Polydopamine-Supported Lipid Bilayers
Abstract
:1. Introduction
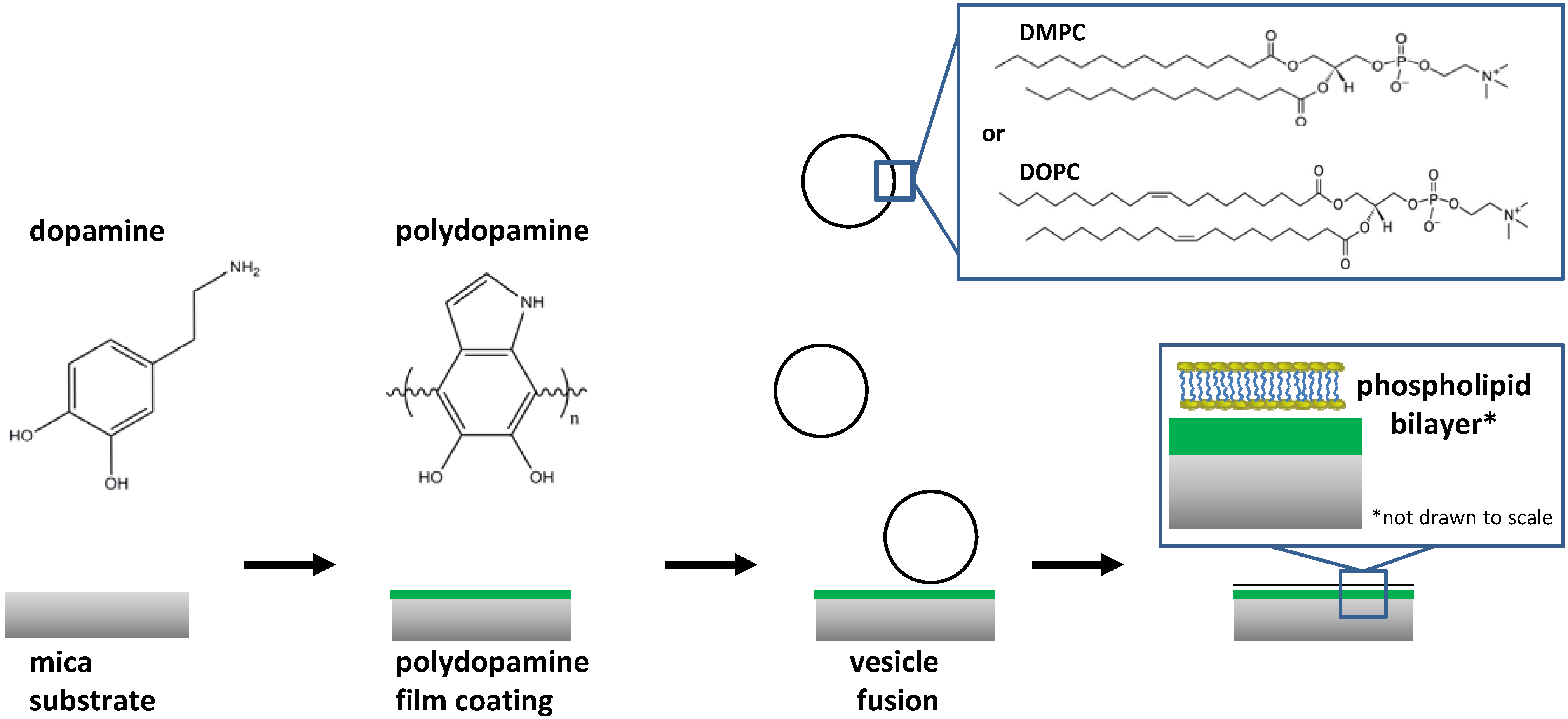
2. Results and Discussion
2.1. Polydopamine Film Thickness

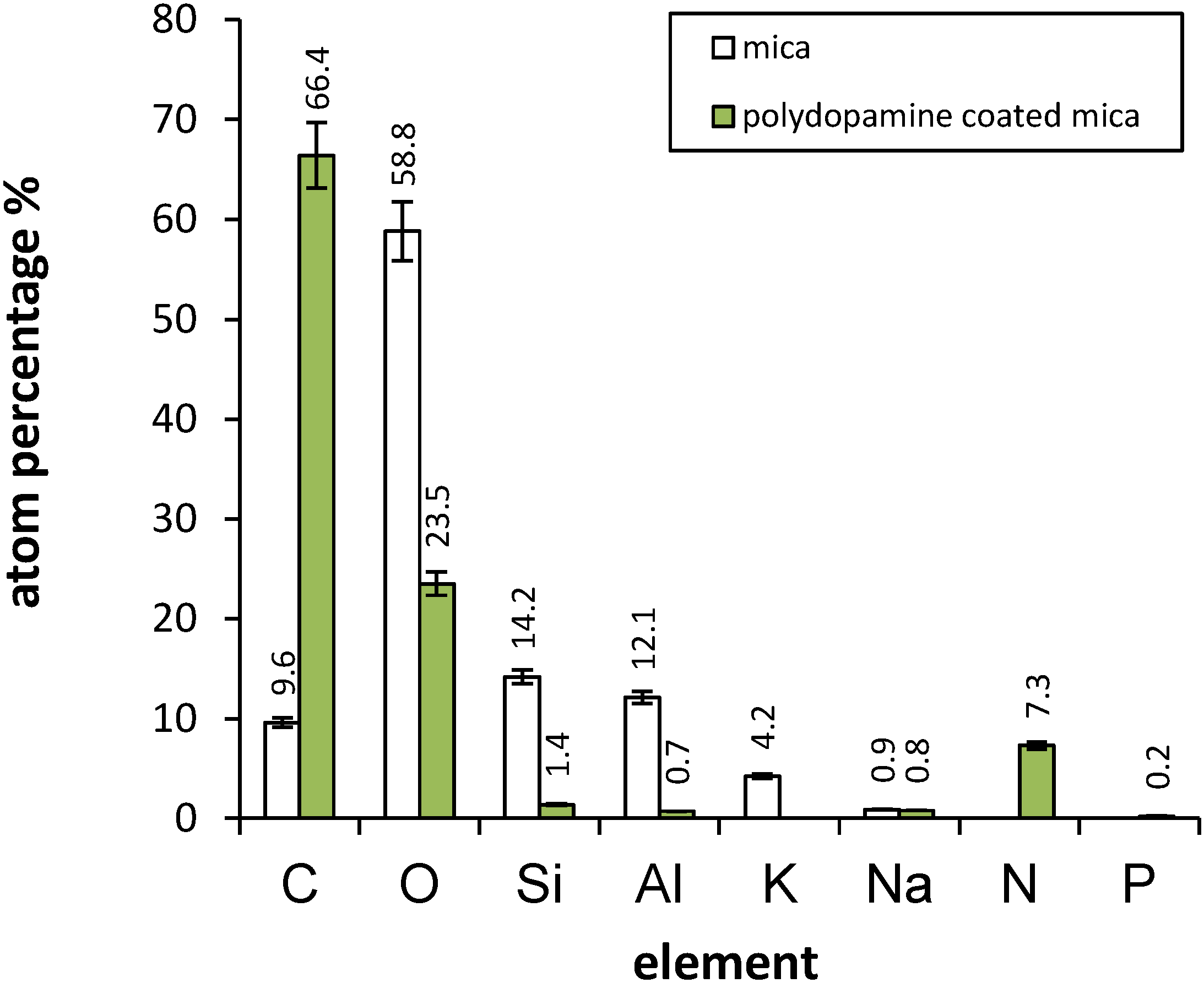
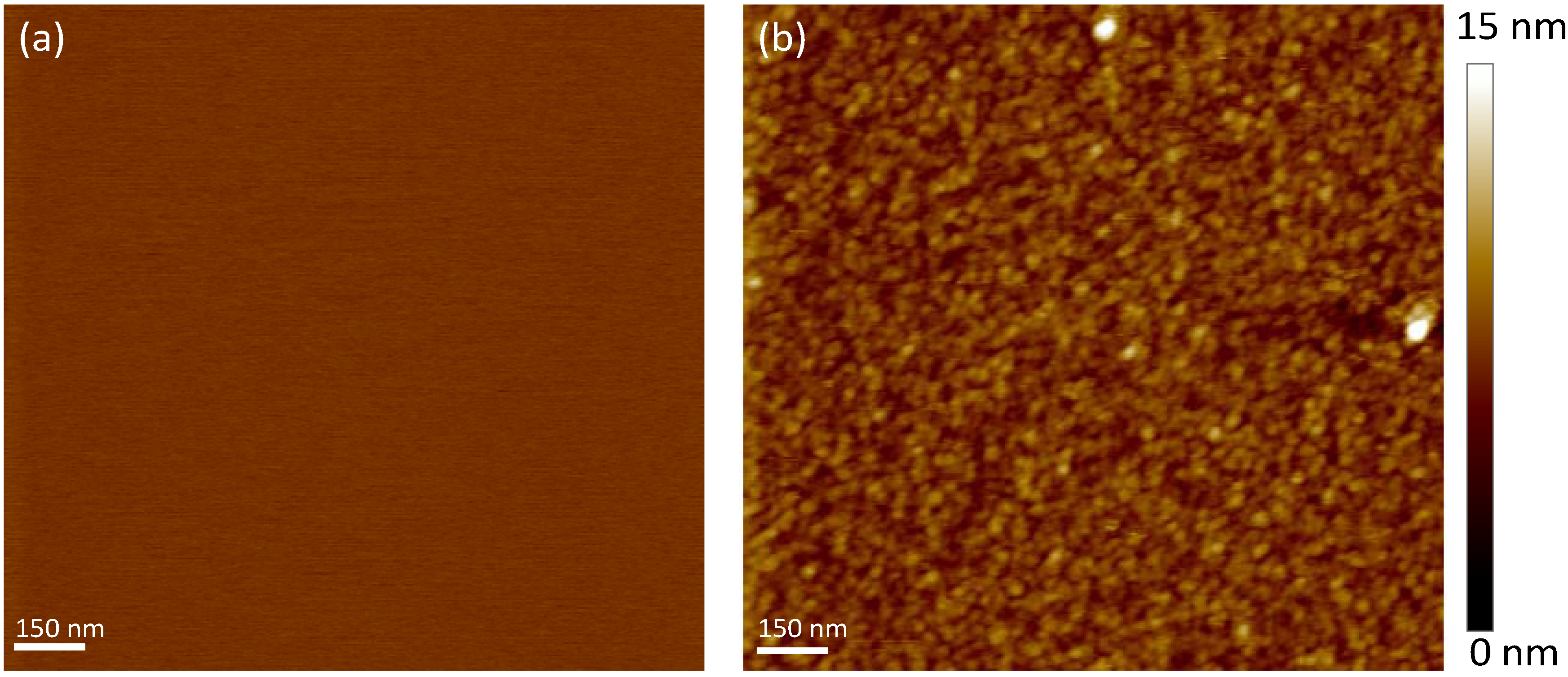
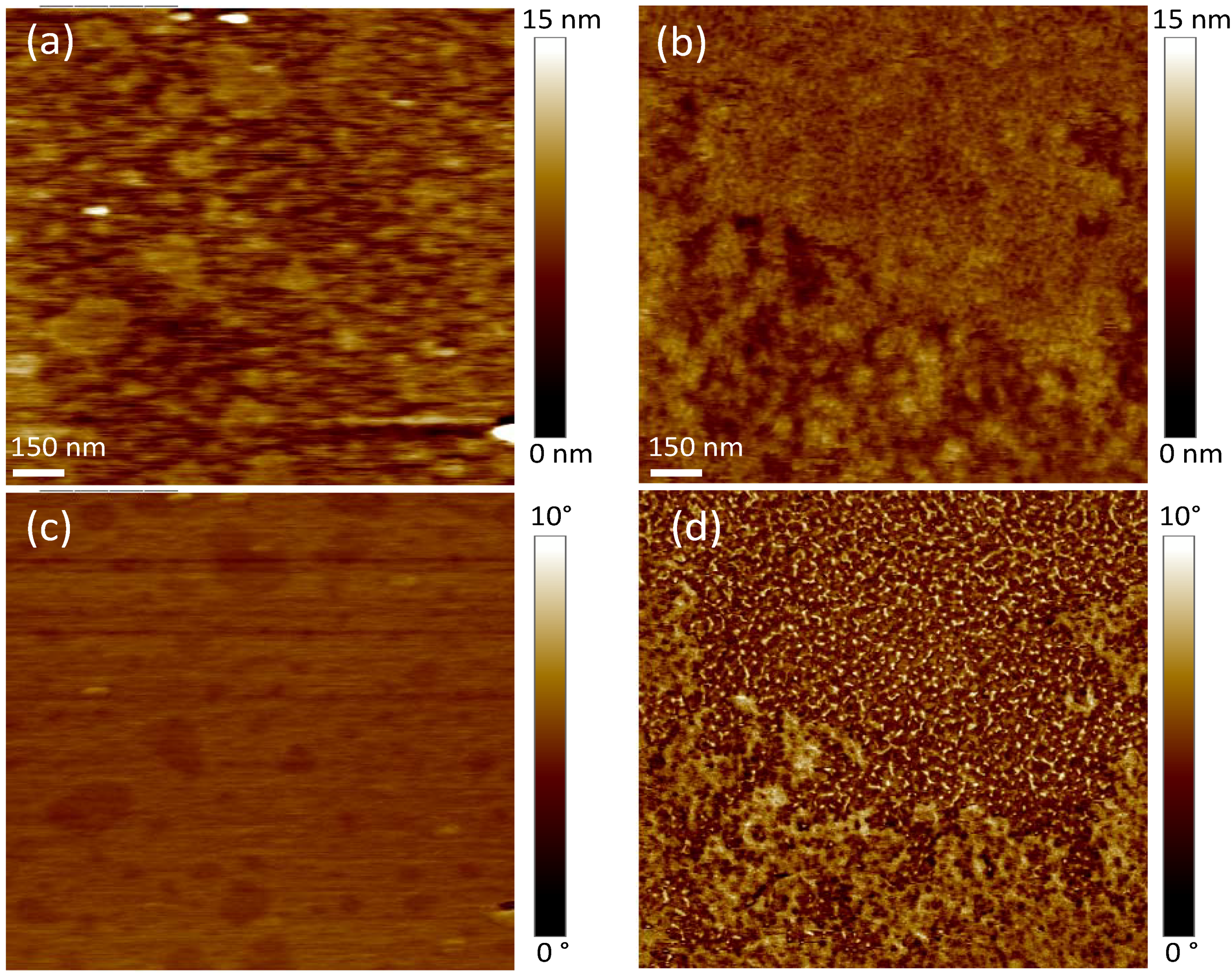
2.2. Mica and Polydopamine Film Characterization
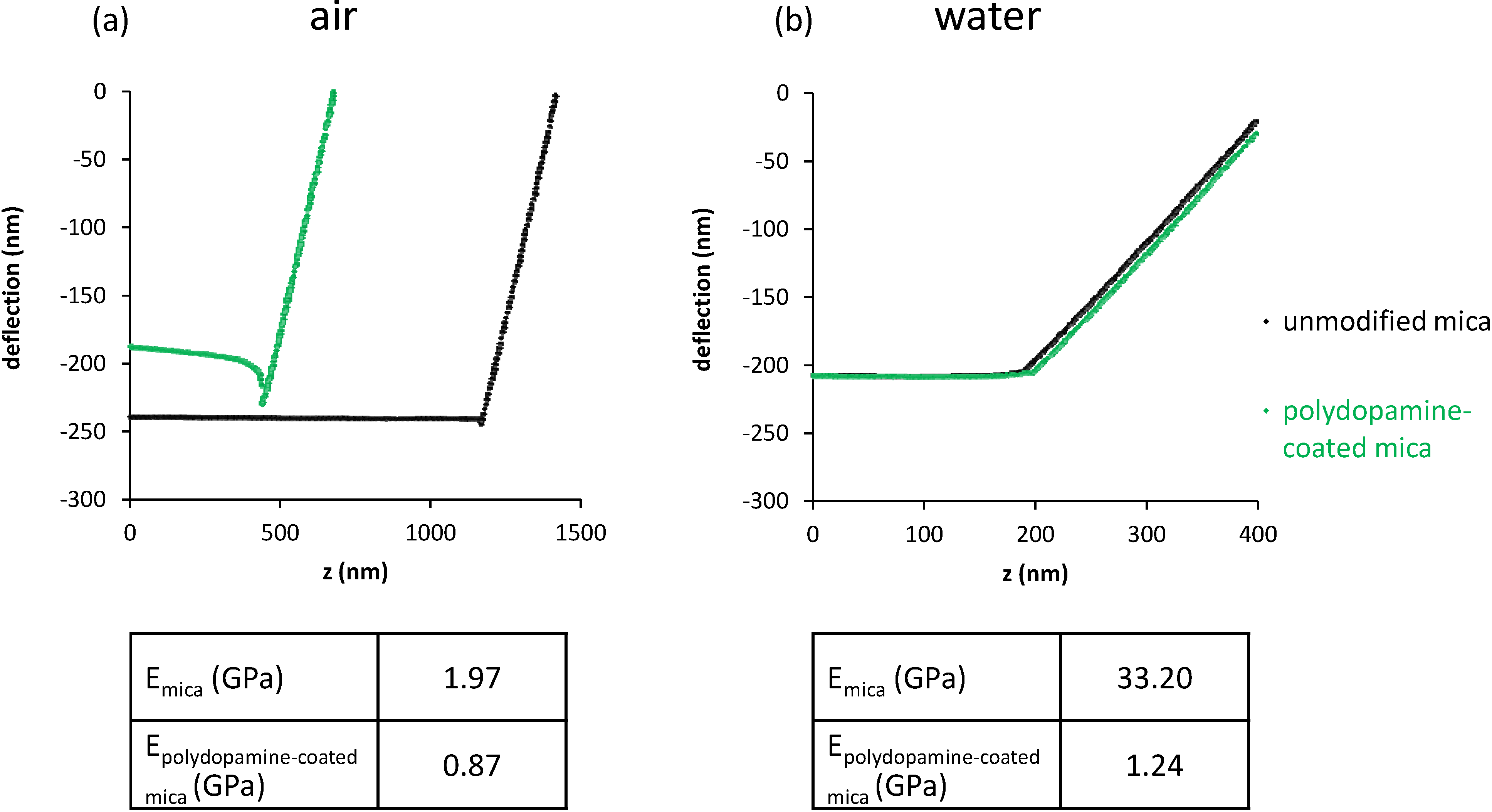
2.3. Characterization of DMPC and DOPC Bilayers on Polydopamine-Coated Mica

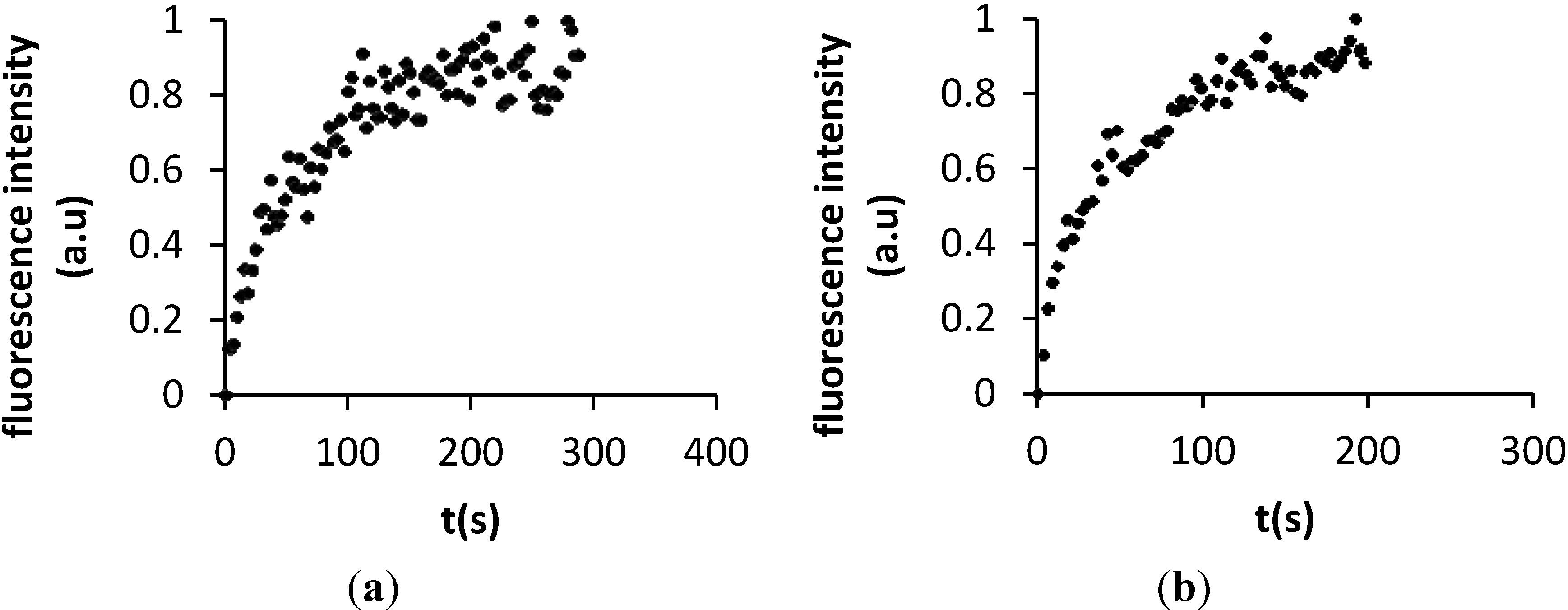
| Lipid | Polymer | Support | D (μm2/s) | Reference |
|---|---|---|---|---|
| DMPC | PAA | glass, quartz or silicon oxide | 2.56 ± 0.84 | [30] |
| DMPC/cholesterol/PEG-DMPE (1 mol %) | cellulose | glass | 3.3 ± 0.2 | [44] |
| DOPC | PEG | glass | 2.16 ± 0.07 | [45] |
| DOPC | (CHI/HA)5 | glass | 2.8 ± 0.2 | [45] |
| PC/PE/PS/cholesterol | maleic acid | silicon oxide | 0.26–2.6 | [46] |
3. Experimental Section
3.1. Materials
3.2. Coating of Mica with Polydopamine
3.3. Deposition of Phospholipid Bilayers
3.4. Ellipsometry
3.5. X-Ray Photoelectron Spectroscopy
3.6. Atomic Force Microscopy (AFM)
3.7. Fluorescence Microscopy and Fluorescence Recovery after Photobleaching (FRAP)
4. Conclusions
Acknowledgments
References
- Brian, A.A.; McConnell, H.M. Allogeneic stimulation of cytotoxic T cells by supported planar membranes. Proc. Natl. Acad. Sci. USA 1984, 81, 6159–6163. [Google Scholar] [CrossRef] [PubMed]
