Biocompatibility of Different Nerve Tubes
Abstract
:1. Introduction
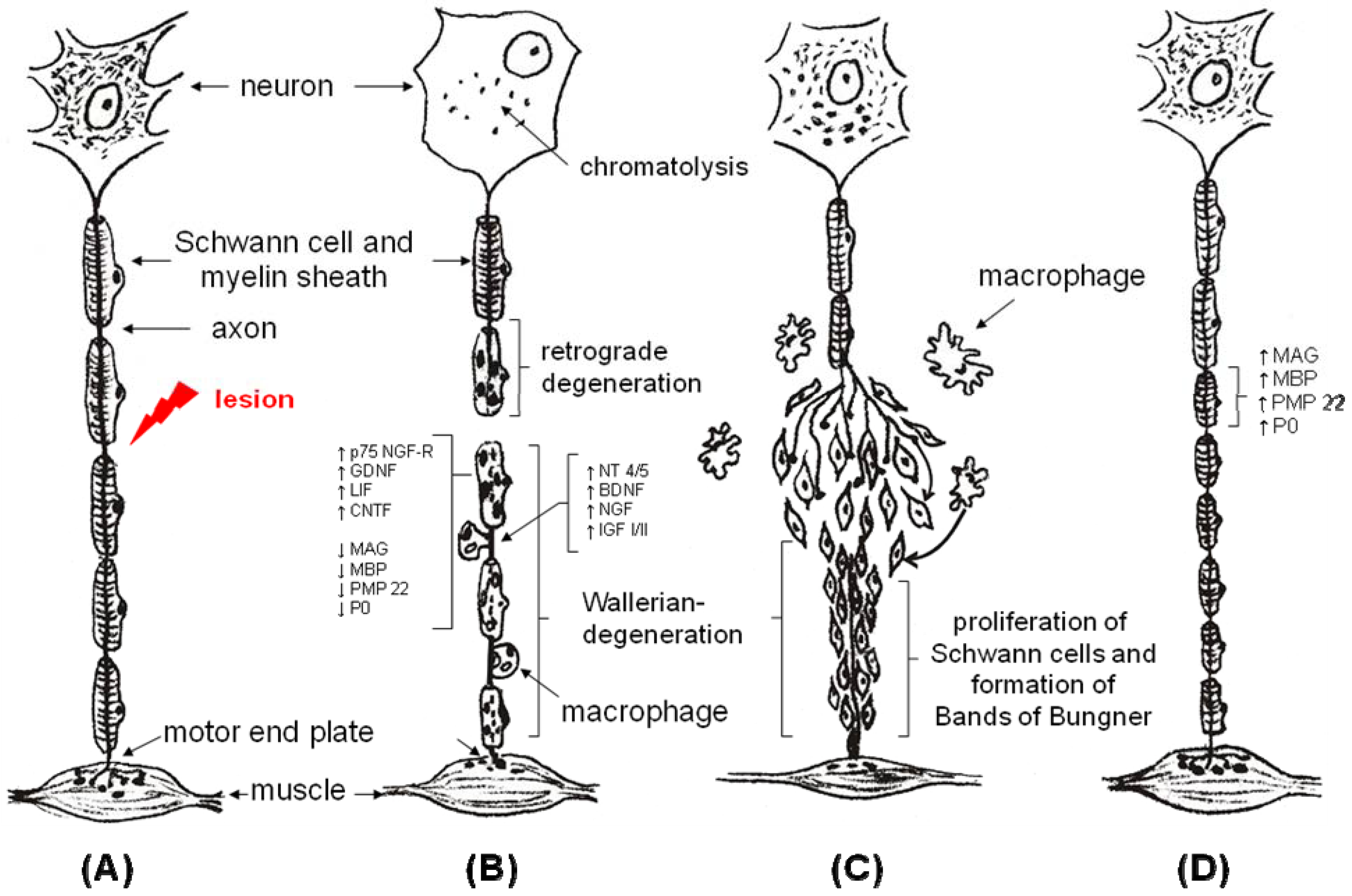
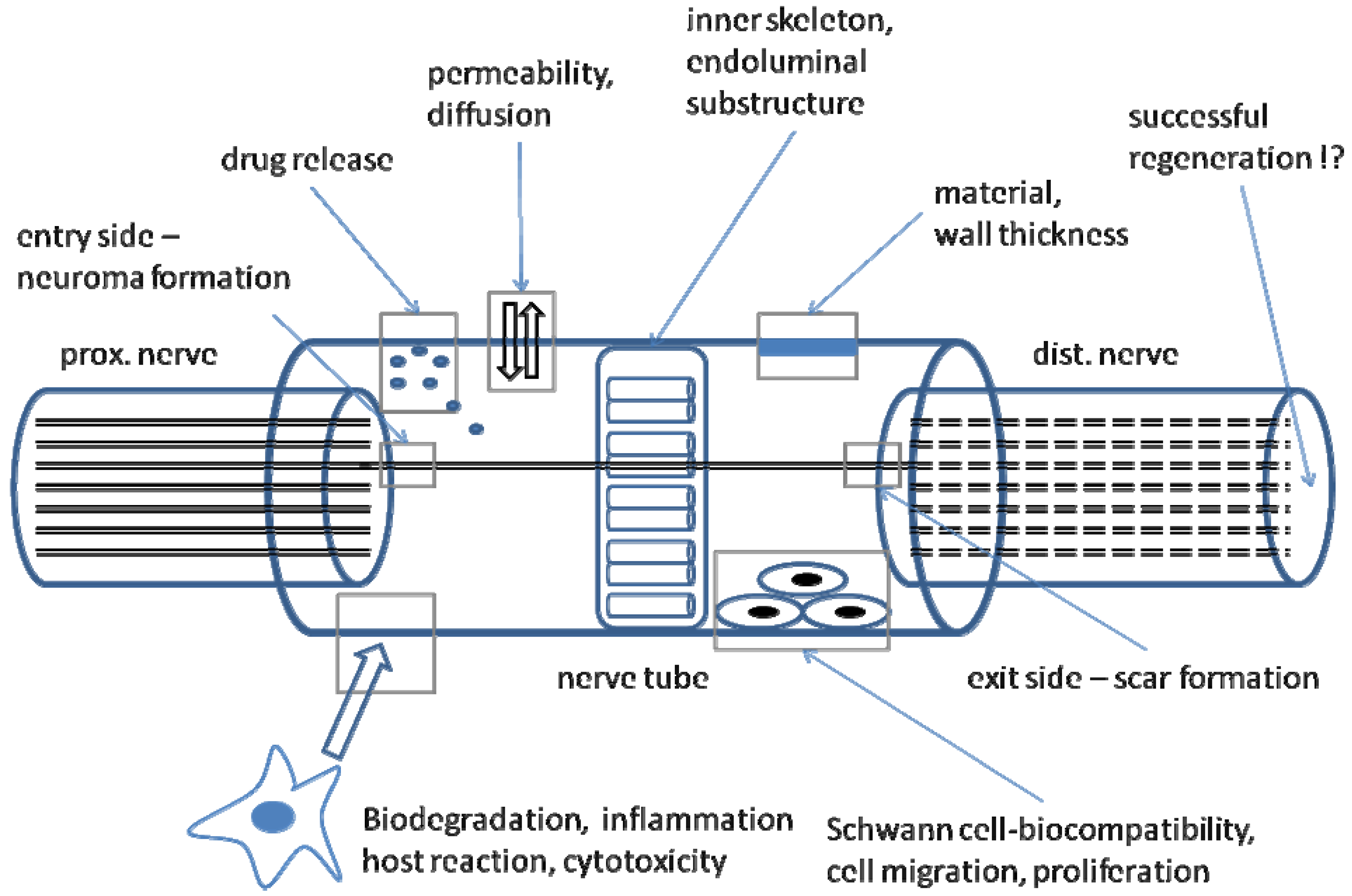
2. The Tube Concept
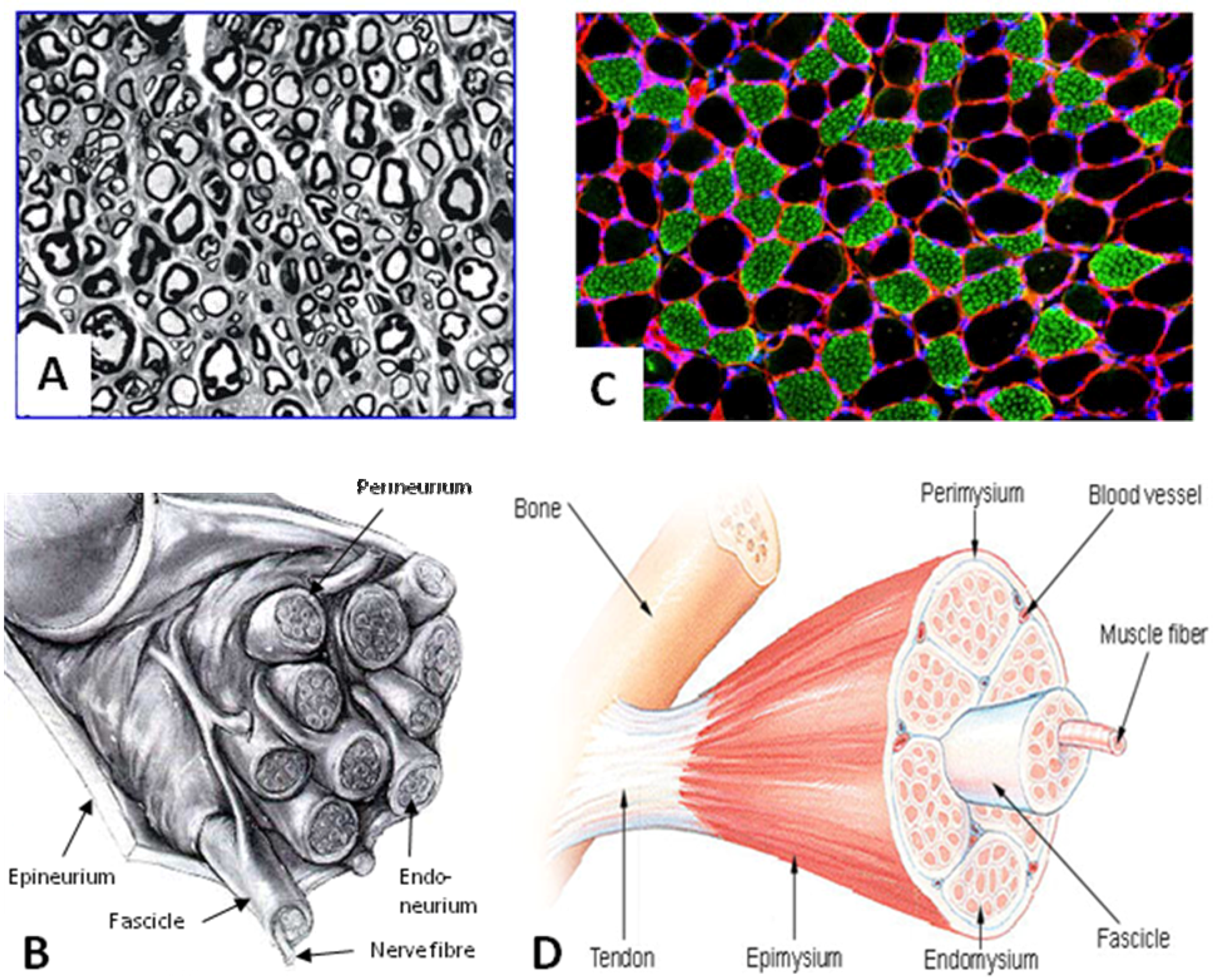
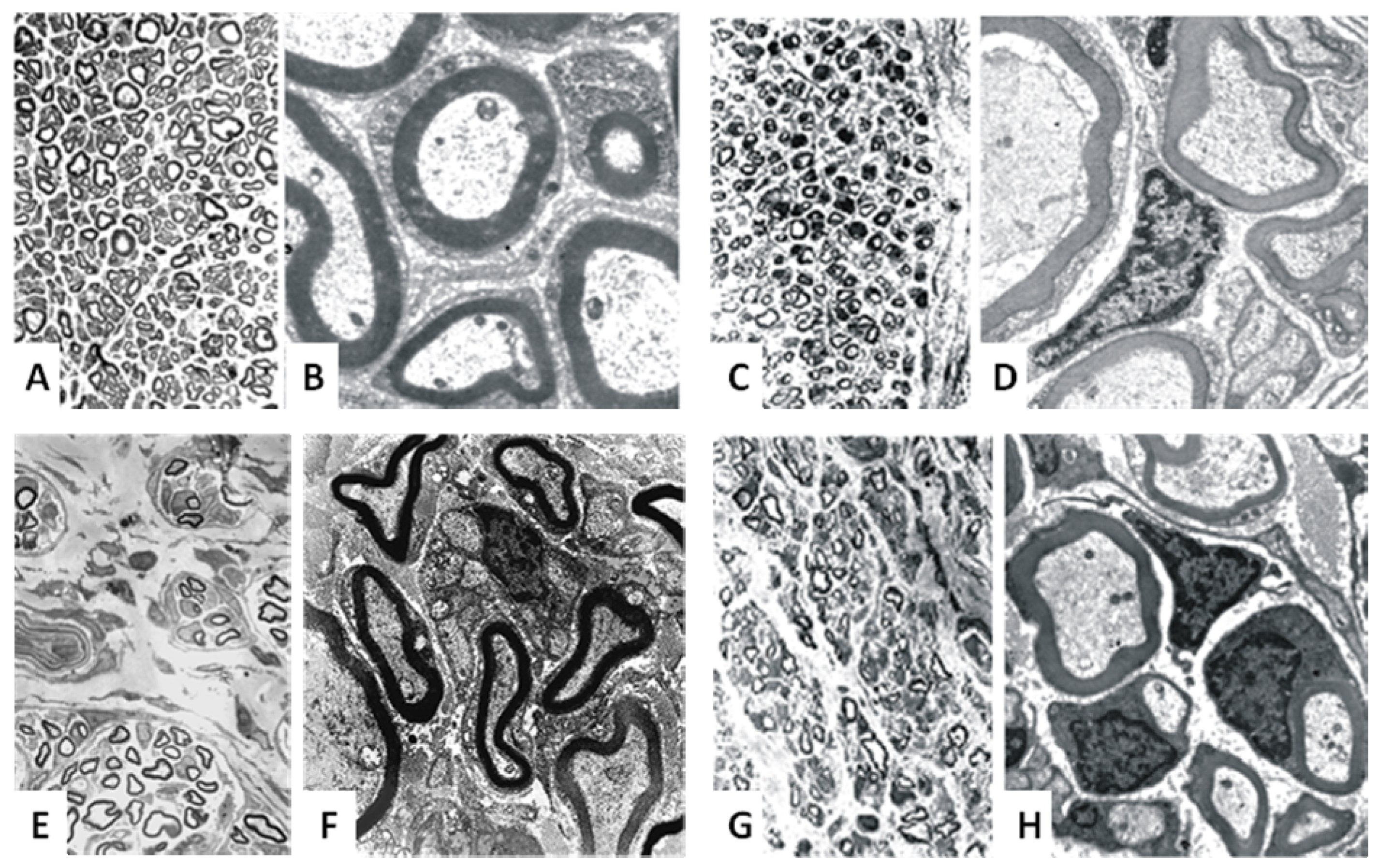
3. Biodegradability
4. Revascularization and Physical Properties
| Biological Nerve Grafts | Animal model (A) / Clinical trial (C) |
| A, C |
| A, C |
| A, C (NeuraGen®, Neuramatrix®) |
| A |
| A, C (AxoGen®) |
| Synthetic Guidance Channels | Animal model (A) / Clinical trial (C) |
| A, C (Neurolac®) |
| A, C (Neurotube®) |
| A |
| A |
| A,C |
| A |
5. Silicone Grafts
6. Acellular Muscle Grafts
7. Vein Grafts
8. Fibrin Grafts
9. Collagen Grafts
10. Glass Fibre Grafts
11. Polyglycolic Acid Grafts
12. Poly(D,L-lactide-ε-caprolactone) Grafts
13. Poly(2-hydroxyethylmethacrylate-co-methyl methacrylate) Grafts
14. Poly(3-hydroxybutyrate) Grafts
15. Acellular Nerve Allografts
16. Conclusions
References and Notes
- Ijpma, F.F.; Nicolai, J.P.; Meek, M.F. Sural nerve donor-site morbidity: Thirty-four years of follow-up. Ann. Plast. Surg. 2006, 57, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Fansa, H.; Keilhoff, G. Factors influencing nerve regeneration. Handchir. Mikrochir. Plast. Chir. 2003, 35, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.J.; Hess, J.R.; Myckatyn, T.M.; Hayashi, A.; Hunter, D.A.; Mackinnon, S.E. Repair of motor nerve gaps with sensory nerve inhibits regeneration in rats. Laryngoscope 2006, 116, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Moradzadeh, A.; Borschel, G.H.; Luciano, J.P.; Whitlock, E.L.; Hayashi, A.; Hunter, D.A.; Mackinnon, S.E. The impact of motor and sensory nerve architecture on nerve regeneration. Exp. Neurol. 2008, 212, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Terzis, J.K.; Sun, D.D.; Thanos, P.K. Historical and basic science review: Past, present, and future of nerve repair. J. Reconstr. Microsurg. 1997, 13, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Heath, C.A.; Rutkowski, G.E. The development of bioartificial nerve grafts for peripheral-nerve regeneration. Trends. Biotechnol. 1998, 16, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Schroder, J.M.; Seiffert, K.E. The fine structure of the neuromatous neurotization of nerve transplants. Virchows Arch. B Cell Pathol. 1970, 5, 219–235. [Google Scholar] [PubMed]
- De Ruiter, G.C.; Spinner, R.J.; Malessy, M.J.; Moore, M.J.; Sorenson, E.J.; Currier, B.L.; Yaszemski, M.J.; Windebank, A.J. Accuracy of motor axon regeneration across autograft, single-lumen, and multichannel poly(lactic-co-glycolic acid) nerve tubes. Neurosurgery 2008, 63, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Stang, F.; Fansa, H.; Wolf, G.; Reppin, M.; Keilhoff, G. Structural parameters of collagen nerve grafts influence peripheral nerve regeneration. Biomaterials 2005, 26, 3083–3091. [Google Scholar] [CrossRef] [PubMed]
- Lohmeyer, J.A.; Shen, Z.L.; Walter, G.F.; Berger, A. Bridging extended nerve defects with an artifcial nerve graft containing Schwann cells pre-seeded on polyglactin filaments. Int. J. Artif. Organs. 2007, 30, 64–74. [Google Scholar] [PubMed]
- Gunatillake, P.A.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur. Cell Mater. 2003, 20, 1–16. [Google Scholar]
- Pego, A.P.; Vleggeert-Lankamp, C.L.; Deenen, M.; Lakke, E.A.; Grijpma, D.W.; Poot, A.A.; Marani, E.; Feijen, J. Adhesion and growth of human Schwann cells on trimethylene carbonate (co)polymers. J. Biomed. Mater. Res. A 2003, 67, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, N.; Suzuki, Y.; Dezawa, M.; Kataoka, K.; Ohta, M.; Cho, H.; Ide, C. Peripheral nerve regeneration by transplantation of BMSC-derived Schwann cells as chitosan gel sponge scaffolds. J. Biomed. Mater. Res. A 2009, 89A, 1128–1124. [Google Scholar]
- Fansa, H.; Keilhoff, G.; Forster, G.; Seidel, B.; Wolf, G.; Schneider, W. Acellular muscle with Schwann-cell implantation: an alternative biologic nerve conduit. J. Reconstr. Microsurg. 1999, 15, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.R.; Brandt, K.; Katz, S.; Chauvin, P.; Otto, L.; Bogle, M.; Wang, B.; Meszlenyi, R.K.; Lu, L.; Mikos, A.G.; Patrick, C.W., Jr. Bioactive poly(L-lactic acid) conduits seeded with Schwann cells for peripheral nerve regeneration. Biomaterials 2002, 23, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Sinis, N.; Schaller, H.E.; Schulte-Eversum, C.; Schlosshauer, B.; Doser, M.; Dietz, K.; Rosner, H.; Muller, H.W.; Haerle, M. Nerve regeneration across a 2-cm gap in the rat median nerve using a resorbable nerve conduit filled with Schwann cells. J. Neurosurg. 2005, 103, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Keilhoff, G.; Fansa, H.; Smalla, K.H.; Schneider, W.; Wolf, G. Neuroma: A donor-age independent source of human Schwann cells for tissue engineered nerve grafts. Neuroreport 2000, 11, 3805–3809. [Google Scholar] [CrossRef] [PubMed]
