Freeze-Casting of Porous Biomaterials: Structure, Properties and Opportunities
Abstract
:1. Introduction
2. Processing Principles and Materials

3. Structure, Properties, and Their Control
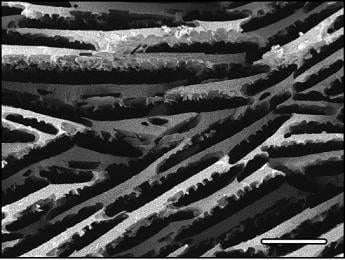
3.1. Porous structure: Pore dimensions, morphologies and orientation





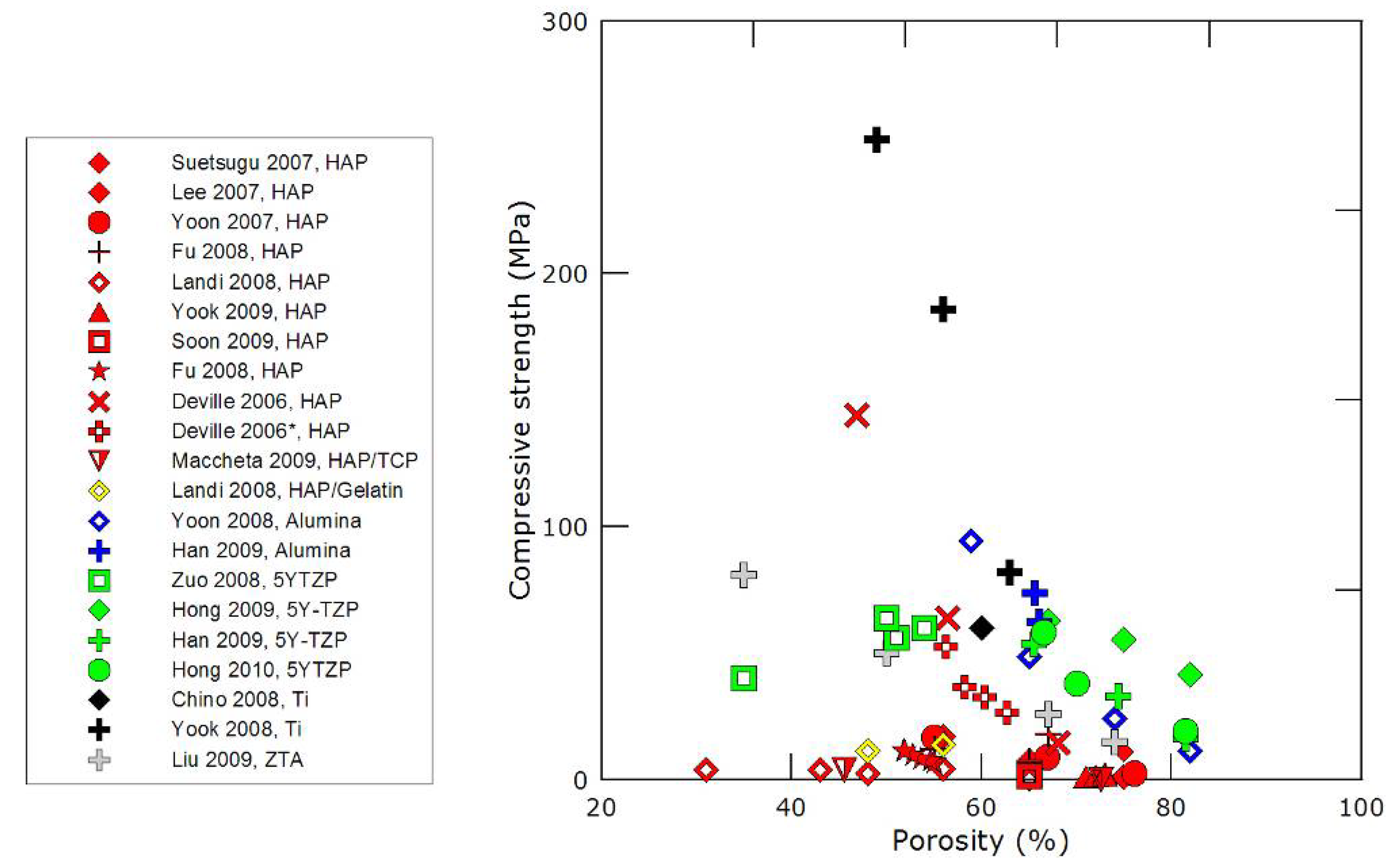
3.2. Mechanical properties
- -
- the nature of the material, although all ceramics have usually extremely high compressive strength values,
- -
- the dimensions of the pores and pore channels: the smaller the channels, the higher the strength,
- -
- the directionality and morphology of the pore channels. Unidirectionally frozen samples exhibit, without surprise, a strongly anisotropic response. The morphology of the pore channels is also of particular importance. Using camphene instead of water will result in materials with a very different porous structure, which seems to be less favorable in terms of mechanical strength.
