Growth and Structure of ZnO Nanorods on a Sub-Micrometer Glass Pipette and Their Application as Intracellular Potentiometric Selective Ion Sensors
Abstract
:1. Introduction
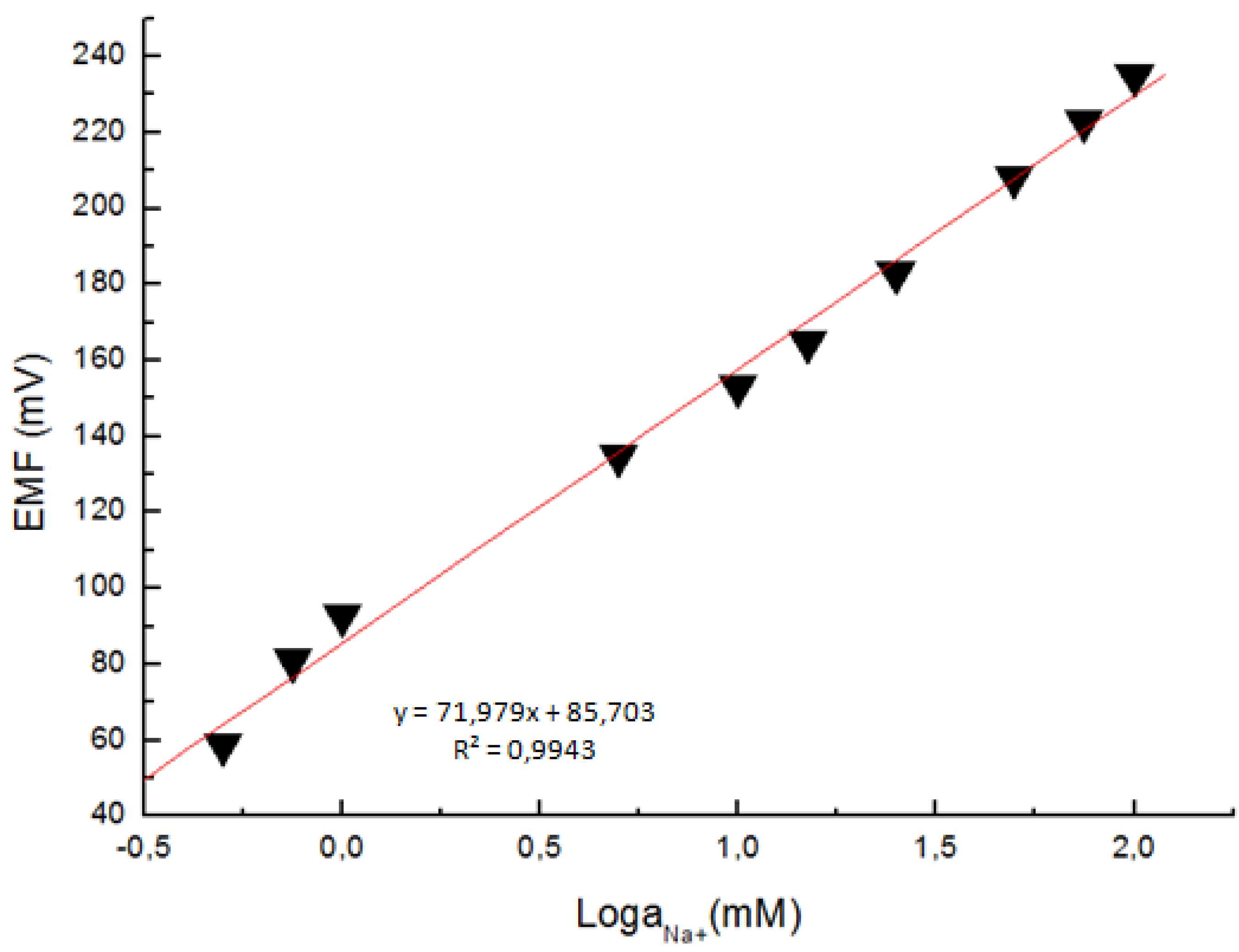
2. Results and Discussion
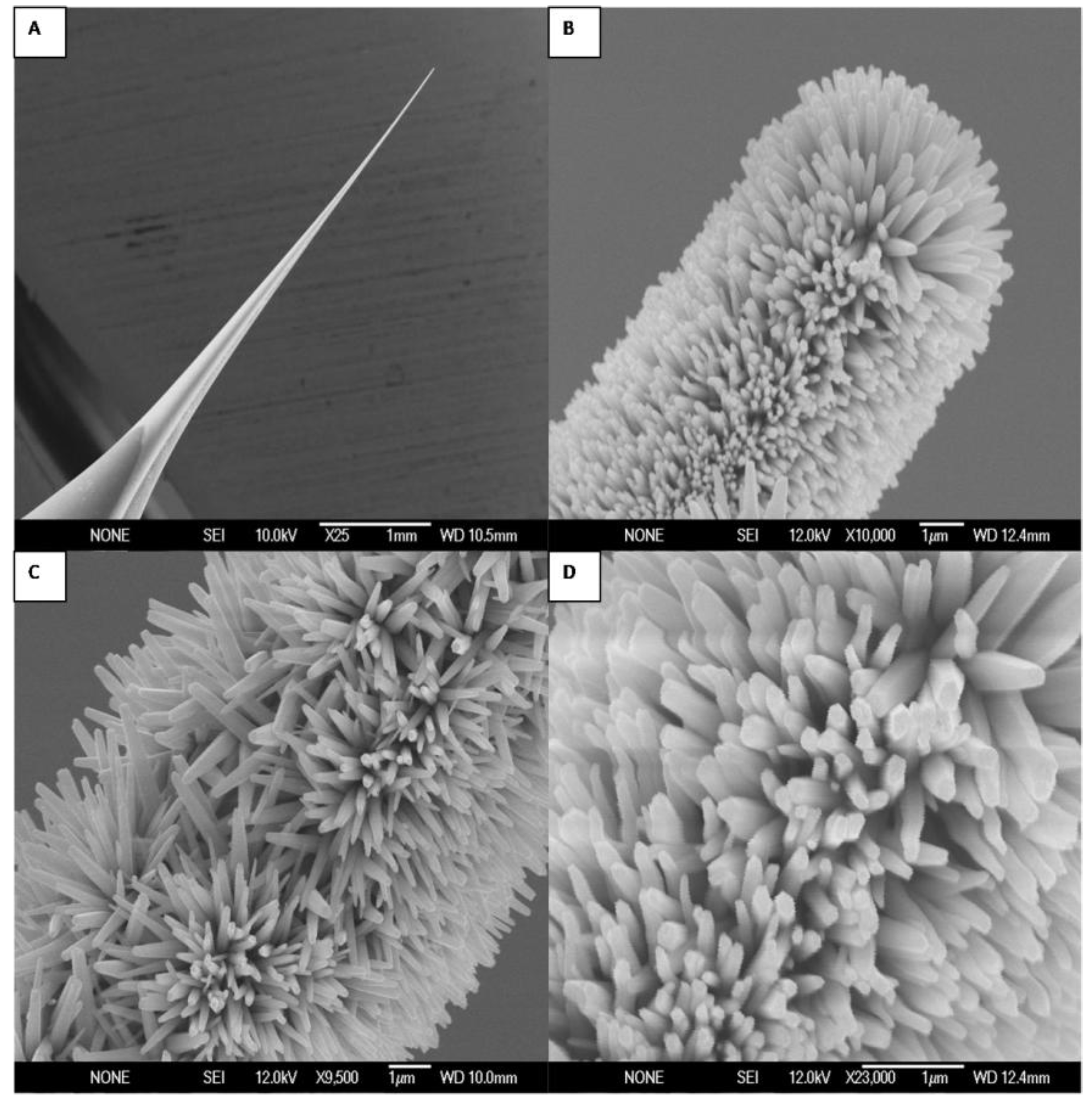
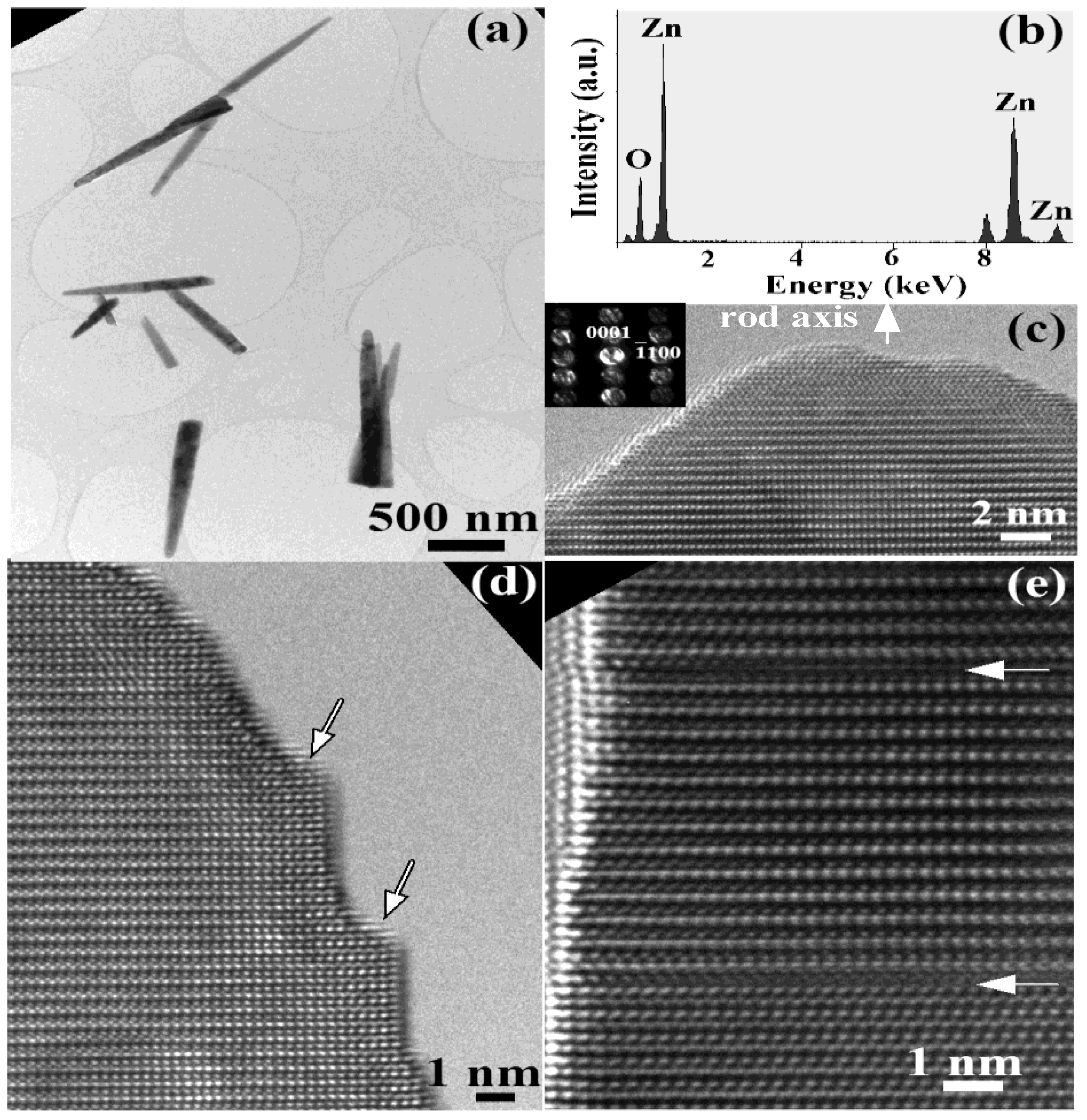
3. Experimental Section
3.1. Growth and characterization of ZnO nanostructures
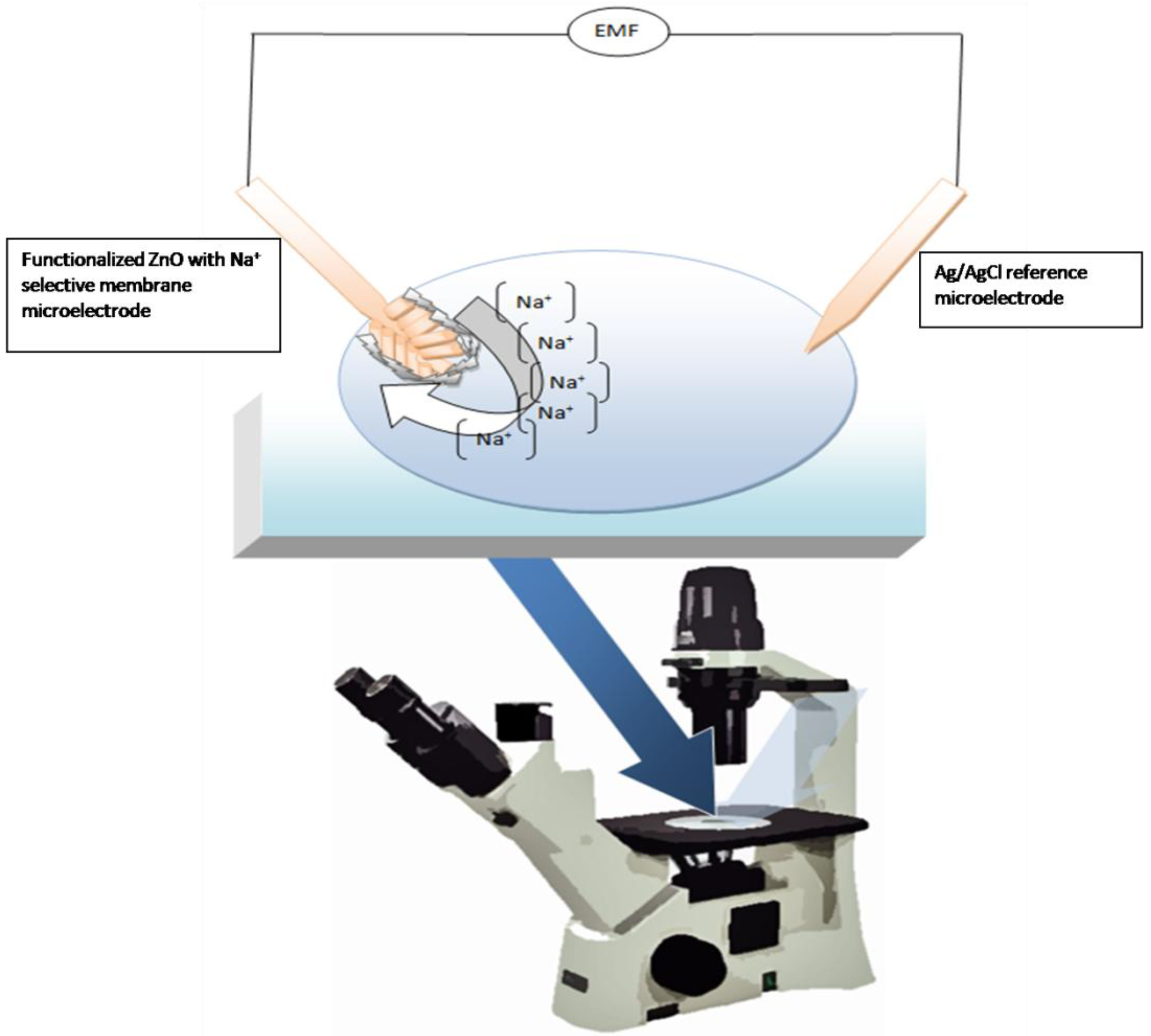
3.2. Immobilization of membrane and electrochemical measurements
3.3. Cellular preparations
4. Conclusions
References
- Heo, Y.W.; Norton, D.P.; Tien, L.C.; Kwon, Y.; Kang, B.S.; Ren, F.; Pearton, S.J.; LaRoche, J.R. ZnO nanowire growth and devices. Mater. Sci. Eng. 2004, 47, 1–47. [Google Scholar] [CrossRef]
- Fan, Z.; Lu, J.G. Zinc oxide nanostructures: Synthesis and properties. J. Nanosci. Nanotechnol. 2005, 5, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.T.; Muster, J.; Krstic, V.; Park, J.G.; Park, Y.W.; Roth, S.; Burghard, M. Field-effect transistor made of individual V2O5 nanofibers. Appl. Phys. Lett. 2000, 76, 1875–1877. [Google Scholar] [CrossRef]
- Stone, N.J.; Ahmed, H. Silicon single electron memory cell. Appl. Phys. Lett. 1998, 73, 2134–2136. [Google Scholar] [CrossRef]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.H.; Mao, S.; Feick, H.; Yan, H.; Wu, Y.; Kind, H.; Weber, E.; Russo, R.; Yang, P. Room-temperature ultraviolet nanowire nanolasers. Science 2001, 292, 1897–1899. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Kong, X.Y.; Ding, Y.; Gao, P.; Hughes, W.L.; Yang, R.; Zhang, Y. Semiconducting and piezoelectric oxide nanostructures induced by polar surface. Adv. Funct. Mater. 2004, 14, 943–956. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Z.L. Structure analysis of nanowires and nanobelts by transmission electron microscopy. J. Phys.Chem. B 2004, 108, 12280–12291. [Google Scholar]
- Tien, L.C.; Sadik, P.W.; Norton, D.P.; Voss, L.F.; Pearton, S.J.; Wang, H.T.; Kang, B.S.; Ren, F.; Jun, J.; Lin, J. Hydrogen sensing at room temperature with Pt-coated ZnO thin films and nanorods. Appl. Phys. Lett. 2005, 87, 222106–222108. [Google Scholar]
- Hsueh, T.J.; Chang, S.J.; Hsu, C.L.; Lin, Y.R.; Chen, I.C. Highly sensitive ZnO nanowire ethanol sensor with Pd adsorption. Appl. Phys. Lett. 2007, 91, 053111–053113. [Google Scholar]
- Li, Q.H.; Gao, T.; Wang, Y.G.; Wang, T.H. Adsorption and desorption of oxygen probed from ZnO nanowire films by photocurrent measurements. Appl. Phys. Lett. 2005, 86, 123117–123119. [Google Scholar] [CrossRef]
- Hung, X.J.; Choi, Y.K. Chemical sensors based on nanostructured materials. Sensor. Actuator. B-Chem. 2007, 122, 659–671. [Google Scholar]
- Li, C.C.; Du, Z.F.; Li, L.M.; Yu, H.C.; Wan, Q.; Wang, T.H. Surface-depletion controlled gas sensing of ZnO nanorods grown at room temperature. Appl. Phys. Lett. 2007, 91, 032101–032103. [Google Scholar]