- Gravvanis, A.I.; Lavdas, A.A.; Papalois, A.; Tsoutsos, D.A.; Matsas, R. The beneficial effect of genetically engineered Schwann cells with enhanced motility in peripheral nerve regeneration: review. Acta Neurochir. Suppl. 2007, 100, 51–56. [Google Scholar] [PubMed]
- Gravvanis, A.I.; Lavdas, A.; Papalois, A.E.; Franceschini, I.; Tsoutsos, D.A.; Dubois-Dalcq, M.; Matsas, R.; Ioannovich, J.D. Effect of genetically modified Schwann cells with increased motility in end-to-side nerve grafting. Microsurgery 2005, 25, 423–432. [Google Scholar] [CrossRef] [PubMed]
- May, F.; Matiasek, K.; Vroemen, M.; Caspers, C.; Mrva, T.; Arndt, C.; Schlenker, B.; Gais, P.; Brill, T.; Buchner, A.; Blesch, A.; Hartung, R.; Stief, C.; Gansbacher, B.; Weidner, N. GDNF-transduced Schwann cell grafts enhance regeneration of erectile nerves. Eur. Urol. 2008, 54, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Pfister, L.A.; Papaloizos, M.; Merkle, H.P.; Gander, B. Nerve conduits and growth factor delivery in peripheral nerve repair. J. Peripher. Nerv. Syst. 2007, 12, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Ward, M.; Liang, E.; Young, M.J.; Langer, R. Stimulation of neurite outgrowth by neurotrophins delivered from degradable hydrogels. Biomaterials 2006, 27, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Goraltchouk, A.; Scanga, V.; Morshead, C.M.; Shoichet, M.S. Incorporation of protein-eluting microspheres into biodegradable nerve guidance channels for controlled release. J. Control. Release 2006, 110, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Peng, J.; Guo, Q.; Zhang, L.; Li, Z.; Zhao, B.; Sui, X.; Wang, Y.; Xu, W.; Lu, S. Improvement of peripheral nerve regeneration in acellular nerve grafts with local release of nerve growth factor. Microsurgery 2009, 29, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Valmikinathan, C.M.; Defroda, S.; Yu, X. Polycaprolactone and bovine serum albumin based nanofibers for controlled release of nerve growth factor. Biomacromolecules 2009, 10, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Keilhoff, G.; Stang, F.; Wolf, G.; Fansa, H. Bio-compatibility of type I/III collagen matrix for peripheral nerve reconstruction. Biomaterials 2003, 24, 2779–2787. [Google Scholar] [CrossRef] [PubMed]
- Fansa, H.; Schneider, W.; Keilhoff, G. Revascularization of tissue-engineered nerve grafts and invasion of macrophages. Tissue Eng. 2001, 7, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H.; Tokiwa, Y. Confirmation of colonization of degrading bacterium strain SC-17 on poly(3-hydroxybutyrate) cast film. J. Environ. Polym. Degrad. 2009, 3, 187–197. [Google Scholar] [CrossRef]
- Vleggeert-Lankamp, C.L.; de Ruiter, G.C.; Wolfs, J.F.; Pego, A.P.; van den Berg, R.J.; Feirabend, H.K.; Malessy, M.J.; Lakke, E.A. Pores in synthetic nerve conduits are beneficial to regeneration. J. Biomed. Mater. Res. A 2007, 80, 965–982. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.J.; Gomez, N.; Perego, G.; Navarro, X. Highly permeable polylactide-caprolactone nerve guides enhance peripheral nerve regeneration through long gaps. Biomaterials 1999, 20, 1489–1500. [Google Scholar] [CrossRef] [PubMed]
- Pego, A.P.; Poot, A.A.; Grijpma, D.W.; Feijen, J. Copolymers of trimethylene carbonate and epsilon-caprolactone for porous nerve guides: synthesis and properties. J. Biomater. Sci. Polym. Ed. 2001, 12, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Uzman, B.G.; Villegas, G.M. Mouse sciatic nerve regeneration through semipermeable tubes: A quantitative model. J. Neurosci. Res. 1983, 9, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Jenq, C.B.; Coggeshall, R.E. Permeable tubes increase the length of the gap that regenerating axons can span. Brain Res. 1987, 408, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, A.N.; Fini, M.; Rocca, M.; Giavaresi, G.; Giardino, R. Guided regeneration with resorbable conduits in experimental peripheral nerve injuries. Int. Orthop. 2000, 24, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Meek, M.F.; Robinson, P.H.; Stokroos, I.; Blaauw, E.H.; Kors, G.; Den Dunnen, W.F. Electronmicroscopical evaluation of short-term nerve regeneration through a thin-walled biodegradable poly(DLLA-epsilon-CL) nerve guide filled with modified denatured muscle tissue. Biomaterials 2001, 22, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Holland, A.; Shanks, R. Poly(caprolactone) thin film preparation, morphology, and surface texture. J. Appl. Polym. Sci. 2007, 103, 1287–1294. [Google Scholar] [CrossRef]
