CO2 Separation and Capture Properties of Porous Carbonaceous Materials from Leather Residues
Abstract
:1. Introduction
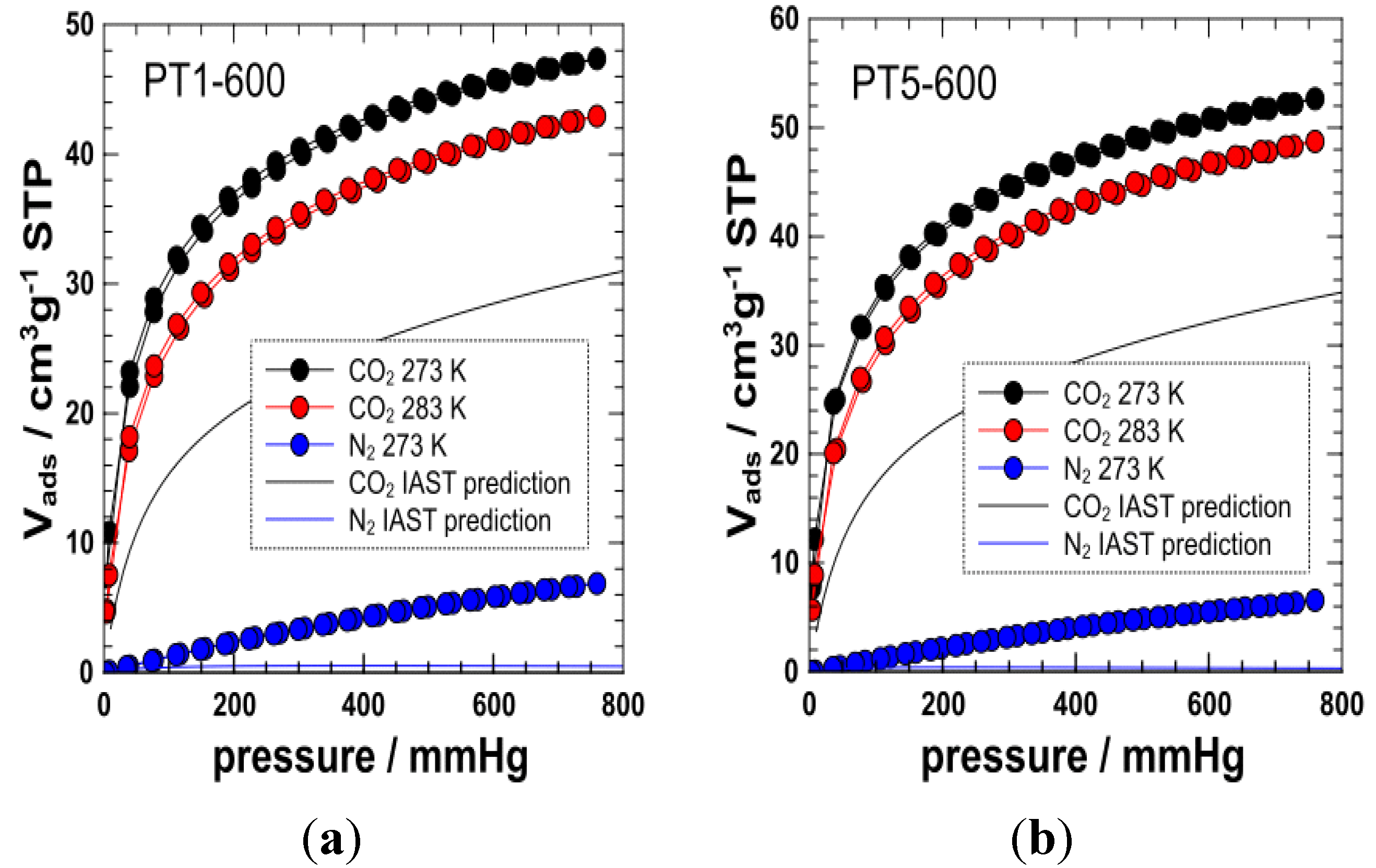
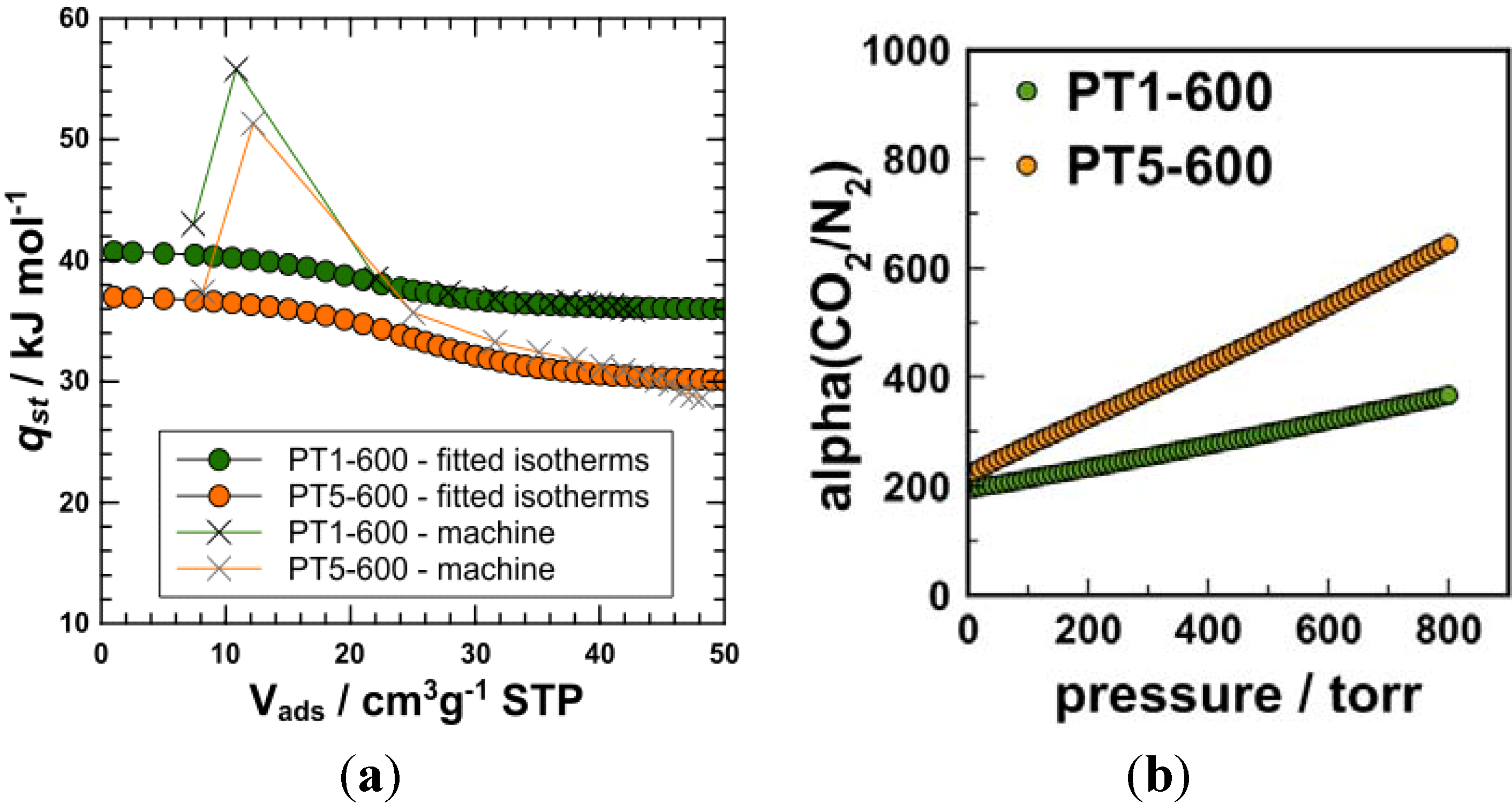
2. Results and Discussion
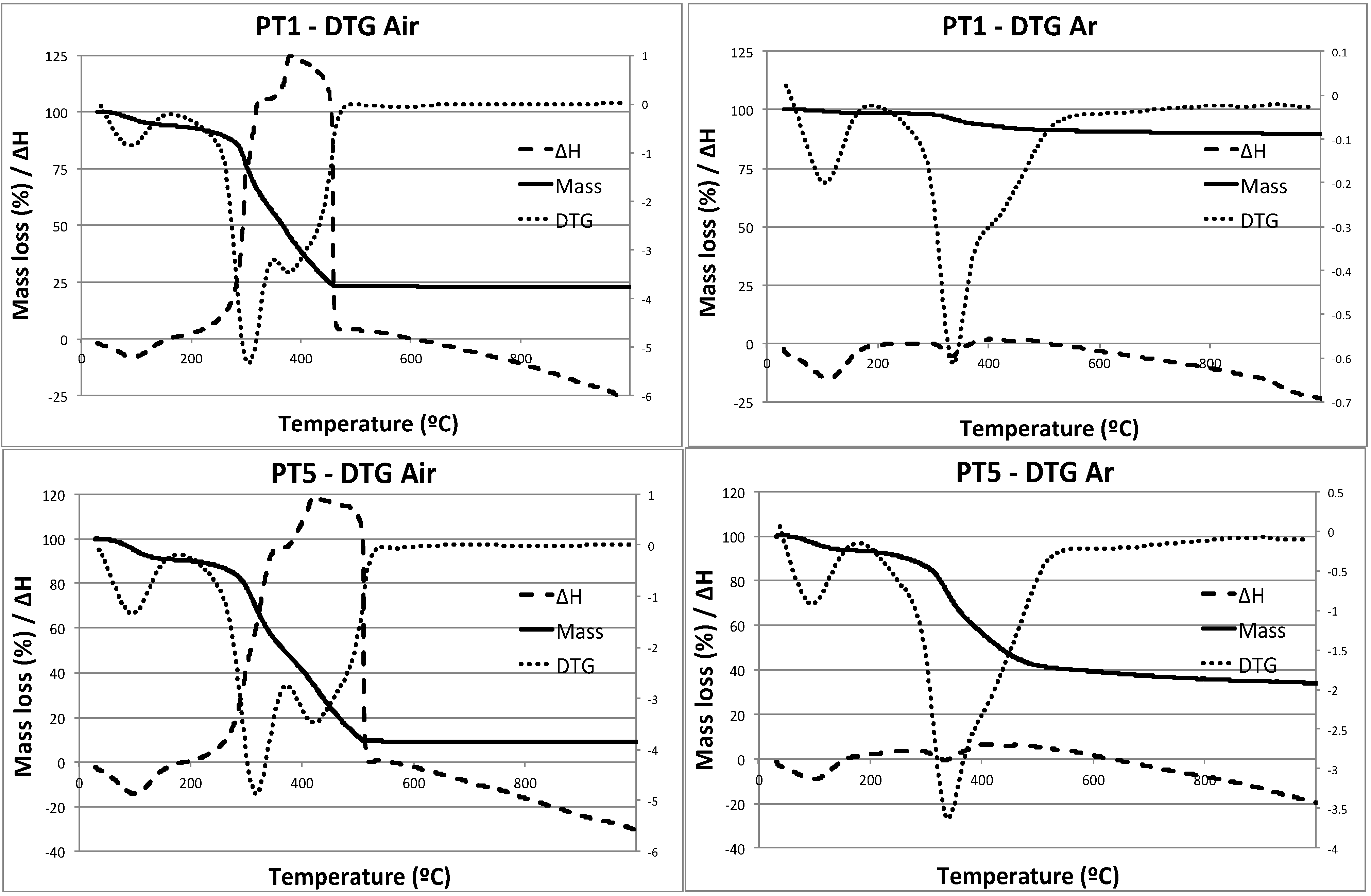
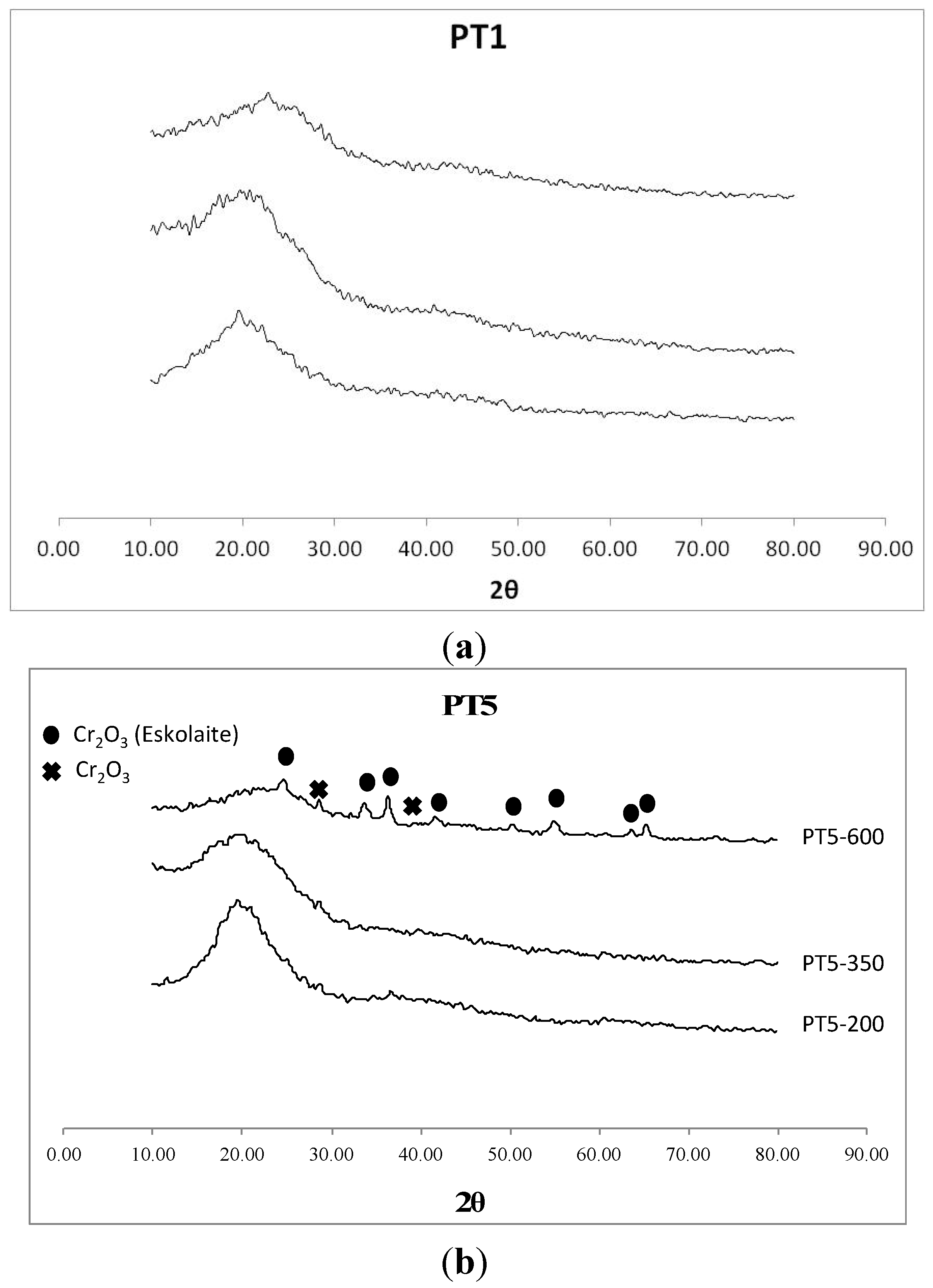
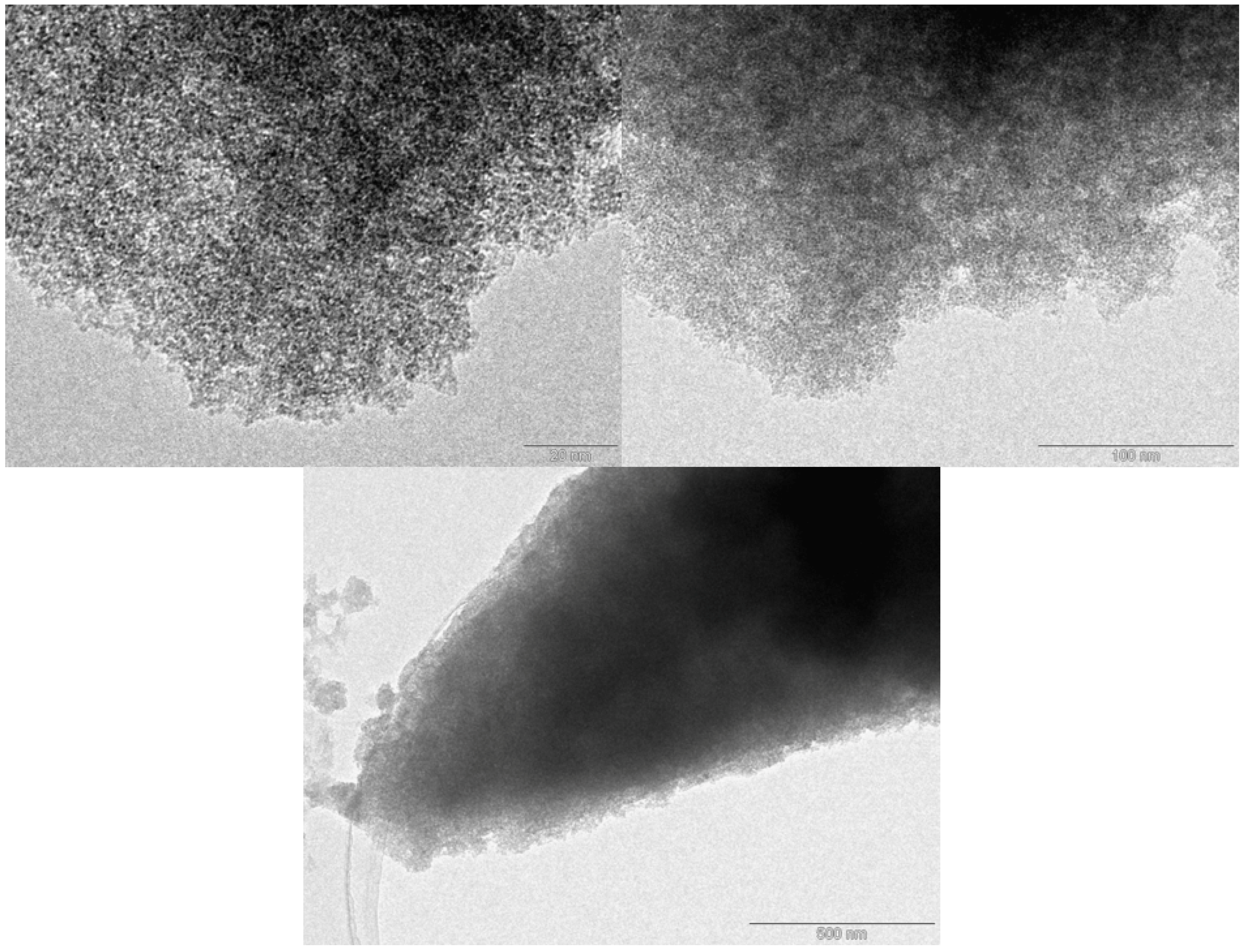
| Material | SBET (m2/g) | Pore Volume (cm3/g) |
