’s Dissertation, Federal University of Rio Grande do Sul, Porto Alegre, Brazil, 2014; 91p. [Google Scholar]
- Bardal, E. Corrosion and Protection; The Norwegian University of Science and Technology: Trondheim, Norway, 2003; 315p. [Google Scholar]
- Gentil, V. Corrosion, 6th ed.; LTC: Rio de Janeiro, Brazil, 2011; 360p. [Google Scholar]
- Mccafferty, E. Introduction to Corrosion Science; Springer Science & Business Media: New York, NY, USA, 2010; 575p. [Google Scholar]
- Loureiro, J.P. Characterization of Duplex Stainless Steel UNS S31803 by the Non-Destructive Technique of Pulsed Eddy Currents. Bachelor’s Thesis, Metallurgical Engineering, Federal University of Rio de Janeiro—UFRJ, Rio de Janeiro, RJ, Brazil, 2010; 101p. [Google Scholar]
- Souza, D.D.B.G.; Vilarinho, L.O. Influence of present phases in corrosion and mechanical behavior of UNS S31803 duplex stainless steel welded by conventional short circuit MIG/MAG process. J. Mater. Res. Technol. 2020, 9, 11244–11254. [Google Scholar] [CrossRef]
- American Society for Testing Materials G31. Teste de Corrosão de Imersão de Metais. In Annual Book of ASTM; ASTM: West Conshohocken, PA, USA, 1999. [Google Scholar]
- American Society for Testing Materials G48-11. Standard Test Methods for Pitting and Crevice Corrosion Resistance of Stainless Steels and Related Alloys by Use of Ferric Chloride Solution. In Annual Book of ASTM; ASTM: West Conshohocken, PA, USA, 2020. [Google Scholar]
- Associação Brasileira de Normas Técnicas NBR 6892; Metallic Materials—Tensile Test Part 1: Test Method at Room Temperature. ISO: Geneva, Switzerland, 2018; 70p.
- NACE Standard RP0775-2005; Preparation, Installation, Analysis, and Interpretation of Corrosion Coupons in Oilfield Operations. Item N° 21017. NACE International: Houston, TX, USA, 2005; ISBN 1-57590-086-6.
- Associação Brasileira de Normas Técnicas NBR 6507-1; Metallic Materials—Vickers Hardness Test—Parte 1: Test Method. ISO: Geneva, Switzerland, 2019; 30p.
- Armas, I.A.; Moreuil, S.D. Duplex Stainless Steels; ISTE Ltd.: London, UK; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; 447p. [Google Scholar]
- Gonçalves, K.A.M.B.; Oliveira, T.R.; Alcântara, C.M.; Santos, D.B. Microstructure and mechanical properties of duplex stainless steels UNS S31803 and UNS S32304 after cold rolling and annealing. In ABM Week; ABM: São Paulo, Brazil, 2016; pp. 1–13. [Google Scholar]
- Tschiptschin, A.P.; Varela, L.B.; Pinedo, C.E.; Lic, X.Y.; Dong, H. Development and microstructure characterization of single and duplex nitriding of UNS S31803 duplex stainless steel. Surf. Coat. Technol. 2017, 327, 83–92. [Google Scholar] [CrossRef]
- Mendonça, C.S.P.; Ribeiro, V.A.S.; Silva, M.R.; Oliveira, V.D.; Rodrigues, C.A.; Melo, M.L.N.M.; Correa, E.O.E.; Silva, E.M. Influence of aging heat treatment at 850 °C in the microstructure, mechanical and magnetic properties of Duplex steel UNS S31803. Rev. Mater. 2013, 18, 1373–1381. [Google Scholar] [CrossRef]