- Meek, M.F.; Den Dunnen, W.F.; Schakenraad, J.M.; Robinson, P.H. Long-term evaluation of functional nerve recovery after reconstruction with a thin-walled biodegradable poly (DL-lactide-epsilon-caprolactone) nerve guide, using walking track analysis and electrostimulation tests. Microsurgery 1999, 19, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Walton, R.L.; Brown, R.E.; Matory, W.E., Jr.; Borah, G.L.; Dolph, J.L. Autogenous vein graft repair of digital nerve defects in the finger: A retrospective clinical study. Plast. Reconstr. Surg. 1989, 84, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.B. Vein conduits with interposition of nerve tissue for peripheral nerve defects. J. Reconstr. Microsurg. 1995, 11, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Fansa, H.; Keilhoff, G.; Wolf, G.; Schneider, W. Tissue engineering of peripheral nerves: A comparison of venous and acellular muscle grafts with cultured Schwann cells. Plast. Reconstr. Surg. 2001, 107, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Norris, R.W.; Glasby, M.A.; Gattuso, J.M.; Bowden, R.E. Peripheral nerve repair in humans using muscle autografts. A new technique. J. Bone Joint Surg. Br. 1988, 70, 530–533. [Google Scholar] [PubMed]
- Hall, S. Axonal regeneration through acellular muscle grafts. J. Anat. 1997, 190, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Keilhoff, G.; Pratsch, F.; Wolf, G.; Fansa, H. Bridging extra large defects of peripheral nerves: Possibilities and limitations of alternative biological grafts from acellular muscle and Schwann cells. Tissue Eng. 2005, 11, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Kalbermatten, D.F.; Pettersson, J.; Kingham, P.J.; Pierer, G.; Wiberg, M.; Terenghi, G. New fibrin conduit for peripheral nerve repair. J. Reconstr. Microsurg. 2009, 25, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, E.L.; Tuffaha, S.H.; Luciano, J.P.; Yan, Y.; Hunter, D.A.; Magill, C.K.; Moore, A.M.; Tong, A.Y.; Mackinnon, S.E.; Borschel, G.H. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve 2009, 39, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Farole, A.; Jamal, B.T. A bioabsorbable collagen nerve cuff (NeuraGen) for repair of lingual and inferior alveolar nerve injuries: A case series. J. Oral Maxillofac. Surg. 2008, 66, 2058–2062. [Google Scholar] [CrossRef] [PubMed]
- Allmeling, C.; Jokuszies, A.; Reimers, K.; Kall, S.; Choi, C.Y.; Brandes, G.; Kasper, C.; Scheper, T.; Guggenheim, M.; Vogt, P.M. Spider silk fibres in artificial nerve constructs promote peripheral nerve regeneration. Cell Prolif. 2008, 41, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.A.; Breidenbach, W.C.; Brown, R.E.; Jabaley, M.E.; Mass, D.P. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast. Reconstr. Surg. 2000, 106, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Inada, Y.; Morimoto, S.; Takakura, Y.; Nakamura, T. Regeneration of peripheral nerve gaps with a polyglycolic acid-collagen tube. Neurosurgery 2004, 55, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Inada, Y.; Hosoi, H.; Yamashita, A.; Morimoto, S.; Tatsumi, H.; Notazawa, S.; Kanemaru, S.; Nakamura, T. Regeneration of peripheral motor nerve gaps with a polyglycolic acid-collagen tube: technical case report. Neurosurgery 2007, 61, E1105–E1107. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Inada, Y.; Fukuda, S.; Yoshitani, M.; Nakada, A.; Itoi, S.; Kanemaru, S.; Endo, K.; Shimizu, Y. Experimental study on the regeneration of peripheral nerve gaps through a polyglycolic acid-collagen (PGA-collagen) tube. Brain Res. 2004, 1027, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.R.; Brandt, K.; Niederbichler, A.D.; Chauvin, P.; Herrman, S.; Bogle, M.; Otta, L.; Wang, B.; Patrick, C.W., Jr. Clinical long-term in vivo evaluation of poly(L-lactic acid) porous conduits for peripheral nerve regeneration. J. Biomater. Sci. Polym. Ed. 2000, 11, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.R.; Brandt, K.; Widmer, M.S.; Lu, L.; Meszlenyi, R.K.; Gupta, P.K.; Mikos, A.G.; Hodges, J.; Williams, J.; Gurlek, A.; Nabawi, A.; Lohman, R.; Patrick, C.W., Jr. In vivo evaluation of poly(L-lactic acid) porous conduits for peripheral nerve regeneration. Biomaterials 1999, 20, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Montenegro, R.; Freier, T.; Midha, R.; Belkas, J.S.; Shoichet, M.S. Coil-reinforced hydrogel tubes promote nerve regeneration equivalent to that of nerve autografts. Biomaterials 2006, 27, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Dalton, P.D.; Flynn, L.; Shoichet, M.S. Manufacture of poly(2-hydroxyethyl methacrylate-co-methyl methacrylate) hydrogel tubes for use as nerve guidance channels. Biomaterials 2002, 23, 3843–3851. [Google Scholar] [CrossRef] [PubMed]