- -
- the integrity of the structure. This point is of particular importance, and is discussed in greater details below.

- -
- Using small particle size is clearly not desirable here, nano is not good! The best values of compressive strength of freeze-cast samples have been obtained with particle sizes in the micrometer range, yielding defect-free structures.
- -
- Large pore channels are often pursued, requiring very low freezing kinetics. Such conditions more often induce diffusion defects.
- -
- The formation of these defects is directly correlated to the diffusivity of the particles. A workaround solution can nevertheless be found, for instance, by increasing the viscosity of the suspension. Interestingly, these defects have not been observed when camphene is used instead of water. The diffusivity of particles in camphene is likely to be different, and little is known about the nucleation conditions in camphene. Unfortunately, the morphology of the porous structure obtained with camphene does not seem to be optimal, with regards to the mechanical response.
- -
- The behavior of the system with regards to the aforementioned problem will be highly dependent on the characteristics of the powder and the solvent, but also the formulation of the initial suspensions and the various additives used (surfactant, binder, etc.). Each system needs to be carefully assessed for its sensitivity to this phenomenon, and the formulation and processing conditions adjusted to achieve defect-free structures.
4. Opportunities for Biomaterials Applications
- -
- The process is versatile: any type of ceramic or polymeric materials can be used, so that the materials composition can be adjusted to the targeted application, almost independently of its structure. For instance, the degradation rate of bioglass scaffolds can be modulated by varying the glass content of the initial formulation [50].
- -
- The process is environmentally-friendly, in particular when water is used as a solvent.
- -
- The compressive strength values can be extremely high, if proper control of the process is achieved, even with intrinsically weak materials such as calcium phosphate.
- -
- The structure is highly controllable at several levels. Of particular interest is the directionality of the structure, exhibiting striking similarities with natural materials. The pore size can be adjusted to the range usually considered to be required for tissue engineering.
- -
- The porous scaffolds can easily be functionalized, for instance by incorporating active species from the beginning of the process. This has been demonstrated by incorporating enzymes in freeze-cast materials [78], although it is limited to the case where no high temperature consolidation step are used.
- -
- A careful structure/property relationship assessment, which is still lacking today, although interesting progress has been made. Such results will provide the necessary guidelines to adjust the process and tailor the structure to the actual functional requirements.
- -
- In vitro and in vivo tests to validate the potential of these materials and the various hypotheses related to the structure, such as the allegedly facilitated fluids and cell penetration into the scaffolds arising from the directionality of the structure.