- Tamm, L.K.; McConnell, H.M. Supported phospholipid bilayers. Biophys. J. 1985, 47, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, V.; Domanska, M.K.; Murray, D.; Wan, C.; Tamm, L.K. Supported lipid bilayers: Development and applications in chemical biology. In Wiley Encyclopedia of Chemical Biology; John Wiley & Sons: Hoboken, NJ, USA, 2008; Volume 4, pp. 411–422. [Google Scholar]
- Castellana, E.T.; Cremer, P.S. Solid supported lipid bilayers: From biophysical studies to sensor design. Surf. Sci. Rep. 2006, 61, 429–444. [Google Scholar] [CrossRef]
- Richter, R.P.; Brisson, A.R. Following the formation of supported lipid bilayers on mica: A study combining AFM, QCM-D, and ellipsometry. Biophys. J. 2005, 88, 3422–3433. [Google Scholar] [CrossRef] [PubMed]
- Schönherr, H.; Johnson, J.M.; Lenz, P.; Frank, C.W.; Boxer, S.G. Vesicle adsorption and lipid bilayer formation on glass studied by atomic force microscopy. Langmuir 2004, 20, 11600–11606. [Google Scholar] [CrossRef] [PubMed]
- Reimhult, E.; Zäch, M.; Höök, F.; Kasemo, B. A multitechnique study of liposome adsorption on Au and lipid bilayer formation on SiO2. Langmuir 2006, 22, 3313–3319. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Sackmann, E. Polymer-supported membranes as models of the cell surface. Nature 2005, 437, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E. Supported membranes: Scientific and practical applications. Science 1996, 271, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.Y.; Park, C.K.; Seitz, M.; Israelachvili, J. Polymer-cushioned bilayers. II. An investigation of interaction forces and fusion using the surface forces apparatus. Biophys. J. 1999, 77, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.H. Mussel power. Nature 2008, 7, 8–9. [Google Scholar] [CrossRef]
- Sileika, T.S.; Kim, H.-D.; Maniak, P.; Messersmith, P.B. Antibacterial performance of polydopamine-modified polymer surfaces containing passive and active components. ACS Appl. Mater. Interfaces 2011, 3, 4602–4610. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shi, X.; Ma, H.; Lv, Y.; Zhang, L.; Mao, Z. The preparation and antibacterial effects of dopa-cotton/AgNPs. Appl. Surf. Sci. 2011, 257, 6799–6803. [Google Scholar] [CrossRef]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.H.; Ryu, J.; Hong, S.K.; Lee, H.; Park, C.B. General functionalization route for cell adhesion on non-wetting surfaces. Biomaterials 2010, 31, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Elucidating the structure of poly(dopamine). Langmuir 2012, 28, 6428–6435. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-covalent self-assembly and covalent polymerization co-contribute to polydopamine formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Nirasay, S.; Mouget, Y.; Marcotte, I.; Claverie, J.P. Supported bilayer on a nanopatterned membrane as model PAMPA membranes. Int. J. Pharm. 2011, 421, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhu, B.; Xu, Y. Surface characteristics of a self-polymerized dopamine coating deposited on hydrophobic polymer films. Langmuir 2011, 27, 14180–14187. [Google Scholar] [CrossRef] [PubMed]
- Shlyakhtenko, L.S.; Gall, A.A.; Weimer, J.J.; Hawn, D.D.; Lyubchenko, Y.L. Atomic force microscopy imaging of DNA covalently immobilized on a functionalized mica substrate. Biophys. J. 1999, 77, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, W.; Jiang, Z.; Dong, X.; Wang, B.; Zhong, Y. Ultrathin and stable active layer of dense composite membrane enabled by poly(dopamine). Langmuir 2009, 25, 7368–7374. [Google Scholar] [CrossRef] [PubMed]
- Mellott, N.P.; Brantley, S.L.; Hamilton, J.P.; Pantano, C.G. Evaluation of surface preparation methods for glass. Surf. Interface Anal. 2001, 31, 362–368. [Google Scholar] [CrossRef]
- Richter, R.P.; Bérat, R.; Brisson, A.R. Formation of solid-supported lipid bilayers: An integrated view. Langmuir 2006, 22, 3497–3505. [Google Scholar] [CrossRef] [PubMed]