- Kalbermatten, D.F.; Kingham, P.J.; Mahay, D.; Mantovani, C.; Pettersson, J.; Raffoul, W.; Balcin, H.; Pierer, G.; Terenghi, G. Fibrin matrix for suspension of regenerative cells in an artificial nerve conduit. J. Plast. Reconstr. Aesthet. Surg. 2008, 61, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Lee, S.K.; Lee, J.H. Peripheral nerve regeneration using a three dimensionally cultured schwann cell conduit. J. Craniofac. Surg. 2007, 18, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Xing, D.; Peng, Z.; Rao, T. Enhanced rat sciatic nerve regeneration through silicon tubes implanted with valproic acid. J. Reconstr. Microsurg. 2008, 24, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, G. The tube concept in nerve repair. Tech. Hand Up Extrem. Surg. 1997, 1, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, L.B.; Anagnostaki, L.; Lundborg, G. Tissue response to silicone tubes used to repair human median and ulnar nerves. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2001, 35, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, G.; Rosen, B.; Dahlin, L.; Holmberg, J.; Rosen, I. Tubular repair of the median or ulnar nerve in the human forearm: A 5-year follow-up. J. Hand Surg. Br. 2004, 29, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.D.; Ellisman, M.H. Axons regenerated through silicone tube splices. I. Conduction properties. Exp. Neurol. 1986, 92, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Hsieh, C.L.; Tsai, C.C.; Chen, T.H.; Cheng, W.C.; Hu, C.L.; Yao, C.H. Peripheral nerve regeneration using silicone rubber chambers filled with collagen, laminin and fibronectin. Biomaterials 2000, 21, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Bunting, S.; Di, S.L.; Deb, S.; Hall, S. Bioresorbable glass fibres facilitate peripheral nerve regeneration. J. Hand Surg. Br. 2005, 30, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Jeans, L.; Healy, D.; Gilchrist, T. An evaluation using techniques to assess muscle and nerve regeneration of a flexible glass wrap in the repair of peripheral nerves. Acta Neurochir. Suppl. 2007, 100, 25–28. [Google Scholar] [PubMed]
- Gilchrist, T.; Glasby, M.A.; Healy, D.M.; Kelly, G.; Lenihan, D.V.; McDowall, K.L.; Miller, I.A.; Myles, L.M. In vitro nerve repair--in vivo. The reconstruction of peripheral nerves by entubulation with biodegradeable glass tubes--a preliminary report. Br. J. Plast. Surg. 1998, 51, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Meek, M.F.; Coert, J.H. US Food and Drug Administration/Conformit Europe- approved absorbable nerve conduits for clinical repair of peripheral and cranial nerves. Ann. Plast. Surg. 2008, 60, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Merle, M.; Dellon, A.L.; Campbell, J.N.; Chang, P.S. Complications from silicon-polymer intubulation of nerves. Microsurgery 1989, 10, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Braga-Silva, J. The use of silicone tubing in the late repair of the median and ulnar nerves in the forearm. J. Hand Surg. Br. 1999, 24, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, G.; Dahlin, L.B.; Danielsen, N. Ulnar nerve repair by the silicone chamber technique. Case report. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1991, 25, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, G.; Rosen, B.; Abrahamson, S.O.; Dahlin, L.; Danielsen, N. Tubular repair of the median nerve in the human forearm. Preliminary findings. J. Hand Surg. Br. 1994, 19, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, L.J.; Yannas, I.V.; Arrizabalaga, A.; Hsu, H.P.; Norregaard, T.V.; Spector, M. Early peripheral nerve healing in collagen and silicone tube implants: Myofibroblasts and the cellular response. Biomaterials 1998, 19, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Fansa, H.; Keilhoff, G.; Plogmeier, K.; Frerichs, O.; Wolf, G.; Schneider, W. Successful implantation of Schwann cells in acellular muscles. J. Reconstr. Microsurg. 