References and Notes
- Deville, S. Freeze-Casting of porous ceramics: A review of current achievements and issues. Adv. Eng. Mater. 2008, 10, 155–169. [Google Scholar] [CrossRef]
- Gutierrez, M.C.; Ferrer, M.; del Monte, F. Ice-Templated materials: Sophisticated structures exhibiting enhanced functionalities obtained after unidirectional freezing and ice-segregation-induced self-assembly. Chem. Mater. 2008, 20, 634–648. [Google Scholar] [CrossRef]
- Blacher, S.; Maquet, V.; Pirard, R.; Pirard, J.P.; Jerome, R. Image analysis, impedance spectroscopy and mercury porosimetry characterisation of freeze-drying porous materials. Colloids Surf. A 2001, 187, 375–383. [Google Scholar] [CrossRef]
- Chen, G.; Ushida, T.; Tateishi, T. Hybrid biomaterials for tissue engineering—A preparative method for PLA or PLGA-collagen hybrid sponges. Adv. Mater. 2000, 12, 455–457. [Google Scholar] [CrossRef]
- Chung, T.W.; Yang, J.; Akaike, T.; Cho, K.Y.; Nah, J.W.; Kim, S.I.; Cho, C.S. Preparation of alginate/galactosylated chitosan scaffold for hepatocyte attachment. Biomaterials 2002, 23, 2827–2834. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.J.; Fujimoto, K.L.; Sacks, M.S.; Wagner, W.R. Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials 2005, 26, 3961–3971. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.P.; Grijpma, D.W.; Feijen, J. Preparation of interconnected highly porous polymeric structures by a replication and freeze-drying process. J. Biomed. Mater. Res. B 2003, 67, 732–740. [Google Scholar] [CrossRef]
- Kang, H.W.; Tabata, Y.; Ikada, Y. Fabrication of porous gelatin scaffolds for tissue engineering. Biomaterials 1999, 20, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Sim, S.J.; Lee, D.H.; Kim, D.; Lee, Y.K.; Chung, D.J. Preparation and properties of PHEA/chitosan composite hydrogel. Polym. J. 2004, 36, 943–948. [Google Scholar] [CrossRef]
- Kurose, T.; Takahashi, T.; Koyama, K. A new process to make a porous PTFE structure from aqueous PTFE dispersion with the help of hydrogel. J. Porous Mater. 2004, 11, 173–181. [Google Scholar] [CrossRef]
- Lavik, E.B.; Klassen, H.; Warfvinge, K.; Langer, R.; Young, M.J. Fabrication of degradable polymer scaffolds to direct the integration and differentiation of retinal progenitors. Biomaterials 2005, 26, 3187–3196. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Gao, C.Y.; Mao, Z.W.; Zhou, J.; Shen, J.C.; Hu, X.Q.; Han, C.M. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 2003, 24, 4833–4841. [Google Scholar] [CrossRef] [PubMed]
- Madihally, S.V.; Matthew, H.W.T. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Nakamatsu, J.; Torres, F.G.; Troncoso, O.P.; Min-Lin, Y.; Boccaccini, A.R. Processing and characterization of porous structures from chitosan and starch for tissue engineering scaffolds. Biomacromolecules 2006, 7, 3345–3355. [Google Scholar] [CrossRef] [PubMed]
- Patist, C.M.; Mulder, M.B.; Gautier, S.E.; Maquet, V.; Jerome, R.; Oudega, M. Freeze-dried poly(D,L-lactic acid) macroporous guidance scaffolds impregnated with brain-derived neurotrophic factor in the transected adult rat thoracic spinal cord. Biomaterials 2004, 25, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Stokols, S.; Tuszynski, M.H. Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury. Biomaterials 2006, 27, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Zmora, S.; Glicklis, R.; Cohen, S. Tailoring the pore architecture in 3-D alginate scaffolds by controlling the freezing regime during fabrication. Biomaterials 2002, 23, 4087–4094. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.-H.; Koh, Y.-H.; Park, C.-S.; Kim, H.-E. Generation of large pore channels for bone tissue engineering using camphene-based freeze casting. J. Am. Ceram. Soc. 2007, 90, 1744–1752. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, K.; Zeng, Y.-P. Effects of gelatin addition on the microstructure of freeze-cast porous hydroxyapatite ceramics. Ceram. Int. 2009, 35, 2151–2154. [Google Scholar] [CrossRef]