|---|---|---|
| PT1* | – | – |
| PT1-350* | < 10 | 0.09 |
| PT1-600* | 29 | 0.10 |
| PT2* | – | – |
| PT2-350* | < 10 | 0.06 |
| PT2-600* | 21 | 0.07 |
| PT3* | – | – |
| PT3-350* | < 5 | – |
| PT3-600* | < 5 | – |
| PT4* | – | – |
| PT4-350* | < 5 | – |
| PT4-600* | – | – |
| PT5-350* | < 5 | – |
| PT5-600* | 10 | 0.05 |
3. Experimental Section
3.1. Materials Preparation

3.2. Characterization of Materials
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Constanza, R.; Daly, H.E. Natural capital and sustainable development. Conserv. Biol. 1992, 6, 37–46. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Pfaltzgraff, L.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.; Kopsahelis, N.; Stamatelou, K.; Dickson, F.; et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2012, 6, 426–464. [Google Scholar] [CrossRef]
- Cabeza, L.; Taylo, M.M.; Di Maio, G.L.; Brown, E.; Marmer, W.N.; Carrió, R.; Celma, J.; Cot, J. Processing of leather waste: pilot scale studies on chrome shavings. Isolation of potentially valuable protein products and chromium. Waste Manag. 1998, 18, 211–218. [Google Scholar] [CrossRef]
- Rao, J.R.; Thanikaivelan, P.; Sreeram, K.J.; Nair, B.U. Green route for the disposal of chrome shavings (chromium containing solid waste) in tanning industry. Environ. Sci. Technol. 2002, 36, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- United Nations Industrial Development Organization (UNIDO). Wastes Generated in the Leather Products Industry. Available on line: http://www.unido.org/fileadmin/import/userfiles/timminsk/leatherpanel14ctcwastes.pdf (accessed on 31 August 2013).