- Ghosh, R.; Dutta, M.; Basak, D. Self-seeded growth and ultraviolet photoresponse properties of ZnO nanowire arrays. Appl. Phys. Lett. 2007, 91, 073108–073110. [Google Scholar]
- Qiu, Y.F; Yang, S.H. ZnO nanotetrapods: Controlled vapor-phase synthesis and application for humidity sensing. Adv. Funct. Mater. 2007, 17, 1345–1352. [Google Scholar]
- Park, J.Y.; Song, D.E.; Kim, S.S. An approach to fabricating chemical sensors based on ZnO nanorod arrays. Nanotechnology 2008, 19, 105503–105508. [Google Scholar]
- Hille, B. Ion Channel of Excitable Membranes, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2001. [Google Scholar]
- McDonough, A.A. Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R851–R861. [Google Scholar] [CrossRef] [PubMed]
- Hodgkin, A.L.; Katz, B. The effect of sodium ions on the electrical activity of the giant axon of the squid. J. Physiol. (Lond.) 1949, 108, 37–77. [Google Scholar] [CrossRef]
- Knight, K.K.; Wentzlaff, D.M.; Snyder, P.M. Intracellular sodium regulates proteolytic activation of the epithelial sodium channel. J. Biol. Chem. 2008, 283, 27477–27482. [Google Scholar] [CrossRef] [PubMed]
- Ho, I.H.; Murrell-Lagnado, R.D. Molecular mechanism for sodium-dependent activation of G protein-gated K+ channels. J. Physiol. 