- Yook, S.-W.; Kim, H.-E.; Yoon, B.-H.; Soon, Y.-M.; Koh, Y.-H. Improvement of compressive strength of porous hydroxyapatite scaffolds by adding polystyrene to camphene-based slurries. Mater. Lett. 2009, 63, 955–958. [Google Scholar] [CrossRef]
- Yoon, B.-H.; Park, C.-S.; Kim, H.-E.; Koh, Y.-H. In-situ fabrication of porous hydroxyapatite (HA) scaffolds with dense shells by freezing HA/camphene slurry. Mater. Lett. 2008, 62, 1700–1703. [Google Scholar] [CrossRef]
- Soon, Y.-M.; Shin, K.-H.; Koh, Y.-H.; Lee, J.-H.; Kim, H.-E. Compressive strength and processing of camphene-based freeze cast calcium phosphate scaffolds with aligned pores. Mater. Lett. 2009, 63, 1548–1550. [Google Scholar] [CrossRef]
- Fu, Q.; Rahaman, M.; Bal, B.; Brown, R. Proliferation and function of MC3T3-E1 cells on freeze-cast hydroxyapatite scaffolds with oriented pore architectures. J. Mater. Sci.: Mater. Med. 2009, 20, 1159–1165. [Google Scholar] [CrossRef]
- Fu, Q.; Rahaman, M.N.; Bal, B.S.; Brown, R.F. In vitro cellular response to hydroxyapatite scaffolds with oriented pore architectures. Mater. Sci. Eng., C 2009, 29, 2147–2153. [Google Scholar] [CrossRef]
- Fu, Q.; Rahaman, M.N.; Dogan, F.; Bal, S.B. Freeze casting of porous hydroxyapatite scaffolds. I. Processing and general microstructure. J. Biomed. Mater. Res. B 2008, 86B, 125–135. [Google Scholar] [CrossRef]
- Fu, Q.; Rahaman, M.N.; Dogan, F.; Bal, S.B. Freeze cast hydroxyapatite for bone tissue engineering applications. Biomed. Mater. 2008, 3, 025005. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Rahaman, M.N.; Dogan, F.B.; Bal, S.B. Freeze casting of porous hydroxyapatite scaffolds. II. Sintering, microstructure, and mechanical behavior. J. Biomed. Mater. Res. 2008, 86B, 514–522. [Google Scholar] [CrossRef]
- Deville, S.; Saiz, E.; Nalla, R.K.; Tomsia, A.P. Freezing as a path to build complex composites. Science 2006, 311, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Deville, S.; Saiz, E.; Tomsia, A.P. Freeze casting of hydroxyapatite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 5480–5489. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-J.; Koh, Y.-H.; Yoon, B.-H.; Kim, H.-E.; Kim, H.-W. Highly porous hydroxyapatite bioceramics with interconnected pore channels using camphene-based freeze casting. Mater. Lett. 2007, 61, 2270–2273. [Google Scholar] [CrossRef]
- Suetsugu, Y.; Hotta, Y.; Iwasashi, M.; Sakane, M.; Kikuchi, M.; Ikoma, T.; Higaki, T.; Ochiai, N.; Tanaka, J. Structural and tissue reaction properties of novel hydroxyapatite ceramics with unidirectional pores. Key Eng. Mater. 2007, 330–332 II, 1003–1006. [Google Scholar] [CrossRef]
- Zuo, K.H.; Zeng, Y.-P.; Jiang, D. Effect of polyvinyl alcohol additive on the pore structure and morphology of the freeze-cast hydroxyapatite ceramics. Mater. Sci. Eng., C 2010, 30, 283–287. [Google Scholar] [CrossRef]
- Macchetta, A.; Turner, I.G.; Bowen, C.R. Fabrication of HA/TCP scaffolds with a graded and porous structure using a camphene-based freeze-casting method. Acta Biomater. 2009, 5, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.-Y.; Fernandes, H.R.; Ventura, J.M.; Kannan, S.; Ferreira, J.M.F. Nano-TiO2-Coated unidirectional porous glass structure prepared by freeze drying and solution infiltration. J. Am. Ceram. Soc. 2007, 90, 1265–1268. [Google Scholar] [CrossRef]
- Mallick, K.K. Freeze casting of porous bioactive glass and bioceramics. J. Am. Ceram. Soc. 2009, 92, S85–S94. [Google Scholar] [CrossRef]
- Song, J.-H.; Koh, Y.-H.; Kim, H.-E.; Li, L.-H.; Bahn, H.-J. Fabrication of a porous bioactive glass-ceramic using room-temperature freeze casting. J. Am. Ceram. Soc. 2006, 89, 2649–2653. [Google Scholar] [CrossRef]
