Creating Surface Properties Using a Palette of Hydrophobins
Abstract
:1. Introduction
2. Biological Functions of Hydrophobins
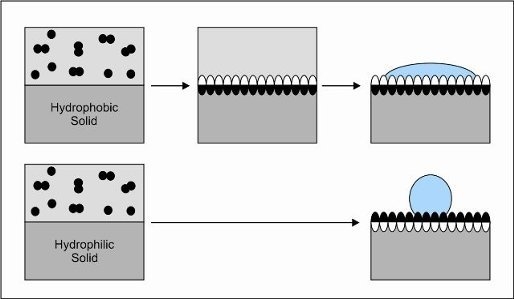
3. Interfacial Self-Assembly of Hydrophobins
4. Structure of Class I and II Hydrophobins
| Hydrophobin | Fungus | Surface activity (mJ·m-2) | Hydrophilic side (θ) | Hydrophobic side (θ) | Rodlets | Reference |
|---|---|---|---|---|---|---|
| Class I | ||||||
| SC3 | S. commune | 27–32 | 36 ± 3 | 115 ± 12 | yes | [12,20,58] |
| deglycosylated SC3a | S. commune | 32 | 66 ± 6 | ND | ND | [58] |
| RGD-SC3 | S. commune | 32 | 44 ± 2 | 122 ± 4 | yes | [58] |
| TrSC3 | S. commune | 32 | 73 ± 3 | 119 ± 3 | yes | [58] |
| RGD-TrSC3 | S. commune | 30 | 68 ± 3 | 120 ± 3 | yes | [58] |
| SC4 | S. commune | 35 | 48 ± 3 | 115 ± 3 | yes | [36,98] |
| ABH1 | A. bisporus | ND | 63 ± 8 | 113 ± 4 | yes | [31] |
| ABH3 | A. bisporus | 37 | 59 ± 5 | 117 ± 3 | yes | [26] |
| HGFIb | G. frondosa | 45 | 62 ± 2.5 | ND | yes | [91] |
| Class II | ||||||
| HFBI | T. reesei | 42 | 59 ± 13 | 60–64 | no | [20] |
| HFBII | T. reesei | 35 | - | 60–70 | no | [20] |
| CRP | C. parasitica | 32 | 22 ± 2 | ≥90c | no | [12,25] |
| CFTH1 | C. fusiformis | 33 | 60 ± 5 | 105 ± 2 | no | [99] |

4.1. Conformational Changes during Self-Assembly at Hydrophilic-Hydrophobic Interface
4.1.1. Class I hydrophobins
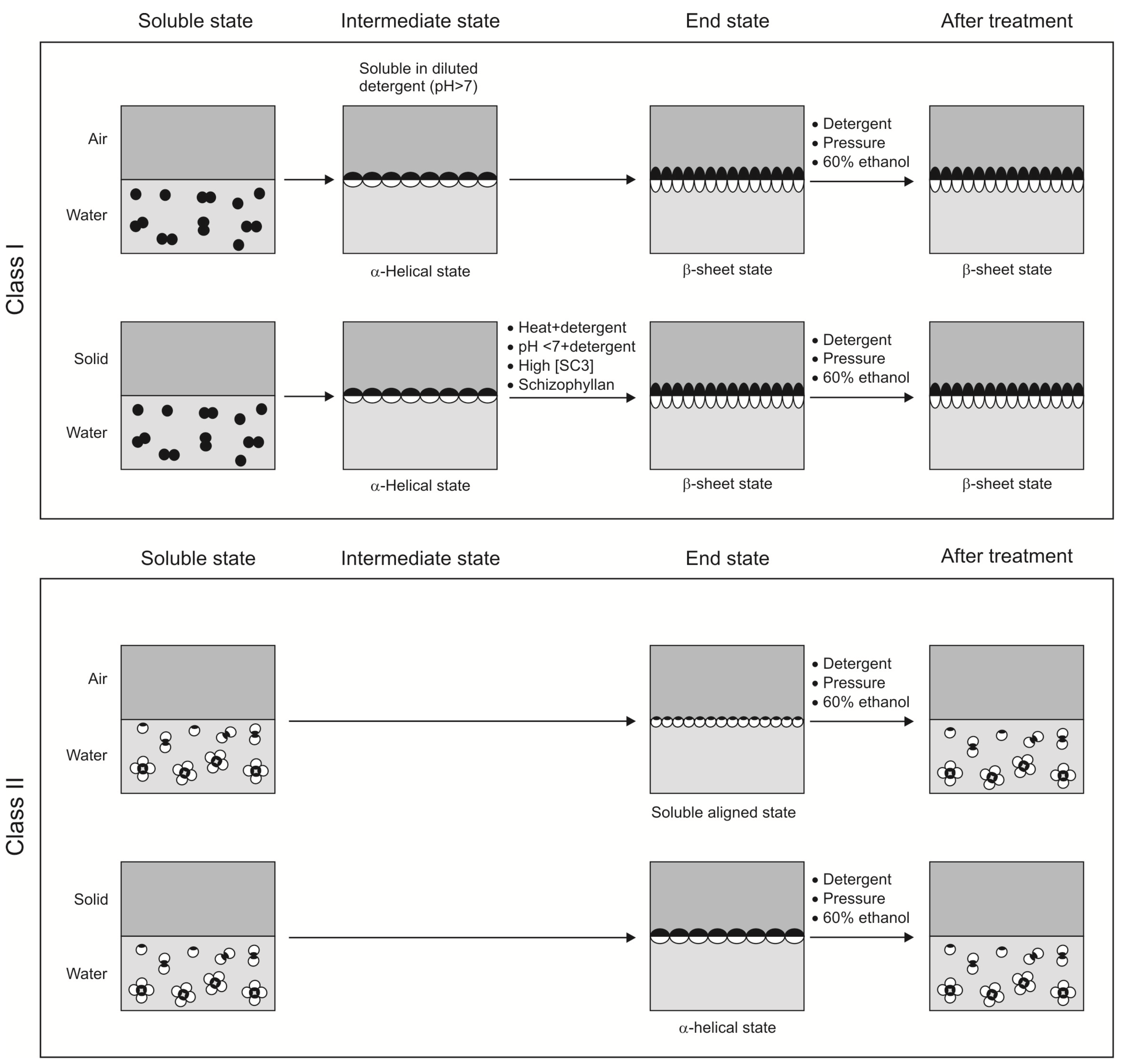
4.1.2. Class II hydrophobins
4.2. Engineered Hydrophobins
5. Applications
5.1. Liquids
5.2. Solid Surfaces
6. Conclusions
References
- Kasemo, B. Biological surface science. Surf. Sci. 2002, 500, 656–677. [Google Scholar] [CrossRef]
- De Chiffre, L.; Kunzmann, H.; Peggs, G.N.; Lucca, D.A. Surfaces in precision engineering, microengineering and nanotechnology. CIRP Annals—Manufacturing Technol. 2003, 52, 561–577. [Google Scholar] [CrossRef]
- Kurella, A.; Dahotre, N.B. Surface modification for bioimplants: The role of laser surface engineering. J. Biomater. App. 2005, 20, 5–50. [Google Scholar] [CrossRef]
- Hanawa, T. An overview of biofunctionalization of metals in Japan. J. R. Soc. Interface 2009, 6, S361–S369. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D.; Bryant, S.J. Biomaterials: Where we have been and where we are going. Annu. Rev. Biomed. Eng. 2004, 6, 41–75. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.C.; Healy, K.E. Controlling biological interfaces on the nanometer length scale. J. Biomed. Mater. Res. A. 2009, 90, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Variola, F.; Vetrone, F.; Richert, L.; Jedrzejowski, P.; Yi, J.H.; Zalzal, S.; Clair, S.; Sarkissian, A.; Perepichka, D.F.; Wuest, J.D.; Rosei, F.; Nanci, A. Improving biocompatibility of implantable metals by nanoscale modification of surfaces: An overview of strategies, fabrication methods, and challenges. Small 2009, 5, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Kusnezow, W.; Hoheisel, J.D. Solid supports for microarray immunoassays. J. Mol. Recognit. 2003, 16, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, L.H.; Ramström, O.; Yan, M. Engineering nanomaterial surfaces for biomedical applications. Exp. Biol. Med. 2009, 234, 1128–1139. [Google Scholar] [CrossRef]
