Characterization of an Exopolymeric Flocculant Produced by a Brachybacterium sp.
Abstract
:1. Introduction
2. Results and Discussion
2.1. Actinobacteria Identification
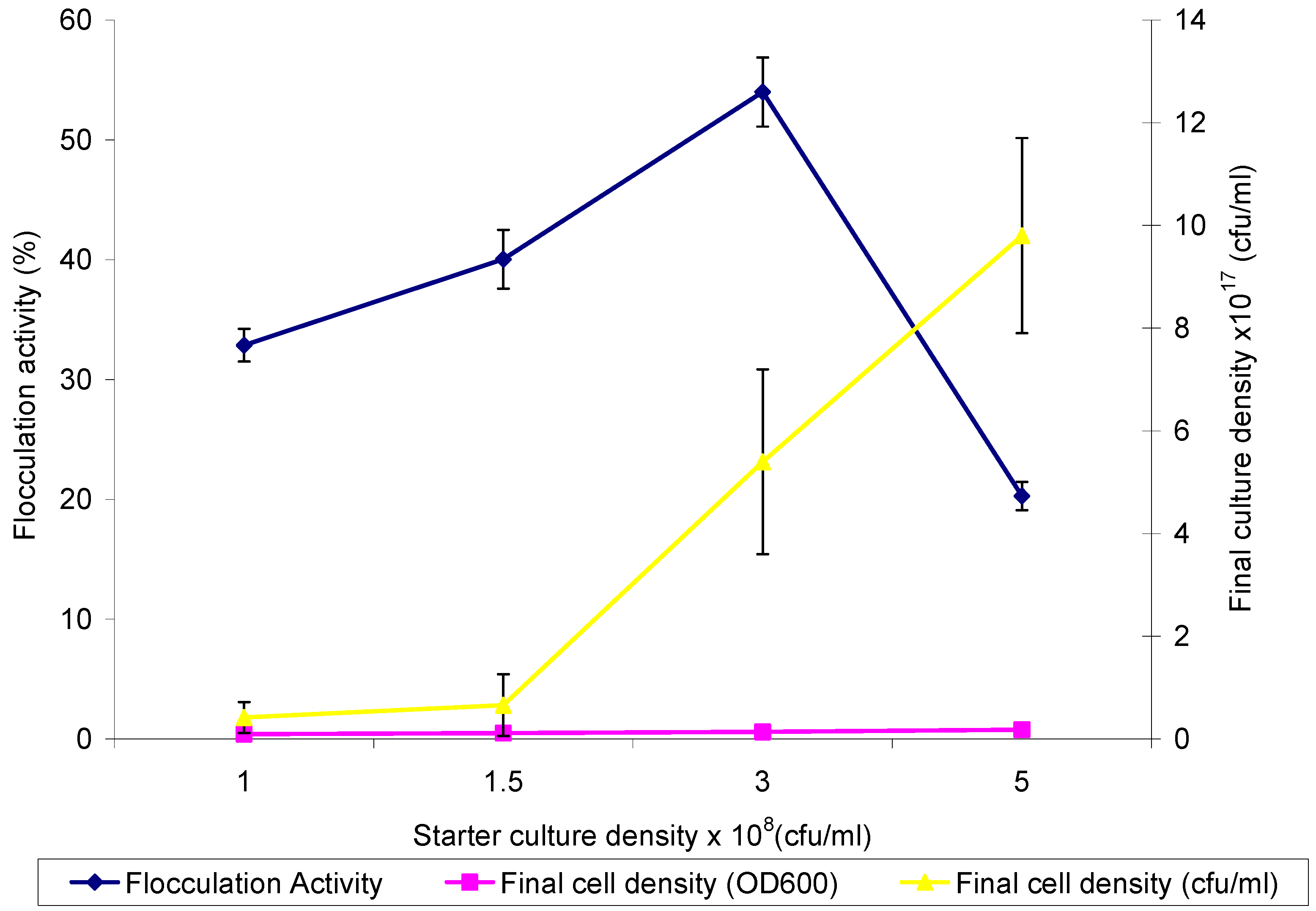
2.2. Optimum Inoculum Cell Density for Bioflocculant Production
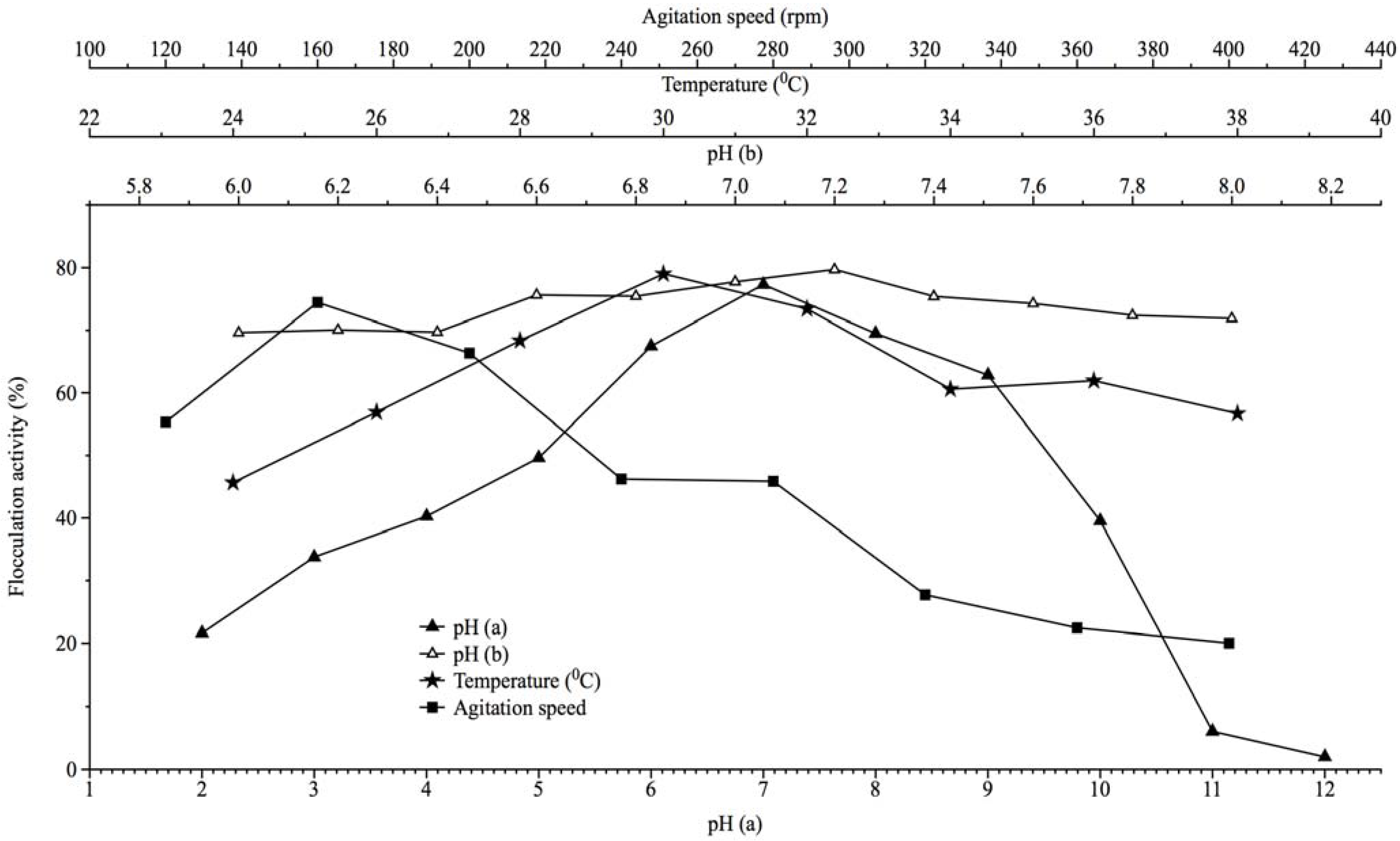
2.3. Effect of Culture Conditions on Bioflocculant Production
2.4. The Effect of Nutritional Factors on Bioflocculant Production
| Carbon source | Glucose | Lactose | Fructose | Sucrose | Maltose | Starch | – |
|---|---|---|---|---|---|---|---|
| Max. flocculation activity (%) | 77.3 ± 1.67 | 63.3 ± 2.14 | 54.9 ± 0.77 | 68.4 ± 2.01 | 83.1 ± 1.31 | 56.2 ± 1.25 | – |