- Yoon, B.-H.; Choi, W.-Y.; Kim, H.-E.; Kim, J.-H.; Koh, Y.-H. Aligned porous alumina ceramics with high compressive strengths for bone tissue engineering. Scripta Mater. 2008, 58, 537–540. [Google Scholar] [CrossRef]
- Han, J.; Hu, L.; Zhang, Y.; Zhou, Y. Fabrication of ceramics with complex porous structures by the impregnate freeze-casting process. J. Am. Ceram. Soc. 2009, 92, 2165–2167. [Google Scholar] [CrossRef]
- Han, J.; Hong, C.; Zhang, X.; Du, J.; Zhang, W. Highly porous ZrO2 ceramics fabricated by a camphene-based freeze-casting route: Microstructure and properties. J. Eur. Ceram. Soc. 2010, 30, 53–60. [Google Scholar] [CrossRef]
- Hong, C.; Zhang, X.; Han, J.; Du, J.; Han, W. Ultra-high-porosity zirconia ceramics fabricated by novel room-temperature freeze-casting. Scripta Mater. 2009, 60, 563–566. [Google Scholar] [CrossRef]
- Hong, C.; Zhang, X.; Han, J.; Du, J.; Zhang, W. Camphene-based freeze-cast ZrO2 foam with high compressive strength. Mater. Chem. Phys. 2010, 119, 359–362. [Google Scholar] [CrossRef]
- Cao, Y.; He, J. Preparation and formation mechanism of porous ultralightweight zirconia by ice templating. Chin. J. Mater. Res. 2009, 23, 518–523. [Google Scholar]
- Liu, G.; Zhang, D.; Meggs, C.; Button, T.W. Porous Al2O3-ZrO2 composites fabricated by an ice template method. Scripta Mater. 2010, 62, 466–468. [Google Scholar] [CrossRef]
- Yunoki, S.; Ikoma, T.; Monkawa, A.; Ohta, K.; Kikuchi, M.; Sotome, S.; Shinomiya, K.; Tanaka, J. Control of pore structure and mechanical property in hydroxyapatite/collagen composite using unidirectional ice growth. Mater. Lett. 2006, 60, 999–1002. [Google Scholar] [CrossRef]
- Landi, E.; Valentini, F.; Tampieri, A. Porous hydroxyapatite/gelatine scaffolds with ice-designed channel-like porosity for biomedical applications. Acta Biomater. 2008, 4, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Turco, G.; Marsich, E.; Bellomo, F.; Semeraro, S.; Donati, I.; Brun, F.; Grandolfo, M.; Accardo, A.; Paoletti, S. Alginate/hydroxyapatite biocomposite for bone ingrowth: A trabecular structure with high and isotropic connectivity. Biomacromolecules 2009, 10, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Ye, J.; Wang, Y. Alginate/poly (lactic-co-glycolic acid)/calcium phosphate cement scaffold with oriented pore structure for bone tissue engineering. J. Biomed. Mater. Res. A 2009, 89, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Maquet, V.; Boccaccini, A.R.; Pravata, L.; Notingher, I.; Jerome, R. Porous poly([alpha]-hydroxyacid)/Bioglass(R) composite scaffolds for bone tissue engineering. I: preparation and in vitro characterisation. Biomaterials 2004, 25, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Blaker, J.J.; Maquet, V.; Jerome, R.; Boccaccini, A.R.; Nazhat, S.N. Mechanical properties of highly porous PDLLA/Bioglass(R) composite foams as scaffolds for bone tissue engineering. Acta Biomater. 2005, 1, 643–652. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, A.M.; Saad, E.A.; El-Hady, B.M.A.; Farag, M.M. Synthesis of silicate glass/Poly(L-Lactide) composite scaffolds by freeze-extraction technique: Characterization and in vitro bioactivity evaluation. Ceram. Int. 2010, 36, 995–1009. [Google Scholar] [CrossRef]
- Chino, Y.; Dunand, D.C. Directionally freeze-cast titanium foam with aligned, elongated pores. Acta Mater. 2008, 56, 105–113. [Google Scholar] [CrossRef]
- Bignon, A.; Chouteau, J.; Chevalier, J.; Fantozzi, G.; Carret, J.-P.; Chavassieux, P.; Boivin, G.; Melin, M.; Hartmann, D. Effect of micro and macroporosity of bone substitute on their mechanical properties and cellular response. J. Mater. Sci.: Mater. Med. 2003, 14, 1089–1097. [Google Scholar] [CrossRef]
- Bettge, M.; Niculescu, H.; Gielisse, P.J. Engineered Porous Ceramics Using a Directional Freeze-Drying Process. In Proceedings of the 28th International Spring Seminar on Electronics Technology, Wiener Neustadt, Germany, 19–20 May 2005; pp. 28–34.