- Domke, J.; Radmacher, M. Measuring the elastic properties of thin polymer films with the atomic force microscope. Langmuir 1998, 14, 3320–3325. [Google Scholar] [CrossRef]
- Cappella, B.; Dietler, G. Force-distance curves by atomic force microscopy. Surf. Sci. Rep. 1999, 34, 1–104. [Google Scholar] [CrossRef]
- Warschawski, D.E.; Arnold, A.A.; Beaugrand, M.; Gravel, A.; Chartrand, É.; Marcotte, I. Choosing membrane mimetics for NMR structural studies of transmembrane proteins. Biochim. Biophys. Acta 2011, 1808, 1957–1974. [Google Scholar] [CrossRef]
- Marsh, D. CRC Handbook of Lipid Bilayers; CRC Press: Boca Raton, FL, USA, 1990; pp. 1–387. [Google Scholar]
- Baumgart, T.; Offenhäusser, A. Polysaccharide-supported planar bilayer lipid model membranes. Langmuir 2003, 19, 1730–1737. [Google Scholar] [CrossRef]
- El-khouri, R.J.; Bricarello, D.A.; Watkins, E.B.; Kim, C.Y.; Miller, C.E.; Patten, T.E.; Parikh, A.N.; Kuhl, T.L. pH responsive polymer cushions for probing membrane environment interactions. Nano Lett. 2011, 11, 2169–2172. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.L.; Jablin, M.S.; Vidyasagar, A.; Saiz, J.; Watkins, E.; Toomey, R.; Hurd, A.J.; Majewski, J. Model lipid membranes on a tunable polymer cushion. Phys. Rev. Lett. 2009, 102, 1–4. [Google Scholar]