1999, 15, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Fansa, H.; Schneider, W.; Wolf, G.; Keilhoff, G. Host responses after acellular muscle basal lamina allografting used as a matrix for tissue engineered nerve grafts1. Transplantation 2002, 74, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Frerichs, O.; Fansa, H.; Schicht, C.; Wolf, G.; Schneider, W.; Keilhoff, G. Reconstruction of peripheral nerves using acellular nerve grafts with implanted cultured Schwann cells. Microsurgery 2002, 22, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Fansa, H.; Keilhoff, G. Comparison of different biogenic matrices seeded with cultured Schwann cells for bridging peripheral nerve defects. Neurol. Res. 2004, 26, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Keilhoff, G.; Stang, F.; Goihl, A.; Wolf, G.; Fansa, H. Transdifferentiated mesenchymal stem cells as alternative therapy in supporting nerve regeneration and myelination. Cell Mol. Neurobiol. 2006, 26, 1235–1252. [Google Scholar] [CrossRef] [PubMed]
- Keilhoff, G.; Goihl, A.; Stang, F.; Wolf, G.; Fansa, H. Peripheral nerve tissue engineering: Autologous Schwann cells vs. transdifferentiated mesenchymal stem cells. Tissue Eng. 2006, 12, 1451–1465. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.H.; Bowden, R.E.; Gattuso, J.M.; Norris, R.W. Comparison of results of repair of digital nerves by denatured muscle grafts and end-to-end sutures. J. Hand Surg. Br. 1991, 16, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.H.; Palande, D.D.; Subramanian, A.; Narayanakumar, T.S.; Curtis, J.; Turk, J.L. Denatured autologous muscle graft in leprosy. Lancet 1991, 338, 1239–1240. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.H.; Bowden, R.E.; Narayanakumar, T.S.; Gschmeissner, S.E. Peripheral nerve reconstruction using denatured muscle autografts for restoring protective sensation in hands and feet of leprosy patients. Indian J. Lepr. 1996, 68, 83–91. [Google Scholar] [PubMed]
- Stang, F. Tissue engineering alternativer Nerventransplantate aus Kollagenröhrchen und kultivierten Schwann-Zellen für die Rekonstruktion peripherer Nerven. Dissertation, Otto-von-Guericke Universität Magdeburg, Magdeburg, 2006. [Google Scholar]
- Informationsdienst Wissenschaft, 2009. http://idw-online.de/pages/de/newsimage?id=79033&size=thumbnail (accessed 30 September 2009).
- SEER Training Modules, U.S. National Institutes of Health, National Cancer Institute, 2009. http://training.seer.cancer.gov/anatomy/muscular/structure.html (accessed 30 September 2009).
- Wrede, L. Ueberbrueckung eines Nervendefektes mittels Seidennaht und lebendem Venenstueckes. Dtsch. Med. Wochenschr. 1909, 35, 1125–1230. [Google Scholar]
- Chang, J.; Jones, N. Twelve simple maneuvers to optimize digital replantation and revascularization. Tech. Hand Up. Extrem. Surg. 2004, 8, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Albala, D.M.; Lawson, J.H. Recent clinical and investigational applications of fibrin sealant in selected surgical specialties. J. Am. Coll. Surg. 2006, 202, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Ornelas, L.; Padilla, L.; Di, S.M.; Schalch, P.; Esperante, S.; Infante, R.L.; Bustamante, J.C.; Avalos, P.; Varela, D.; Lopez, M. Fibrin glue: An alternative technique for nerve coaptation—Part II. Nerve regeneration and histomorphometric assessment. J. Reconstr. Microsurg. 2006, 22, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Menovsky, T.; Beek, J.F. Laser, fibrin glue, or suture repair of peripheral nerves: A comparative functional, histological, and morphometric study in the rat sciatic nerve. J. Neurosurg. 2001, 95, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Laidmae, I.; McCormick, M.E.; Herod, J.L.; Pastore, J.J.; Salum, T.; Sawyer, E.S.; Janmey, P.A.; Uibo, R. Stability, sterility, coagulation, and immunologic studies of salmon coagulation proteins with potential use for mammalian wound healing and cell engineering. Biomaterials 2006, 27, 5771–5779. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.E.; Janmey, P.A.; McCormick, M.E.; Sawyer, E.S.; Flanagan, L.A. Enhanced neurite growth from mammalian neurons in three-dimensional salmon fibrin gels. Biomaterials 2007, 28, 2097–2108. [Google Scholar] [CrossRef] [PubMed]
- Bunge, M.B.; Bunge, R.P.; Kleitman, N.; Dean, A.C. Role of peripheral nerve extracellular matrix in Schwann cell function and in neurite regeneration. Dev. Neurosci. 1989, 11, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Thumann, G.; Viethen, A.; Gaebler, A.; Walter, P.; Kaempf, S.; Johnen, S.; Salz, A.K. The in vitro and in vivo behaviour of retinal pigment epithelial cells cultured on ultrathin collagen membranes. Biomaterials 2009, 30, 287–294. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, H.E. Biological Matrices and Tissue Reconstruction; Stark, G.B., Horch, R., Tanoszos, E., Eds.; Springer: Heidelberg, Germany, 1998; p. 237. [Google Scholar]