- Deville, S.; Saiz, E.; Tomsia, A.P. Freeze casting of hydroxyapatite scaffolds for bone tissue regeneration. Biomaterials 2006, 27, 5480–5489. [Google Scholar] [CrossRef] [PubMed]
- Deville, S.; Saiz, E.; Tomsia, A.P. Ice-templated porous alumina structures. Acta Mater. 2007, 55, 1965–1974. [Google Scholar] [CrossRef]
- Moritz, T.; Richter, H.-J. Ceramic bodies with complex geometries and ceramic shells by freeze casting using ice as mold material. J. Am. Ceram. Soc. 2006, 89, 2394–2398. [Google Scholar] [CrossRef]
- Moritz, T.; Richter, H.-J. Ice-mould freeze casting of porous ceramic components. J. Eur. Ceram. Soc. 2007, 27, 4595–4601. [Google Scholar] [CrossRef]
- Sofie, S.W. Fabrication of functionally graded and aligned porosity in thin ceramic substrates with the novel freeze-tape-casting process. J. Am. Ceram. Soc. 2007, 90, 2024–2031. [Google Scholar] [CrossRef]
- Yumin, Z.; Luyang, H.; Jiecai, H. Preparation of a dense/porous bilayered ceramic by applying an electric field during freeze casting. J. Am. Ceram. Soc. 2009, 92, 1874–1876. [Google Scholar] [CrossRef]
- Deville, S.; Maire, E.; Lasalle, A.; Bogner, A.; Gauthier, C.; Leloup, J.; Guizard, C. In situ X-Ray radiography and tomography observations of the solidification of aqueous alumina particles suspensions. Part I: Initial Instants. J. Am. Ceram. Soc. 2009, 92, 2471–2488. [Google Scholar] [CrossRef]
- Buckley, C.T.; O'Kelly, K.U. Fabrication and characterization of a porous multidomain hydroxyapatite scaffold for bone tissue engineering investigations. J. Biomed. Mater. Res. B 2010, in press. [Google Scholar]
- Yook, S.-W.; Kim, H.-E.; Koh, Y.-H. Fabrication of porous titanium scaffolds with high compressive strength using camphene-based freeze casting. Mater. Lett. 2009, 63, 1502–1504. [Google Scholar] [CrossRef]
- Araki, K.; Halloran, J.W. New freeze-casting technique for ceramics with sublimable vehicles. J. Am. Ceram. Soc. 2004, 87, 1859–1863. [Google Scholar] [CrossRef]
- Araki, K.; Halloran, J.W. Room-temperature freeze casting for ceramics with nonaqueous sublimable vehicles in the naphthalene-camphor eutectic system. J. Am. Ceram. Soc. 2004, 87, 2014–2019. [Google Scholar] [CrossRef]
- Araki, K.; Halloran, J.W. Porous ceramic bodies with interconnected pore channels by a novel freeze casting technique. J. Am. Ceram. Soc. 2005, 88, 1108–1114. [Google Scholar] [CrossRef]
- Koh, Y.-H.; Lee, E.-J.; Yoon, B.-H.; Song, J.-H.; Kim, H.-E.; Kim, H.-W. Effect of polystyrene addition on freeze casting of ceramic/camphene slurry for ultra-high porosity ceramics with aligned pore channels. J. Am. Ceram. Soc. 2006, 89, 3646–3653. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Fu, Q. Manipulation of porous bioceramic microstructures by freezing of suspensions containing binary mixtures of solvents. J. Am. Ceram. Soc. 2008, 91, 4137–4140. [Google Scholar] [CrossRef]
- Chen, R.; Wang, C.-A.; Huang, Y.; Ma, L.; Lin, W. Ceramics with special porous structures fabricated by freeze-gelcasting: using tert-Butyl alcohol as a template. J. Am. Ceram. Soc. 2007, 90, 3478–3484. [Google Scholar] [CrossRef]