- Wu, X.; Yang, Y.; Sun, Y.; Liu, Y.; Li, J.; Jiang, Y. The temperature-dependent pitting and repassivation behaviors of UNS S31803 duplex stainless steel in chloride solutions. J. Mater. Res. Technol. 2019, 149, 29–36. [Google Scholar] [CrossRef]
- Lacerda, J.C.; Cândido, L.C.; Godefroid, L.B. Corrosion behavior of UNS S31803 steel with changes in the volume fraction of ferrite and the presence of chromium nitride. Mater. Sci. Eng. A 2015, 648, 428–435. [Google Scholar] [CrossRef]
- Ortiz, N.; Curiel, F.F.; López, V.H.; Ruiz, A. Evaluation of the intergranular corrosion susceptibility of UNS S31803 duplex stainless steel with thermoelectric power measurements. Corros. Sci. 2013, 69, 236–244. [Google Scholar] [CrossRef]
- Roberge, P.R. Corrosion Engineering—Principles and Practice; The McGraw-Hill Companies: New York, NY, USA, 2008; 754p. [Google Scholar]
- Plieth, W. Electrochemistry for Materials Science, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2008; p. 433. [Google Scholar]
- Colli, M.N. Study of the Influence of Temperature on the Selective Corrosion of S31803 Steel in 1M HCl Solution. Bachelor’s Thesis, Materials Engineering, University Center of FEI—São Bernardo do Campos, São Paulo, SP, Brazil, 2012; 83p. [Google Scholar]
- Souza, E.C.; Rossitti, S.M.; Rollo, J.M. Influence of chloride ion concentration and temperature on the electrochemical properties of passive films formed on a superduplex stainless steel. Mater. Charact. 2010, 61, 240–244. [Google Scholar] [CrossRef]
- Li, Y.Z.; Wang, X.; Zhang, G.A. Corrosion behavior of 13Cr stainless steel under stress and crevice in 3.5 wt.% NaCl solution. Corros. Sci. 2020, 163, 108290. [Google Scholar] [CrossRef]
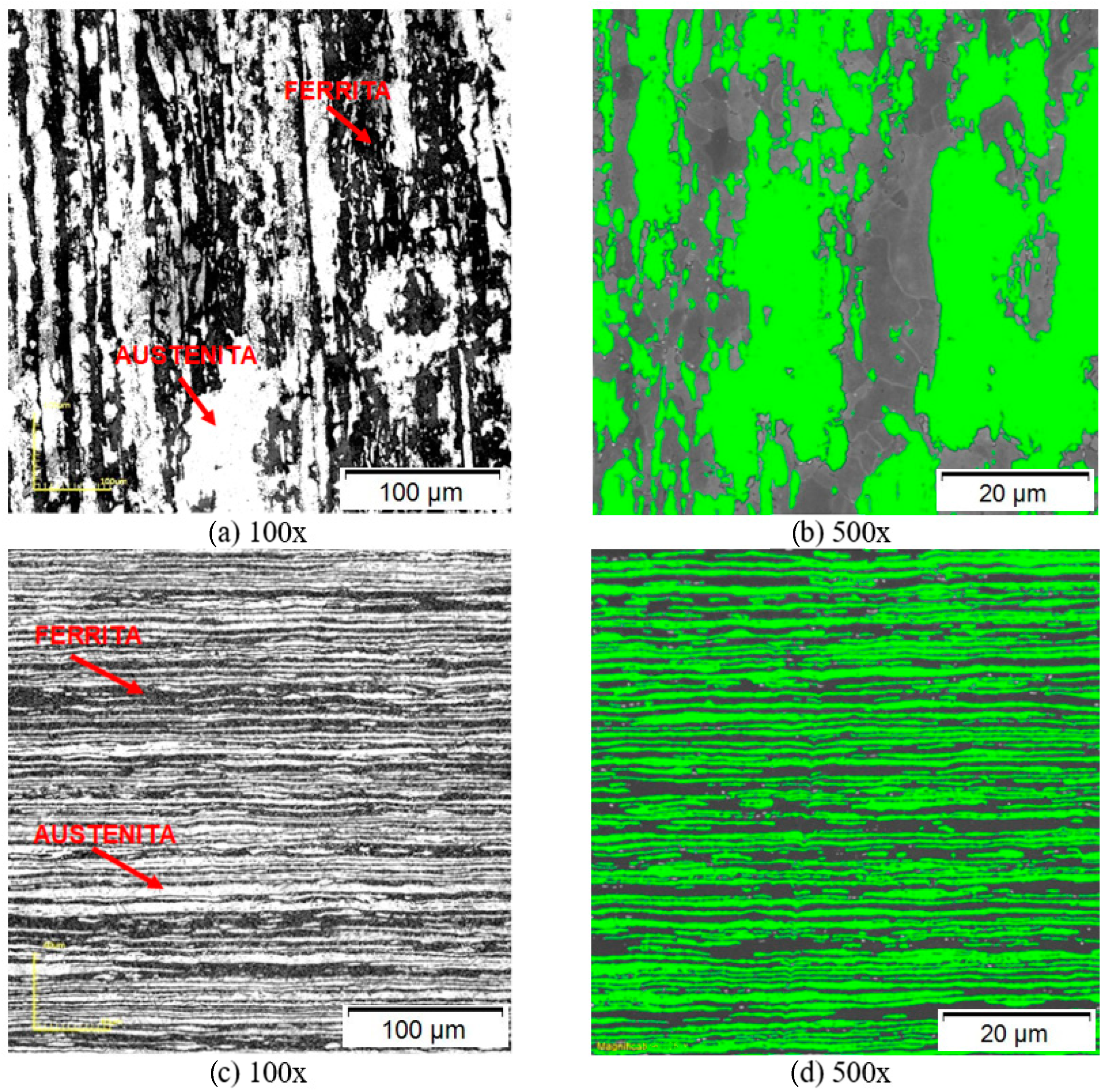




| Lamination | Normal | ||
|---|---|---|---|
| %Ferrite | %Austenite | %Ferrite | %Austenite |
| 46.2 ± 4.3 | 53.8 ± 4.3 | 56.1 ± 1.3 | 43.9 ± 1.3 |
| Solution/Temperature | Corrosion Rate |
|---|---|
| HCl 25 °C | 0.85 |
| HCl 50 °C | 0.92 |
| FeCl3 25 °C | 0.0006 |
| FeCl3 50 °C | 0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, L.M.; Pereira, E.; Amaral, T.B.d.S.; Monteiro, S.N.; de Azevedo, A.R.G. Corrosion Study on Duplex Stainless Steel UNS S31803 Subjected to Solutions Containing Chloride Ions. Materials 2024, 17, 1974. https://doi.org/10.3390/ma17091974
de Souza LM, Pereira E, Amaral TBdS, Monteiro SN, de Azevedo ARG. Corrosion Study on Duplex Stainless Steel UNS S31803 Subjected to Solutions Containing Chloride Ions. Materials. 2024; 17(9):1974. https://doi.org/10.3390/ma17091974
Chicago/Turabian Stylede Souza, Lucas Menezes, Elaine Pereira, Thiago Barreto da Silva Amaral, Sergio Neves Monteiro, and Afonso Rangel Garcez de Azevedo. 2024. "Corrosion Study on Duplex Stainless Steel UNS S31803 Subjected to Solutions Containing Chloride Ions" Materials 17, no. 9: 1974. https://doi.org/10.3390/ma17091974