- Jablin, M.S.; Zhernenkov, M.; Toperverg, B.P.; Dubey, M.; Smith, H.L.; Vidyasagar, A.; Toomey, R.; Hurd, A.J.; Majewski, J. In-plane correlations in a polymer-supported lipid membrane measured by off-specular neutron scattering. Phys. Rev. Lett. 2011, 106, 1–4. [Google Scholar] [CrossRef]
- Jablin, M.S.; Dubey, M.; Zhernenkov, M.; Toomey, R.; Majewski, J. Influence of lipid membrane rigidity on properties of supporting polymer. Biophys. J. 2011, 101, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.L.; Tamm, L.K. Tethered polymer-supported planar lipid bilayers for reconstitution of integral membrane proteins: Silane-polyethyleneglycol-lipid as a cushion and covalent linker. Biophys. J. 2000, 79, 1400–1414. [Google Scholar] [CrossRef] [PubMed]
- Nussio, M.R.; Oncins, G.; Ridelis, I.; Szili, E.; Shapter, J.G.; Sanz, F.; Voelcker, N.H. Nanomechanical characterization of phospholipid bilayer islands on flat and porous substrates: A force spectroscopy study. J. Phys. Chem. B 2009, 113, 10339–10347. [Google Scholar] [CrossRef] [PubMed]
- Charrier, A.; Thibaudau, F. Main phase transitions in supported lipid single-bilayer. Biophys. J. 2005, 89, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Leonenko, Z.V.; Carnini, A.; Cramb, D.T. Supported planar bilayer formation by vesicle fusion: The interaction of phospholipid vesicles with surfaces and the effect