- Catalina, M.; Antunes, A.P.M.; Attenburrow, G.E.; Cot, J.; Covington, A.D.; Phillips, P.S. Sustainable management of waste-reduction of the chromium content of tannery solid waste as a step in the cleaner production of gelatin. J. Solid Waste Technol. Manag. 2007, 33, 43–50. [Google Scholar]
- Catalina, M.; Attenburrow, G.E; Cot, J.; Covington, A.D.; Antunes, A.P.M. Influence of crosslinkers and crosslinking method on the properties of gelatin films extracted from leather solid waste. J. Appl. Polym. Sci. 2011, 119, 2105–2111. [Google Scholar] [CrossRef]
- Catalina, M.; Cot, J.; Balu, A.M.; Serrano-Ruiz, J.C.; Luque, R. Tailor-made biopolymers from leather waste valorisation. Green Chem. 2012, 14, 308–312. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Narayanan, N.T.; Reddy, A.L.M.; Gupta, B.K.; Chandrasekaran, B.; Talapatra, S.; Ajayan, P.M.; Thanikaivelan, P. Transforming collagen wastes into doped nanocarbons for sustainable energy applications. Green Chem. 2012, 14, 1689–1695. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef]
- Lu, W.; Sculley, J.P.; Yuan, D.; Krishna, R.; Wei, Z.; Zhou, H.C. Polyamine-tethered porous polymer networks for carbon dioxide capture from flue gas. Angew. Chem. Int. Ed. 2012, 51, 7480–7484. [Google Scholar] [CrossRef]
- McDonald, T.M.; Lee, W.R.; Mason, J.A.; Wiers, B.M.; Hong, C.S.; Long, J.R. Capture of carbon dioxide from air and flue gas in the alkylamine-appended metal-organic framework mmen-Mg2(dobpdc). J. Am. Chem.Soc. 2012, 134, 7056–7065. [Google Scholar] [CrossRef]
- Rodriguez-Reinoso, F.; Lopez-Gonzalez, J.D.; Berenguer, C. Activated carbons from almond shells—I: Preparation and characterization by nitrogen adsorption. Carbon 1982, 20, 513–518. [Google Scholar] [CrossRef]
- Lozano-Castelló, D.; Cazorla-Amoros, D.; Linares-Solano, A. Usefulness of CO2 adsorption at 273 K for the characterization of porous carbons. Carbon 2004, 42, 1233–1236. [Google Scholar] [CrossRef]
- Yu, L.; Falco, C.; Weber, J.; White, R.; Howe, J.Y.; Titirici, M.M. Carbohydrate-derived hydrothermal carbons: A thorough characterization study. Langmuir 2012, 28, 12373–12283. [Google Scholar] [CrossRef]
- Baccile, N.; Weber, J.; Falco, C.; Titirici, M.M. Sustainable Carbon Materials from Hydrothermal Processes; Titirici, M.M., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013; p. 190. [Google Scholar]
- Ritter, N.; Senkovska, I.; Kaskel, S.; Weber, J. Intrinsically microporous poly(imide)s: Structure-porosity relationship studied by gas sorption and X-ray scattering. Macromolecules 2011, 44, 2025–2033. [Google Scholar] [CrossRef]
- Budarin, V.; Clark, J.H.; Hardy, J.J.E.; Luque, R.; Macquarrie, D.J.; Milkowski, K.; Tavener, S.J.; Wilson, A.J. Starbons: New starch-derived mesoporous carbonaceous materials with tunable properties. Angew. Chem. Int. Ed. 2006, 45, 3782–3786. [Google Scholar] [CrossRef]
- Xing, X.; Liu, C.; Zhou, Z.; Zhang, L.; Zhou, J.; Zhou, S.; Yan, Z.; Gao, H.; Wang, G.; Qiao, S.Z. Superior CO2 uptake of N-doped activated carbon through hydrogen-bonding interaction. Energy Environ. Sci. 2012, 5, 7323–7327. [Google Scholar] [CrossRef]
- Maroto-Valer, M.M.; Tang, Z.; Zhang, Y. CO2 capture by activated and impregnated anthracites. Fuel Process. Technol. 2005, 86, 1487–1502. [Google Scholar] [CrossRef]
- Grondein, A.; Belanger, D. Chemical modification of carbon powders with aminophenyl and aryl-aliphatic amine groups by reduction of in situ generated diazonium cations: Applicability of the grafted powder towards CO2 capture. Fuel 2011, 90, 2684–2693. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, J.; Feng, D.; Wu, Z.; Wu, Q.; Park, S.S.; Ha, C.; Zhao, D. Facile synthesis of porous carbon nitride spheres with hierarchical three-dimensional mesostructures for CO2 capture. Nano Res. 2010, 3, 632–642. [Google Scholar] [CrossRef]