- Kemp, S.W.; Syed, S.; Walsh, S.K.; Zochodne, D.W.; Midha, R. Collagen nerve conduits promote enhanced axonal regeneration, schwann cell association, and neovascularization compared to silicone conduits. Tissue Eng. Part A 2009, 15, 1975–1988. [Google Scholar] [CrossRef] [PubMed]
- Harley, B.A.; Spilker, M.H.; Wu, J.W.; Asano, K.; Hsu, H.P.; Spector, M.; Yannas, I.V. Optimal degradation rate for collagen chambers used for regeneration of peripheral nerves over long gaps. Cells Tissues. Organs 2004, 176, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Takakuda, K.; Kawabata, S.; Aso, Y.; Kasai, K.; Itoh, H.; Shinomiya, K. Evaluation of cross-linking procedures of collagen tubes used in peripheral nerve repair. Biomaterials 2002, 23, 4475–4481. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Hui, T.Y.; Chan, O.C.; So, K.F.; Lu, W.; Cheung, K.M.; Salomatina, E.; Yaroslavsky, A. Photochemical cross-linking for collagen-based scaffolds: A study on optical properties, mechanical properties, stability, and hematocompatibility. Tissue Eng. 2007, 13, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Mundy, G.R.; DeMartino, S.; Rowe, D.W. Collagen and collagen-derived fragments are chemotactic for tumor cells. J. Clin. Invest. 1981, 68, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.L.; Dai, J.; van Golen, K.L.; Keller, E.T.; Long, M.W. Type I collagen receptor (alpha 2 beta 1) signaling promotes the growth of human prostate cancer cells within the bone. Cancer Res. 2006, 66, 8648–8654. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Taira, T.; Noishiki, Y. Collagen engineering for biomaterial use. Clin. Mater. 1992, 9, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, A.; Brook, G.A.; Moellers, S.; Lassner, F.; Sellhaus, B.; Weis, J.; Woeltje, M.; Tank, J.; Beckmann, C.; Fuchs, P.; Damink, L.O.; Schugner, F.; Heschel, I.; Pallua, N. In vitro assessment of axonal growth using dorsal root ganglia explants in a novel three-dimensional collagen matrix. Tissue Eng. 2007, 13, 2971–2979. [Google Scholar] [CrossRef] [PubMed]
- Alluin, O.; Wittmann, C.; Marqueste, T.; Chabas, J.F.; Garcia, S.; Lavaut, M.N.; Guinard, D.; Feron, F.; Decherchi, P. Functional recovery after peripheral nerve injury and implantation of a collagen guide. Biomaterials 2009, 30, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Archibald, S.J.; Shefner, J.; Krarup, C.; Madison, R.D. Monkey median nerve repaired by nerve graft or collagen nerve guide tube. J. Neurosci. 1995, 15, 4109–4123. [Google Scholar] [PubMed]
- Yoshii, S.; Oka, M.; Shima, M.; Taniguchi, A.; Akagi, M. 30 mm regeneration of rat sciatic nerve along collagen filaments. Brain Res. 2002, 949, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Taras, J.S.; Nanavati, V.; Steelman, P. Nerve conduits. J. Hand Ther. 2005, 18, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Ashley, W.W., Jr.; Weatherly, T.; Park, T.S. Collagen nerve guides for surgical repair of brachial plexus birth injury. J. Neurosurg. 2006, 105, 452–456. [Google Scholar] [PubMed]
- Lohmeyer, J.A.; Siemers, F.; Machens, H.G.; Mailander, P. The clinical use of artificial nerve conduits for digital nerve repair: A prospective cohort study and literature review. J. Reconstr. Microsurg. 2009, 25, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Farole, A.; Jamal, B.T. A bioabsorbable collagen nerve cuff (NeuraGen) for repair of lingual and inferior alveolar nerve injuries: A case series. J. Oral Maxillofac. Surg. 2008, 66, 2058–2062. [Google Scholar] [CrossRef] [PubMed]
- Vainionpaa, S.; Rokkanen, P.; Tormala, P. Surgical applications of biodegradable polymers in human tissues. Prog. Polym. Sci. 1989, 14, 679–716. [Google Scholar] [CrossRef]