of gramicidin on bilayer properties using atomic force microscopy. Biochim. Biophys. Acta 2000, 1509, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Vacic, A.; Criscione, J.M.; Rajan, N.K.; Stern, E.; Fahmy, T.M.; Reed, M.A. Determination of molecular configuration by Debye length modulation. J. Am. Chem. Soc. 2011, 133, 13886–13889. [Google Scholar] [CrossRef] [PubMed]
- Cha, T.; Guo, A.; Zhu, X.-Y. Formation of supported phospholipid bilayers on molecular surfaces: Role of surface charge density and electrostatic interaction. Biophys. J. 2006, 90, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Liu, J.; Liu, S.; Zhou, F. Pdop layer exhibiting zwitterionicity: A simple electrochemical interface for governing ion permeability. Chem. Commun. 2010, 46, 5900–5902. [Google Scholar] [CrossRef]
- Engler, E.M.; Andose, J.D.; Schleyer, P.V.R. Critical evaluation of molecular mechanics. J. Am. Chem. Soc. 1973, 95, 8005–8025. [Google Scholar] [CrossRef]
- Soumpasis, D.M. Theorotical analysis of fluorescence photobleaching recovery experiments. Biophys. J. 1983, 41, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Przybylo, M.; Sýkora, J.; Humpolíckova, J.; Benda, A.; Zan, A.; Hof, M. Lipid diffusion in giant unilamellar vesicles is more than 2 times faster than in supported phospholipid bilayers under identical conditions. Langmuir 2006, 22, 9096–9099. [Google Scholar] [CrossRef] [PubMed]