- Sevilla, M.; Valle-Vigón, P.; Fuertes, A.B. N-doped polypyrrole-based porous carbons for CO2 capture. Adv. Funct. Mater. 2011, 21, 2781–2787. [Google Scholar] [CrossRef]
- Gutierrez, M.C.; Carriazo, D.; Ania, C.O.; Parra, J.B.; Ferrer, M.L.; Monte, F.D. Deep eutectic solvents as both precursors and structure directing agents in the synthesis of nitrogen doped hiearchical carbons highly suitable for CO2 capture. Energy Environ. Sci. 2011, 4, 3535–3544. [Google Scholar] [CrossRef]
- Hao, G.; Li, W.; Qian, D.; Lu, A. Rapid synthesis of nitrogen-doped porous carbon monolith for CO2 capture. Adv. Mater. 2010, 22, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Parra, J.B.; Fuertes, A.B. Assessment of the role of micropore size and N-doping in CO2 capture by porous carbons. ACS Appl. Mater. Interfaces 2013, 5, 6360–6368. [Google Scholar] [CrossRef] [PubMed]
- Vishnyakov, A.; Ravikovitch, P.I.; Neimark, A.V. Molecular level models for CO2 sorption in nanopores. Langmuir 1999, 15, 8736–8742. [Google Scholar] [CrossRef]
- Mason, J.A.; Sumida, K.; Herm, Z.R.; Krishna, R.; Long, J.R. Evaluating metal-organic framworks for post-combustion carbon dioxidecapture via temperature swing adsorption. Energy Environ. Sci. 2011, 4, 3030–3040. [Google Scholar] [CrossRef]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of mixed-gas adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]
- Akhtar, F.; Liu, Q.; Hedin, N.; Bergström, L. Strong and binder free structured zeolite sorbents with very high CO2-over-N2 selectivities and high capacities to adsorb CO2 rapidly. Energy Environ. Sci. 2012, 5, 7664–7673. [Google Scholar] [CrossRef]
- Hedin, N.; Andersson, L.; Bergström, L.; Yan, J. Adsorbents for the post-combustion capture of CO2 using rapid temperature swing or vacuum swing adsorption. Appl. Energy 2013, 104, 418–433. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Sustainable porous carbons with a superior performance for CO2 capture. Energy Environ. Sci. 2011, 4, 1765–1771. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. CO2 adsorption by activated templated carbons. J. Colloid Interface Sci. 2012, 366, 147–154. [Google Scholar] [CrossRef]
- SERPELSA Furs SA Homepage. Available online: www.serpelsa.com (accessed on 30 August 2013).
- Max Plank Institute of Colloids and Interfaces, Groups of Porous Polymers. Available online: http://www.mpikg.mpg.de/144866/xDownloads (accessed on 30 August 2013).
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bermúdez, J.M.; Dominguez, P.H.; Arenillas, A.; Cot, J.; Weber, J.; Luque, R. CO2 Separation and Capture Properties of Porous Carbonaceous Materials from Leather Residues. Materials 2013, 6, 4641-4653. https://doi.org/10.3390/ma6104641
Bermúdez JM, Dominguez PH, Arenillas A, Cot J, Weber J, Luque R. CO2 Separation and Capture Properties of Porous Carbonaceous Materials from Leather Residues. Materials. 2013; 6(10):4641-4653. https://doi.org/10.3390/ma6104641
Chicago/Turabian StyleBermúdez, José M., Pablo Haro Dominguez, Ana Arenillas, Jaume Cot, Jens Weber, and Rafael Luque. 2013. "CO2 Separation and Capture Properties of Porous Carbonaceous Materials from Leather Residues" Materials 6, no. 10: 4641-4653. https://doi.org/10.3390/ma6104641
APA StyleBermúdez, J. M., Dominguez, P. H., Arenillas, A., Cot, J., Weber, J., & Luque, R. (2013). CO2 Separation and Capture Properties of Porous Carbonaceous Materials from Leather Residues. Materials, 6(10), 4641-4653. https://doi.org/10.3390/ma6104641