- Schlosshauer, B.; Dreesmann, L.; Schaller, H.E.; Sinis, N. Synthetic nerve guide implants in humans: a comprehensive survey. Neurosurgery 2006, 59, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Keeley, R.D.; Nguyen, K.D.; Stephanides, M.J.; Padilla, J.; Rosen, J.M. The artificial nerve graft: A comparison of blended elastomer-hydrogel with polyglycolic acid conduits. J. Reconstr. Microsurg. 1991, 7, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Merrell, J.C.; Russell, R.C.; Zook, E.G. Polyglycolic acid tubing as a conduit for nerve regeneration. Ann. Plast. Surg. 1986, 17, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C.M. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.M.; Hentz, V.R.; Kaplan, E.N. Fascicular tubulization: A cellular approach to peripheral nerve repair. Ann. Plast. Surg. 1983, 11, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Waitayawinyu, T.; Parisi, D.M.; Miller, B.; Luria, S.; Morton, H.J.; Chin, S.H.; Trumble, T.E. A comparison of polyglycolic acid versus type 1 collagen bioabsorbable nerve conduits in a rat model: an alternative to autografting. J. Hand Surg. Am. 2007, 32, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, S.E.; Dellon, A.L. Clinical nerve reconstruction with a bioabsorbable polyglycolic acid tube. Plast. Reconstr. Surg. 1990, 85, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Crawley, W.A.; Dellon, A.L. Inferior alveolar nerve reconstruction with a polyglycolic acid bioabsorbable nerve conduit. Plast. Reconstr. Surg. 1992, 90, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Dellon, A.L. Reconstruction of a painful post-traumatic medial plantar neuroma with a bioabsorbable nerve conduit: A case report. J. Foot Ankle Surg. 2001, 40, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Navissano, M.; Malan, F.; Carnino, R.; Battiston, B. Neurotube for facial nerve repair. Microsurgery 2005, 25, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Ducic, I.; Maloney, C.T., Jr.; Dellon, A.L. Reconstruction of the spinal accessory nerve with autograft or neurotube? Two case reports. J. Reconstr. Microsurg. 2005, 21, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Donoghoe, N.; Rosson, G.D.; Dellon, A.L. Reconstruction of the human median nerve in the forearm with the Neurotube. Microsurgery 2007, 27, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Rosson, G.D.; Williams, E.H.; Dellon, A.L. Motor nerve regeneration across a conduit. Microsurgery 2009, 29, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Seckel, B.R.; Chiu, T.H.; Nyilas, E.; Sidman, R.L. Nerve regeneration through synthetic biodegradable nerve guides: Regulation by the target organ. Plast. Reconstr. Surg. 1984, 74, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.C.; Huang, Y.T.; Lin, J.H.; Yao, C.H.; Lou, C.W.; Tsai, C.C.; Chen, Y.S. Evaluation of a multi-layer microbraided polylactic acid fiber-reinforced conduit for peripheral nerve regeneration. J. Mater. Sci. Mater. Med. 2009, 20, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Den Dunnen, W.F.; Stokroos, I.; Blaauw, E.H.; Holwerda, A.; Pennings, A.J.; Robinson, P.H.; Schakenraad, J.M. Light-microscopic and electron-microscopic evaluation of short-term nerve regeneration using a biodegradable poly(DL-lactide-epsilon-caprolacton) nerve guide. J Biomed. Mater. Res. 1996, 31, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Bertleff, M.J.; Meek, M.F.; Nicolai, J.P. A prospective clinical evaluation of biodegradable neurolac nerve guides for sensory nerve repair in the hand. J. Hand Surg. Am. 2005, 30, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Meek, M.F.; Bertleff, M.J.; Ritt, M.J.; Robinson, P.H.; Nicolai, J.P. A degradable artificial nerve guide to bridge peripheral nerve defects. Ned. Tijdschr. Geneeskd. 2003, 147, 717–721. [Google Scholar] [PubMed]
- Meek, M.F.; Jansen, K. Two years after in vivo implantation of poly(DL-lactide-epsilon-caprolactone) nerve guides: Has the material finally resorbed? J. Biomed. Mater. Res. A 2008, 89A, 734–738. [Google Scholar]
- Meek, M.F. More than just sunshine with implantation of resorbable (p(DLLA-epsilon-CL)) biomaterials. Biomed. Mater. Eng. 2007, 17, 329–334. [Google Scholar] [PubMed]
- Meek, M.; Den Dunnen, W.; Bartels, H.; Robinson, P.; Schakenraad, J.; Pennings, A. Peripheral nerve regeneration and functional nerve recovery after reconstruction with a thin-walled biodegradable poly (DL-lactide-e-caprolactone) nerve guide. Cell. Mater. 1997, 7, 53–62. [Google Scholar]
- Meek, M.F.; Den Dunnen, W.F. Porosity of the wall of a Neurolac(R) nerve conduit hampers nerve regeneration. Microsurgery 2009, 29, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Belkas, J.S.; Munro, C.A.; Shoichet, M.S.; Midha, R. Peripheral nerve regeneration through a synthetic hydrogel nerve tube. Restor. Neurol. Neurosci. 2005, 23, 19–29. [Google Scholar] [PubMed]
- Belkas, J.S.; Munro, C.A.; Shoichet, M.S.; Johnston, M.; Midha, R. Long-term in vivo biomechanical properties and biocompatibility of poly(2-hydroxyethyl methacrylate-co-methyl methacrylate) nerve conduits. Biomaterials 2005, 26, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Miguel, O.; Fernandez-Berridi, M.; Iruin, J. Survey on transport properties of liquids, vapors, and gases in biodegradable poly(3-hydroxybutyrate) (PHB). J. Appl. Polym. Sci. 1997, 63, 1849–1859. [Google Scholar] [CrossRef]