- Wu, H.; Fan, Y.; Sheng, J.; Sui, S.F. Induction of changes in the secondary structure of globular proteins by a hydrophobic surface. Eur. Biophys. J. 1993, 22, 201–205. [Google Scholar] [CrossRef] [PubMed]
- He, L.Z.; Dexter, A.F.; Middelberg, A.P.J. Biomolecular engineering at interfaces. Chem. Eng. Sci. 2006, 61, 989–1003. [Google Scholar] [CrossRef]
- Wösten, H.A.B.; de Vocht, M.L. Hydrophobins, the fungal coat unravelled. Biochim. Biophys. Acta 2000, 1469, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Scholtmeijer, K.; Janssen, M.I.; van Leeuwen, M.B.M.; van Kooten, T.G.; Hektor, H.J.; Wösten, H.A.B. The use of hydrophobins to fuctionalize surfaces. Biomed. Mater. Eng. 2004, 14, 447–454. [Google Scholar] [PubMed]
- Hektor, H.J.; Scholtmeijer, K. Hydrophobins: Proteins with potential. Curr. Opin. Biotechnol. 2005, 16, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.B.; Szilvay, G.R.; Nakari-Setälä, T.; Penttilä, M.E. Hydrophobins: The protein-amphiphiles of filamentous fungi. FEMS Microbiol. Rev. 2005, 29, 877–896. [Google Scholar] [CrossRef] [PubMed]
- Wösten, H.A.B. Hydrophobins: Multipurpose proteins. Annu. Rev. Microbiol. 2001, 55, 625–646. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, M.J.; Talbot, N.J. Hydrophobins and repellents: Proteins with fundamental roles in fungal morphogenesis. Fungal. Genet. Biol. 1998, 23, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Van der Vegt, W.; van der Mei, H.C.; Wösten, H.A.B.; Wessels, J.G.H.; Busscher, H.J.A. Comparison of the surface activity of the fungal hydrophobin SC3p with those of other proteins. Biophys. Chem. 1996, 57, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Wösten, H.A.B.; van Wetter, M.A.; Lugones, L.G.; van der Mei, H.C.; Busscher, H.J.; Wessels, J.G.H. How a fungus escapes the water to grow into the air. Curr. Biol. 1999, 9, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Askolin, S.; Linder, M.B.; Scholtmeijer, K.; Tenkanen, M.; Penttilä, M.E.; de Vocht, M.L.; Wösten, H.A.B. Interaction and comparison of a class I hydrophobin from Schizophyllum commune and class II hydrophobins from Trichoderma reseei. Biomacromolecules 2006, 7, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Wessels, J.G.H. Developmental regulation of fungal cell wall formation. Annu. Rev. Phytopathol. 1994, 32, 413–437. [Google Scholar] [CrossRef]
- Lugones, L.G.; Wösten, H.A.B.; Wessels, J.G.H. A hydrophobin (ABH3) specifically secreted by vegetatively growing hyphae of Agaricus bisporus (common white botton mushroom). Microbiology 1998, 144, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- Askolin, S.; Penttilä, M.E.; Wösten, H.A.B.; Nakari-Setälä, T. The Trichoderma reesei hydrophobin genes hfb1 and hfb2 have diverse functions in fungal development. FEMS Microbiol. Lett. 2005, 253, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Wessels, J.G.H.; de Vries, O.M.H.; Asgeirsdóttir, S.A.; Schuren, F.H.J. Hydrophobin genes involved in formation of aerial hyphae and fruit bodies in Schizophyllum. Plant Cell 1991, 3, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Van Wetter, M.A.; Schuren, F.H.J.; Schuurs, T.A.; Wessels, J.G.H. Targeted mutation of the SC3 hydrophobin gene of Schizophyllum commune affects formation of aerial hyphae. FEMS Microbiol. Lett. 1996, 140, 265–269. [Google Scholar]