- Goennenwein, S.; Tanaka, M.; Hu, B.; Moroder, L.; Sackmann, E. Functional incorporation of integrins into solid supported membranes on ultrathin films of cellulose: Impact on adhesion. Biophys. J. 2003, 85, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, K.; Jakubek, Z.J.; Johnston, L.J. Supported lipid bilayers on biocompatible polysaccharide multilayers. Langmuir 2011, 27, 14352–14359. [Google Scholar] [CrossRef] [PubMed]
- Renner, L.; Osaki, T.; Chiantia, S.; Schwille, P.; Pompe, T.; Werner, C. Supported lipid bilayers on spacious and pH-responsive polymer cushions with varied hydrophilicity. J. Phys. Chem. B 2008, 112, 6373–6378. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nirasay, S.; Badia, A.; Leclair, G.; Claverie, J.P.; Marcotte, I. Polydopamine-Supported Lipid Bilayers. Materials 2012, 5, 2621-2636. https://doi.org/10.3390/ma5122621
Nirasay S, Badia A, Leclair G, Claverie JP, Marcotte I. Polydopamine-Supported Lipid Bilayers. Materials. 2012; 5(12):2621-2636. https://doi.org/10.3390/ma5122621
Chicago/Turabian StyleNirasay, Souryvanh, Antonella Badia, Grégoire Leclair, Jerome P. Claverie, and Isabelle Marcotte. 2012. "Polydopamine-Supported Lipid Bilayers" Materials 5, no. 12: 2621-2636. https://doi.org/10.3390/ma5122621