1999, 520, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.M.; Salter, M.W. Gain control of NMDA-receptor currents by intracellular sodium. Nature 1998, 396, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Efendiev, R.; Bertorello, A.M.; Zandomeni, R.; Cinelli, A.R.; Pedemonte, C.H. Agonist-dependent regulation of renal Na+, K+-ATPase activity is modulated by intracellular sodium concentration. J. Biol. Chem. 2002, 277, 11489–11496. [Google Scholar] [CrossRef] [PubMed]
- Budelli, G.; Hage, T.A.; Wei, A.; Rojas, P.; Jong, Y.J.; O’Malley, K.; Salkoff, L. Na+-activated K+ channels express a large delayed outward current in neurons during normal physiology. Nat. Neurosci. 2009, 12, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, M.; Kakei, M.; Sato, R.; Shibasaki, T.; Matsuda, H.; Irisawa, H. Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature 1984, 309, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Akanda, N.; Tofighi, R.; Brask, J.; Tamm, C.; Elinder, F.; Ceccatelli, S. Voltage-dependent anion channels (VDAC) in the plasma membrane play a critical role in apoptosis in differentiated hippocampal neurons but not in neural stem cells. Cell Cycle 2008, 7, 3225–3234. [Google Scholar]
- Thompson, G.J.; Langlais, C.; Cain, K.; Conley, E.C.; Cohen, G.M. Elevated extracellular [K+] inhibits death-receptor- and chemical-mediated apoptosis prior to caspase activation and cytochrome c release. Biochem. J. 2001, 357, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Banasiak, K.J.; Burenkova, O.; Haddad, G.G. Activation of voltage-sensitive sodium channels during oxygen deprivation leads to apoptotic neuronal death. Neuroscience 