- Wösten, H.A.B.; de Vries, O.M.H.; Wessels, J.G.H. Interfacial self-assembly of a fungal hydrophobin into a hydrophobic rodlet layer. Plant Cell 1993, 5, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Wösten, H.A.B.; Asgeirsdóttir, S.A.; Krook, J.H.; Drenth, J.H.; Wessels, J.G.H. The fungal hydrophobin Sc3p self-assembles at the surface of aerial hyphae as a protein membrane constituting the hydrophobic layer. Eur. J. Cell Biol. 1994, 63, 122–129. [Google Scholar] [PubMed]
- Lugones, L.G.; Bosscher, J.S.; Scholtmeijer, K.; de Vries, O.M.H.; Wessels, J.G.H. An abundant hydrophobin (ABH1) forms hydrophobic rodlet layers in Agaricus bisporus fruiting bodies. Microbiology 1996, 142, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Stringer, M.A.; Dean, R.A.; Sewall, T.C.; Timberlake, W.E. Rodletless, a new Aspergillus developmental mutant induced by directed gene inactivation. Genes Dev. 1991, 5, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Bell-Pedersen, D.; Dunlap, J.C.; Loros, J.J. The Neurospora circadian clock-controlled gene, ccg-2, is allelic to eas and encodes a fungal hydrophobin required for formation of the conidial rodlet layer. Genes Dev. 1992, 6, 2382–2394. [Google Scholar] [CrossRef] [PubMed]
- Thau, N.; Monod, M.; Crestani, B.; Rolland, C.; Tronchin, G.; Latgé, J.P.; Paris, S. Rodletless mutants of Aspergillus fumigatus. Infect. Immun. 1994, 62, 4380–4388. [Google Scholar] [PubMed]
- Parta, M.; Chang, Y.; Rulong, S.; Pinto-DaSilva, P.; Kwon-Chung, K.J. HYP1, a hydrophobin gene from Aspergillus fumigatus, complements the rodletless phenotype in Aspergillus nidulans. Infect. Immun. 1994, 62, 4389–4395. [Google Scholar] [PubMed]
- Van Wetter, M.A.; Wösten, H.A.B.; Wessels, J.G.H. SC3 and SC4 hydrophobins have distinct roles in formation of aerial structures in dikaryons of Schizophyllum commune. Mol. Microbiol. 2000, 36, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Temple, B.; Horgen, P.A.; Bernier, L.; Hintz, W.E. Cerato-ulmin, a hydrophobin secreted by the causal agents of Dutch elm disease, is a parasitic fitness factor. Fungal Genet. Biol. 1997, 22, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Aimanianda, V.; Bayry, J.; Bozza, S.; Kniemeyer, O.; Perruccio, K.; Elluru, S.R.; Clavaud, C.; Paris, S.; Brakhage, A.A.; Kaveri, S.V.; Srini, V.K.; Romani, L.; Latgé, J.P. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 2009, 460, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Paris, S.; Debeaupuis, J.P.; Crameri, R.; Carey, M.; Charlés, F.; Prévost, M.C.; Schmitt, C.; Philippe, B.; Latgé, J.P. Conidial hydrophobins of Aspergillus fumigatus. Appl. Environ. Microbiol. 2003, 69, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, K.; Takaoka, M.; Uchida, K.; Wakayama, M.; Yamaguchi, H.; Takahashi, K.; Paris, S.; Latge, J.P.; Naoe, S. Histopathology of experimental invasive pulmonary aspergillosis in rats: Pathological comparison of pulmonary lesions induced by specific virulent factor deficient mutants. Microb. Pathog. 1999, 27, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Bruns, S.; Kniemeyer, O.; Hasenberg, M.; Aimanianda, V.; Nietzsche, S.; Thywissen, A.; Jeron, A.; Latgé, J.P.; Brakhage, A.A.; Gunzer, M. Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog. 2010, 6. [Google Scholar] [CrossRef] [PubMed]