- Yang, T.; Lee, J.; Yoon, S.; Park, H. Hydroxyapatite scaffolds processed using a TBA-based freeze-gel casting/polymer sponge technique. J. Mater. Sci. Mater. Med. 2010, in press. [Google Scholar]
- Munch, E.; Saiz, E.; Tomsia, A.P.; Deville, S. Architectural control of freeze-cast ceramics through additives and templating. J. Am. Ceram. Soc. 2009, 92, 1534–1539. [Google Scholar] [CrossRef]
- Han, J.; Hu, L.; Zhang, Y.; Zhou, Y. Fabrication of ceramics with complex porous structures by the impregnate freeze-casting process. J. Am. Ceram. Soc. 2009, 92, 2165–2167. [Google Scholar] [CrossRef]
- Moon, J.-W.; Hwang, H.-J.; Awano, M.; Maeda, K. Preparation of NiO-YSZ tubular support with radially aligned pore channels. Mater. Lett. 2003, 57, 1428–1434. [Google Scholar] [CrossRef]
- Jung, H.-D.; Yook, S.-W.; Kim, H.-E.; Koh, Y.-H. Fabrication of porous titanium scaffolds with gradient in porosity and pore size using sequential freeze casting. Mater. Lett. 2009, 63, 1545–1547. [Google Scholar] [CrossRef]
- Boddapati, S.R.; Bordia, R.K. Aligned Pore Channels in 8 mol% Ytttria-Stabilized Zirconia by Freeze-Casting. In Proceedings of the The American Ceramic Society's 31st International Conference on Advanced Ceramics and Composites, Daytona Beach, FL, USA, 21–26 January 2007; pp. 57–65.
- Deville, S.; Maire, E.; Bernard-Granger, G.; Lasalle, A.; Bogner, A.; Gauthier, C.; Leloup, J.; Guizard, C. Metastable and unstable cellular solidification of colloidal suspensions. Nat. Mater. 2009, 8, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Zuo, K.H.; Zeng, Y.-P.; Jiang, D. Properties of microstructure-controllable porous yttria-stabilized zirconia ceramics fabricated by freeze casting. Int. J. Appl. Ceram. Tech. 2008, 5, 198–203. [Google Scholar] [CrossRef]
- Yook, S.-W.; Yoon, B.-H.; Kim, H.-E.; Koh, Y.-H.; Kim, Y.-S. Porous titanium (Ti) scaffolds by freezing TiH2/camphene slurries. Mater. Lett. 2008, 62, 4506–4508. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Jobbágy, M.; Rapún, N.; Ferrer, M.L.; del Monte, F. A biocompatible bottom-up route for the preparation of hierarchical biohybrid materials. Adv. Mater. 2006, 18, 1137–1140. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Deville, S. Freeze-Casting of Porous Biomaterials: Structure, Properties and Opportunities. Materials 2010, 3, 1913-1927. https://doi.org/10.3390/ma3031913
Deville S. Freeze-Casting of Porous Biomaterials: Structure, Properties and Opportunities. Materials. 2010; 3(3):1913-1927. https://doi.org/10.3390/ma3031913
Chicago/Turabian StyleDeville, Sylvain. 2010. "Freeze-Casting of Porous Biomaterials: Structure, Properties and Opportunities" Materials 3, no. 3: 1913-1927. https://doi.org/10.3390/ma3031913
APA StyleDeville, S. (2010). Freeze-Casting of Porous Biomaterials: Structure, Properties and Opportunities. Materials, 3(3), 1913-1927. https://doi.org/10.3390/ma3031913