2004, 126, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Bortner, C.D.; Cidlowski, J.A. Uncoupling cell shrinkage from apoptosis reveals that Na+ influx is required for volume loss during programmed cell death. J. Biol. Chem. 2003, 278, 39176–39184. [Google Scholar] [CrossRef] [PubMed]
- Bortner, C.D.; Gomez-Angelats, M.; Cidlowski, J.A. Plasma membrane depolarization without repolarization is an early molecular event in anti-Fas-induced apoptosis. J. Biol. Chem. 2001, 276, 4304–4314. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.H.; Fulati, A.; Nur, O.; Willander, M.; Brännmark, C.; Strålfors, P.; Börjesson, S.I.; Elinder, F. Functionalized zinc oxide nanorod with ionophore-membrane coating as an intracellular Ca2+ selective sensor. Appl. Phys. Lett. 2009, 95, 023703–023705. [Google Scholar] [CrossRef]
- Asif, M.H.; Ali, S.U.; Nur, O.; Willander, M.; Brännmark, C.; Strålfors, P.; Englund, U.H.; Elinder, F.; Danielsson, B. Functionalised ZnO-nanorod-based selective electrochemical sensor for intracellular glucose. Biosens. Bioelectron. 2010, 25, 2205–2211. [Google Scholar]
- Michalska, A.; Hulanicki, A.; Lewenstam, A. All-Solid-State Potentiometric Sensors for Potassium and Sodium Based on Poly(pyrrole) Solid Contact. Microchem. J. 1997, 57, 59–64. [Google Scholar]
- Asif, M.H.; Nur, O.; Willander, M.; Yakovleva, M.; Danielsson, B. Studies on calcium ion selectivity of ZnO nanowire sensors using ionophore membrane coatings. Res. Lett. Nanotechnol. 2008, 2008, 1–4. [Google Scholar] [CrossRef]