- Wösten, H.A.B.; Schuren, F.H.J.; Wessels, J.G.H. Interfacial self-assembly of a hydrophobin into an amphipathic protein membrane mediates fungal attachment to hydrophobic surfaces. EMBO J. 1994, 13, 5848–5854. [Google Scholar] [PubMed]
- Talbot, N.J.; Kershaw, M.J.; Wakley, G.E.; de Vries, O.M.H.; Wessels, J.G.H.; Hamer, J.E. MPG1 encodes a fungal hydrophobin involved in surface interactions during infection-related development of Magnaporthe grisea. Plant Cell 1996, 8, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Ebbole, D.J. Hydrophobins and fungal infection of plants and animals. Trends Microbiol. 1997, 5, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Lugones, L.G.; de Jong, J.F.; de Vries, O.M.H.; Jalving, R.; Dijksterhuis, J.; Wösten, H.A.B. The SC15 protein of Schizophyllum commune mediates formation of aerial hyphae and attachment in the absence of the SC3 hydrophobin. Mol. Microbiol. 2004, 53, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Talbot, N.J.; Ebbole, D.J.; Hamer, J.E. Identification and characterization of MPG1, a gene involved in pathogenicity from the rice blast fungus Magnaporthe grisea. Plant Cell 1993, 5, 1575–1590. [Google Scholar] [CrossRef] [PubMed]
- Hamer, J.E.; Talbot, N.J. Infection-related development in the rice blast fungus Magnaporthe grisea. Curr. Opin. Microbiol. 1998, 1, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Spanu, P. HCF-1, a hydrophobin from the tomato pathogen Cladosporium fulvum. Gene 1997, 193, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Van Wetter, M.A.; Wösten, H.A.B.; Sietsma, J.H.; Wessels, J.G.H. Hydrophobin gene expression affects hyphal wall composition in Schizophyllum commune. Fungal Genet. Biol. 2000, 31, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Sunde, M.; Kwain, A.H.Y.; Templeton, M.D.; Beever, R.E.; Mackay, J.P. Structural analysis of hydrophobins. Micron. 2008, 39, 773–784. [Google Scholar] [CrossRef] [PubMed]
- De Vries, O.M.H.; Fekkes, M.P.; Wösten, H.A.B.; Wessels, J.G.H. Insoluble hydrophobin complexes in the walls of Schizophyllum commune and other filamentous fungi. Arch. Microbiol. 1993, 159, 330–335. [Google Scholar] [CrossRef]
- Russo, P.S.; Blum, F.D.; Ipsen, J.D.; Abul-Hajj, Y.J.; Miller, W.G. The surface activity of the phytotoxin cerato-ulmin. Can. J. Bot. 1982, 60, 1414–1422. [Google Scholar] [CrossRef]
- Carpenter, C.E.; Mueller, R.J.; Kazmierczak, P.; Zhang, L.; Villalon, D.K.; van Alfen, N.K. Effect of a virus on accumulation of a tissue-specific cell-surface protein of the fungus Cryphonectria (Endothia) parasitica. Mol. Plant Microbe Int. 1992, 4, 55–61. [Google Scholar] [CrossRef]
- Kwan, A.H.Y.; Winefield, R.D.; Sunde, M.; Matthews, J.M.; Haverkamp, R.G.; Templeton, M.D.; Mackay, J.P. Structural basis for rodlet assembly in fungal hydrophobins. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 3621–3626. [Google Scholar] [CrossRef] [PubMed]