- Tohill, M.; Mantovani, C.; Wiberg, M.; Terenghi, G. Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci. Lett. 2004, 362, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Dezawa, M.; Takahashi, I.; Esaki, M.; Takano, M.; Sawada, H. Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur. J. Neurosci. 2001, 14, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Young, R.C.; Wiberg, M.; Terenghi, G. Poly-3-hydroxybutyrate (PHB): A resorbable conduit for long-gap repair in peripheral nerves. Br. J. Plast. Surg. 2002, 55, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Chen, B.; Lu, S.; Zhao, M.; Guo, Y.; Hou, S. Nerve regeneration and functional recovery after a sciatic nerve gap is repaired by an acellular nerve allograft made through chemical extraction in canines. J. Reconstr. Microsurg. 2007, 23, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Liu, X.; Hu, J.; Jiang, L. Experimental research on revascularization of chemically extracted acellular allogenous nerve graft. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2009, 23, 235–238. [Google Scholar] [PubMed]
- Wang, D.; Liu, X.L.; Zhu, J.K.; Jiang, L.; Hu, J.; Zhang, Y.; Yang, L.M.; Wang, H.G.; Yi, J.H. Bridging small-gap peripheral nerve defects using acellular nerve allograft implanted with autologous bone marrow stromal cells in primates. Brain Res. 2008, 1188, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.S.; Yoo, J.J.; Abouheba, M.; Soker, S.; McDougal, W.S.; Atala, A. Cavernous nerve regeneration using acellular nerve grafts. World J. Urol. 2008, 26, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Aszmann, O.C.; Korak, K.J.; Luegmair, M.; Frey, M. Bridging critical nerve defects through an acellular homograft seeded with autologous schwann cells obtained from a regeneration neuroma of the proximal stump. J. Reconstr. Microsurg. 2008, 24, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.H.; Che, Y.Q.; Tong, X.J.; Zhang, L.X.; Feng, Y.; Xu, A.H.; Tong, L.; Jia, H.; Zhang, X. Improving nerve regeneration of acellular nerve allografts seeded with SCs bridging the sciatic nerve defects of rat. Cell Mol. Neurobiol. 2009, 29, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Peng, J.; Wang, G.; Yang, Q.; Yu, H.; Guo, Q.; Wang, A.; Zhao, B.; Lu, S. Effects of local release of hepatocyte growth factor on peripheral nerve regeneration in acellular nerve grafts. Exp. Neurol. 2008, 214, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Fansa, H.; Dodic, T.; Wolf, G.; Schneider, W.; Keilhoff, G. Tissue engineering of peripheral nerves: Epineurial grafts with application of cultured Schwann cells. Microsurgery. 2003, 23, 72–77. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Petrov, T.; Chung, P.H.; Gordon, T. The expression of the low affinity nerve growth factor receptor in long-term denervated Schwann cells. Glia 1997, 20, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.Y.; Gordon, T. The cellular and molecular basis of peripheral nerve regeneration. Mol. Neurobiol. 1997, 14, 67–116. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Terenghi, G.; Hall, S.M. Effects of delayed re-innervation on the expression of c-erbB receptors by chronically denervated rat Schwann cells in vivo. Glia 1997, 20, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.; Midha, R. Practical considerations concerning the use of stem cells for peripheral nerve repair. Neurosurg. Focus 2009, 26. [Google Scholar] [CrossRef]
- Yannas, I.V.; Zhang, M.; Spilker, M.H. Standardized criterion to analyze and directly compare various materials and models for peripheral nerve regeneration. J. Biomater. Sci. Polym. Ed. 2007, 18, 943–966. [Google Scholar] [CrossRef] [PubMed]
- Schoen, F.J. Biomaterial-associated infection, neoplasia, and calcification. Clinicopathologic features and pathophysiologic concepts. ASAIO Trans. 1987, 33, 8–18. [Google Scholar] [PubMed]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Stang, F.; Keilhoff, G.; Fansa, H. Biocompatibility of Different Nerve Tubes. Materials 2009, 2, 1480-1507. https://doi.org/10.3390/ma2041480
Stang F, Keilhoff G, Fansa H. Biocompatibility of Different Nerve Tubes. Materials. 2009; 2(4):1480-1507. https://doi.org/10.3390/ma2041480
Chicago/Turabian StyleStang, Felix, Gerburg Keilhoff, and Hisham Fansa. 2009. "Biocompatibility of Different Nerve Tubes" Materials 2, no. 4: 1480-1507. https://doi.org/10.3390/ma2041480