- Strålfors, P.; Honnor, R.C. Insulin-induced dephosphorylation of hormone-sensitive lipase Correlation with lipolysis and CAMP-dependent protein kinase activity. Eur. J. Biochem. 1989, 182, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, A.; Öst, A.; Lystedt, E.; Kjolhede, P.; Gustavsson, J.; Nyström, F.H.; Strålfors, P. Insulin resistance in human adipocytes occurs downstream of IRS1 after surgical cell isolation but at the level of phosphorylation of IRS1 in type 2 diabetes. FEBS J. 2005, 272, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Börjesson, S.I.; Parkkari, T.; Hammarström, S.; Elinder, F. Electrostatic tuning of cellular excitability. Biophys. J. 2010, 98, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L. ZnO nanowire and nanobelt platform for nanotechnology. Mater. Sci. Eng. 2009, 64, 33–71. [Google Scholar] [CrossRef]
- Resh, M.D.; Nemenoff, R.A.; Guidotti, G. Insulin stimulation of (Na+, K+)- adenosine triphosphatase-dependent 86Rb+ uptake in rat adipocytes. J. Biol. Chem. 1980, 255, 10938–10945. [Google Scholar] [PubMed]
- Dascal, N. The use of Xenopus oocytes for the study of ion channels. CRC Crit. Rev. Biochem. 1987, 22, 317–387. [Google Scholar] [CrossRef]