- Hakanpää, J.; Paananen, A.; Askolin, S.; Nakari-Setälä, T.; Parkkinen, T.; Penttilä, M.E.; Linder, M.B.; Rouvinen, J. Atomic resolution structure of the HFBII hydrophobin, a self-assembling amphiphile. J. Biol. Chem. 2004, 279, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Hakanpää, J.; Szilvay, G.R.; Kaljunen, H.; Maksimainen, M.; Linder, M.B.; Rouvinen, J. Two crystal structures of Trichoderma reesei hydrophobin HFBI—The structure of a protein amphiphile with and without detergent interaction. Protein Sci. 2006, 15, 2129–2140. [Google Scholar] [CrossRef] [PubMed]
- Hakanpää, J.; Linder, M.B.; Popov, A.; Schmidt, A.; Rouvinen, J. Hydrophobin HFBII in detail: Ultrahigh-resolution structure at 0.75 Ǻ. Acta Crystallogr. D Biol. Crystallogr. 2006, 62, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, M.J.; Thornton, C.R.; Wakley, G.E.; Talbot, N.J. Four conserved intramolecular disulphide linkages are required for secretion and cell wall localization of a hydrophobin during fungal morphogenesis. Mol. Microbiol. 2005, 56, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Kwan, A.H.; Macindoe, I.; Vukasin, P.V.; Morris, V.K.; Kass, I.; Gupte, R.; Mark, A.E.; Templeton, M.D.; Mackay, J.P.; Sunde, M. The Cys3-Cys4 loop of the hydrophobin EAS is not required for rodlet formation and surface activity. J. Mol. Biol. 2008, 382, 708–720. [Google Scholar] [CrossRef] [PubMed]
- De Vocht, M.L.; Reviakine, I.; Wösten, H.A.B.; Brisson, A.; Wessels, J.G.H.; Robillard, G.T. Structural and functional role of the disulphide bridges in the hydrophobin SC3. J. Biol. Chem. 2000, 275, 28428–28432. [Google Scholar] [CrossRef] [PubMed]
- De Vocht, M.L.; Scholtmeijer, K.; van der Vegte, E.W.; de Vries, O.M.H.; Sonveaux, N.; Wösten, H.A.B.; Ruysschaert, J.M.; Hadziloannou, G.; Wessels, J.G.H.; Robillard, G.T. Structural characterization of the hydrophobin SC3, as monomer and after self-assembly at hydrophobic/hydrophilic interfaces. Biophys. J. 1998, 74, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Scholtmeijer, K.; Janssen, M.I.; Gerssen, B.; de Vocht, M.L.; van Leeuwen, B.M.M.; van Kooten, T.G.; Wösten, H.A.B.; Wessels, J.G.H. Surface modifications created by using engineered hydrophobins. Appl. Environ. Microbiol. 2002, 68, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Graveland-Bikker, J.F.; de Kruif, C.G.; Robillard, G.T. Oligomerization of hydrophobin SC3 in solution: From soluble state to self-assembly. Protein Sci. 2004, 13, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; de Vocht, M.L.; de Jonge, J.; Poolman, B.; Robillard, G.T. Structural changes and molecular interactions of hydrophobin SC3 in solution and on a hydrophobic surface. Protein Sci. 2002, 11, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
- De Vocht, M.L.; Reviakine, I.; Ulrich, W.P.; Bergsma-Schutter, W.; Wösten, H.A.B.; Vogel, H.; Brisson, A.; Wessels, J.G.H.; Robillard, G.T. Self-assembly of the hydrophobin SC3 proceeds via two structural intermediates. Protein Sci. 2002, 11, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, F.; Wösten, H.A.B.; Hektor, H.J.; Poolman, B.; Robillard, G.T. The SC3 hydrophobin self-assembles into a membrane with distinct mass transfer properties. Biophys. J. 2005, 88, 3434–3443. [Google Scholar] [CrossRef] [PubMed]
- Butko, P.; Buford, J.P.; Goodwin, J.S.; Stroud, P.A.; McCormick, C.L.; Cannon, G.C. Spectroscopic evidence for amyloid-like interfacial self-assembly of hydrophobin Sc3. Biochem. Biophys. Res. Comm. 2001, 280, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Mackay, J.P.; Matthews, J.M.; Winefield, R.D.; Mackay, L.G.; Haverkamp, R.G.; Templeton, M.D. The hydrophobin EAS is largely unstructured in solution and functions by forming amyloid-like structures. Structure 2001, 9, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Scholtmeijer, K.; de Vocht, M.L.; Rink, R.; Robillard, G.T.; Wösten, H.A.B. Assembly of the fungal SC3 hydrophobin into functional amyloid fibrils depends on its concentration and is promoted by cell wall polysaccharides. J. Biol. Chem. 2009, 284, 26309–26314. [Google Scholar] [CrossRef] [PubMed]
- Szilvay, G.R.; Nakari-Setälä, T.; Linder, M.B. Behavior of Trichoderma reseei hydrophobins in solution: Interactions, dynamics and multimer formation. Biochemistry 2006, 45, 8590–8598. [Google Scholar] [CrossRef] [PubMed]
- Torkkeli, M.; Serimaa, R.; Ikkala, O.; Linder, M.B. Aggregation and self-assembly of hydrophobins from Trichoderma reesei: Low-resolution structural models. Biophys. J. 2002, 83, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Szilvay, G.R.; Kisko, K.; Serimaa, R.; Linder, M.B. The relation between solution association and surface activity of the hydrophobin HFBI from Trichoderma reseei. FEBS Lett. 2007, 581, 2721–2726. [Google Scholar] [CrossRef] [PubMed]
- Paananen, A.; Vuorimaa, E.; Torkkeli, M.; Penttilä, M.E.; Kauranen, M.; Ikkala, O.; Lemmetyinen, H.; Serimaa, R; Linder, M.B. Structural hierarchy in molecular films of two class II hydrophobins. Biochemistry 2003, 42, 5253–5258. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, J.J.; Conley, A.J.; Lienemann, M.; Brandle, J.E.; Linder, M.B.; Menassa, R. Hydrophobin fusions for high-level transient protein expression and purification in Nicotiana benthamiana. Plant Physiol. 2010, 152, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, T.; Linder, M.B.; Nakari-Setälä, T.; Oker-Blom, C. Hydrophobin (HFBI): A potential fusion partner for one-step purification of recombinant proteins from insect cells. Protein Expr. Purif. 2008, 59, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.B.; Qiao, M.; Laumen, F.; Selber, K.; Hyytiä, T.; Nakari-Setälä, T.; Penttilä, M.E. Efficient purification of recombinant proteins using hydrophobins as tags in surfactant-based two-phase systems. Biochemistry 2004, 43, 11873–11882. [Google Scholar] [CrossRef] [PubMed]
- Kostiainen, M.A.; Szilvay, G.R.; Lehtinen, J.; Smith, D.K.; Linder, M.B; Urtti, A.; Ikkala, O. Precisely defined protein-polymer conjugates: Construction of synthetic DNA binding domains on protein by using multivalent dendrons. ACS Nano 2007, 1, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Kostiainen, M.A.; Szilvay, G.R.; Smith, D.K.; Linder, M.B; Urtti, A.; Ikkala, O. Multivalent dendrons for high-affinity adhesion of proteins to DNA. Angew. Chem. Int. Ed. Engl. 2006, 45, 3538–3542. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, P.; Kivioja, J.; Paananen, A.; Kainlauri, M.; Kontturi, K.; Ahopelto, J.; Linder, M.B. Selective nanopatterning using citrate-stabilized Au nanoparticles and cystein-modified amphiphilic protein. Langmuir 2009, 25, 5185–5192. [Google Scholar] [CrossRef] [PubMed]