- Zhou, B.J.; Xu, N.; Wang, Z.L. Dissolving behavior and stability of ZnO wires in biofluids: A study on biodegradability and biocompatibility of ZnO nanostructures. Adv. Mater. 2006, 18, 2432–2435. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Asif, M.H.; Nur, O.; Willander, M.; Strålfors, P.; Brännmark, C.; Elinder, F.; Englund, U.H.; Lu, J.; Hultman, L. Growth and Structure of ZnO Nanorods on a Sub-Micrometer Glass Pipette and Their Application as Intracellular Potentiometric Selective Ion Sensors. Materials 2010, 3, 4657-4667. https://doi.org/10.3390/ma3094657
Asif MH, Nur O, Willander M, Strålfors P, Brännmark C, Elinder F, Englund UH, Lu J, Hultman L. Growth and Structure of ZnO Nanorods on a Sub-Micrometer Glass Pipette and Their Application as Intracellular Potentiometric Selective Ion Sensors. Materials. 2010; 3(9):4657-4667. https://doi.org/10.3390/ma3094657
Chicago/Turabian StyleAsif, Muhammad H., Omer Nur, Magnus Willander, Peter Strålfors, Cecilia Brännmark, Fredrik Elinder, Ulrika H. Englund, Jun Lu, and Lars Hultman. 2010. "Growth and Structure of ZnO Nanorods on a Sub-Micrometer Glass Pipette and Their Application as Intracellular Potentiometric Selective Ion Sensors" Materials 3, no. 9: 4657-4667. https://doi.org/10.3390/ma3094657
APA StyleAsif, M. H., Nur, O., Willander, M., Strålfors, P., Brännmark, C., Elinder, F., Englund, U. H., Lu, J., & Hultman, L. (2010). Growth and Structure of ZnO Nanorods on a Sub-Micrometer Glass Pipette and Their Application as Intracellular Potentiometric Selective Ion Sensors. Materials, 3(9), 4657-4667. https://doi.org/10.3390/ma3094657