- Scholtmeijer, K.; Rink, R.; Hektor, H.J.; Wösten, H.A.B. Expression and Engineering of Fungal Hydrophobins. In Applied Mycology and Biotechnology; Elsevier: Amsterdam, The Netherlands, 2005; Volume 5, Chapter 10. [Google Scholar]
- Linder, M.B. Hydrophobins: Proteins that self assemble at interface. Curr. Opin. Colloid Interface Sci. 2009, 14, 356–363. [Google Scholar] [CrossRef]
- Lumsdon, S.O.; Green, J.; Stieglitz, B. Adsorption of hydrophobin proteins at hydrophobic and hydrophilic interfaces. Colloids Surf. B Biointerfaces 2005, 44, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Haas Jimoh Akanbi, M.; Post, E.; Meter-Arkema, A.; Rink, R.; Robillard, G.T.; Wang, X.; Wösten, H.A.B.; Scholtmeijer, K. Use of hydrophobins in formulation of water insoluble drugs for oral administration. Colloids Surf. B Biointerfaces 2010, 75, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Valo, H.K.; Laaksonen, P.H.; Peltonen, L.J.; Linder, M.B.; Hirvonen, J.T.; Laaksonen, T.J. Multifunctional hydrophobin: Toward functional coatings for drug nanoparticles. ACS Nano 2010, 4, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.; Li, J.; Cannon, G.C.; Morgan, S.E. Nanoscale reduction in surface friction of polymer surfaces modified with Sc3 hydrophobin from Schizophyllum commune. Biomacromolecules 2006, 7, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, L.; Rea, I.; Armenante, A.; Giardina, P.; Giocondo, M.; Rendina, I. Self-assembled biofilm of hydrophobins protects the silicon surface in the KOH wet etch process. Langmuir 2007, 23, 7920–7922. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Wang, L.K.; Feng, X.Z.; Yang, Y.L.; Wang, R.; Wang, C.; Yu, L.; Shao, B.; Qiao, M.Q. Bioactive surface modification of mica and poly(dimethylsiloxane) with hydrophobins for protein immobilization. Langmuir 2007, 23, 4465–4471. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lienemann, M.; Qiao, M.; Linder, M.B. Mechanisms of protein adhesion on surface films of hydrophobin. Langmuir 2010, 26, 8491–8496. [Google Scholar] [CrossRef] [PubMed]
- Corvis, Y.; Walcarius, A.; Rink, R.; Mrabet, N.T.; Rogalska, E. Preparing catalytic surfaces for sensing applications by immobilizing enzymes via hydrophobin layers. Anal. Chem. 2005, 77, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.X.; Qiao, M.Q.; Yin, F.; Shao, B.; Wu, B.Y.; Wang, Y.Y.; Wang, X.S.; Qin, X.; Li, S.; Chen, Q. Amperometric glucose biosensor based on self-assembly hydrophobin with high efficiency of enzyme utilization. Biosens. Bioelectron. 2007, 22, 3021–3027. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.X.; Wang, H.C.; Qin, X.; Wang, X.S.; Qiao, M.Q.; Anzai, J.; Chen, Q. Self-assembled film of hydrophobins on gold surfaces and its application to electrochemical biosensing. Colloids Surf. B Biointerfaces 2009, 71, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Li, X.; Feng, X.Z.; Wang, R.; Wang, C.; Yu, L.; Qiao, M.Q. Surface modification using a novel type I hydrophobin HGFI. Anal. Bioanal. Chem. 2009, 394, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Hou, S.; Wang, L.; Feng, X.; Wang, R.; Yang, Y.; Wang, C.; Yu, L.; Shao, B.; Qiao, M. Two methods for glass surface modification and their application in protein immobilization. Colloids Surf. B Biointerfaces 2007, 60, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.I.; van Leeuwen, M.B.M.; Scholtmeijer, K.; van Kooten, T.G.; Dijkhuizen, L.; Wösten, H.A.B. Coating with genetic engineered hydrophobin promotes growth of fibroblasts on a hydrophobic solid. Biomaterials 2002, 23, 4847–4854. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.I.; van Leeuwen, M.B.M.; van Kooten, T.G.; de Vries, J.; Dijkhuizen, L.; Wösten, H.A.B. Promotion of fibroblast activity by coating with hydrophobins in the β-sheet end state. Biomaterials 2004, 25, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Yang, K.; Qin, M.; Feng, X.Z.; Guan, L.; Yang, Y.; Wang, C. Patterning of cells on functionalized poly(dimethylsiloxane) surface prepared by hydrophobin and collagen modification. Biosens. Bioelectron. 2008, 24, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hou, S.; Feng, X.; Yu, Y.; Ma, J.; Li, L. Patterning of neural stem cells on poly(lactic-co-glycolic acid) film modified by hydrophobin. Colloids Surf. B Biointerfaces 2009, 74, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Askolin, S.; Nakari-Setälä, T.; Tenkanen, M. Overproduction, purification and characterization of the Trichoderma reesei hydrophobin HFBI. Appl. Microbiol. Biotechnol. 2001, 57, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Feng, S.; Huang, Y.; Li, S.; Xu, H.; Zhang, X.; Bai, Y.; Qiao, M. Expression and characterization of a Grifola frondosa hydrophobin in Pichia pastoris. Protein Expr. Purif. 2010, 72, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Wohlleben, W.; Subkowski, T.; Bollschweiler, C.; von Vacano, B.; Liu, Y.; Schrepp, W.; Baus, U. Recombinantly produced hydrophobins from fungal analogues as highly surface-active performance proteins. Eur. Biophys. J. 2010, 39, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Lugones, L.G.; Wösten, H.A.B.; Birkenkamp, K.U.; Sjollema, K.A.; Zagers, J.; Wessels, J.G.H. Hydrophobins line air channels in fruiting bodies of Schizophyllum commune and Agaricus bisporus. Mycol. Res. 1999, 103, 635–640. [Google Scholar] [CrossRef]
- De Vries, O.M.H.; Moore, S.; Arntz, C.; Wessels, J.G.H.; Tudzynski, P. Identification and characterization of a tri-partite hydrophobin from Claviceps fusiformis; A novel type of class II hydrophobin. FEBS J. 1999, 262, 377–385. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zampieri, F.; Wösten, H.A.B.; Scholtmeijer, K. Creating Surface Properties Using a Palette of Hydrophobins. Materials 2010, 3, 4607-4625. https://doi.org/10.3390/ma3094607
Zampieri F, Wösten HAB, Scholtmeijer K. Creating Surface Properties Using a Palette of Hydrophobins. Materials. 2010; 3(9):4607-4625. https://doi.org/10.3390/ma3094607
Chicago/Turabian StyleZampieri, Filippo, Han A. B. Wösten, and Karin Scholtmeijer. 2010. "Creating Surface Properties Using a Palette of Hydrophobins" Materials 3, no. 9: 4607-4625. https://doi.org/10.3390/ma3094607