| Bioflocculant yield (g/L) | 4.53 ± 2.08 | 3.0 ± 2.42 | 3.59 ± 1.08 | 3.05 ± 2.44 | 4.83 ± 1.36 | 2.53 ± 2.23 | – |
| Nitrogen source | Urea | (NH4)2SO4 | (NH4)2NO3 | (NH4)2Cl4 | Peptone | – | – |
| Max. flocculation activity (%) | 91.2 ± 0.82 | 40.2 ± 1.57 | 56.4 ± 1.22 | 32.8 ± 1.31 | 80.4 ± 3.22 | – | – |
| Bioflocculant yield (g/L) | 4.18 ± 0.5 | 4.02 ± 1.8 | 3.61 ± 1.28 | 2.76 ± 2.09 | 4.51 ± 2.3 | – | – |
| Cation source | KCl | NaCl | MgCl2 | CaSO4·H2O | MnCl·4H2O | FeSO4 | FeCl3 |
| Max. flocculation activity (%) | 24.6 ± 0.27 | 36.4 ± 0.91 | 91.1 ± 1.18 | 72.4 ± 2.23 | 42.8 ± 0.47 | 37.4 ± 0.66 | 37 ± 1.11 |
| Bioflocculant yield (g/L) | 1.98 ± 3.19 | 1.82 ± 1.61 | 4.66 ± 1.19 | 3.28 ± 1.99 | 2.46 ± 2.83 | 2.57 ± 3.62 | 2.07 ± 6.16 |
2.5. Bioflocculant Production Time Course

2.6. The Effects of Cations and pH on Flocculation Activity of Purified Bioflocculant

2.7. Compositional Analyses of Purified Bioflocculant
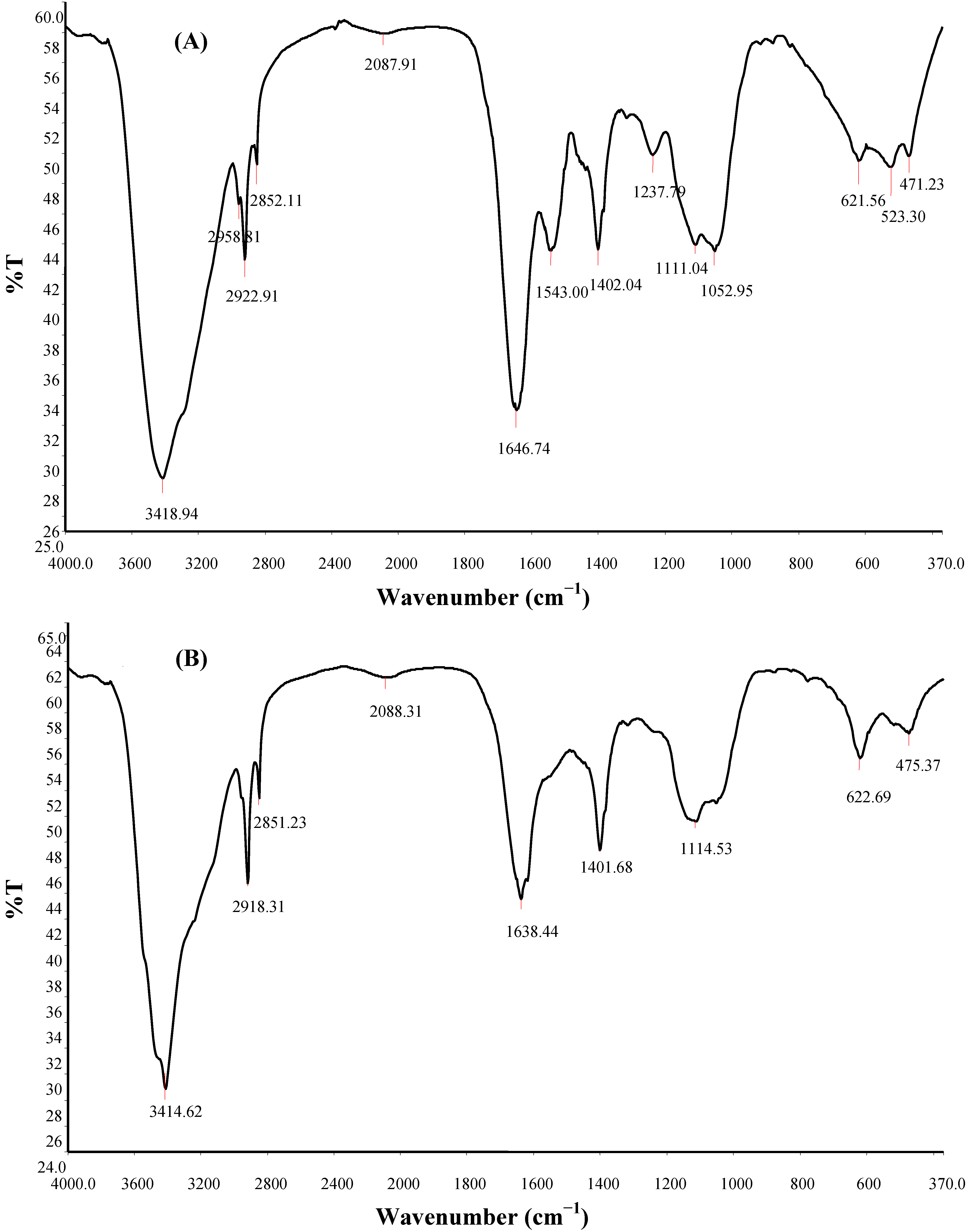
2.8. FTIR Spectroscopy, SEM Micrography and Elemental Composition of Bioflocculant
| Compound | Origin | Group frequency wave number (cm−1) | Assignment/Functional group | ||
|---|---|---|---|---|---|
| Assigned | PPB | CPB | |||
| Hydroxy and ether compounds | O–H | 3570–3200 (broad) | 3414.62 | 3418.94 | Hydroxy group; H-bonded OH stretch |
| Amino compounds and polysaccharides | O–H | 3400–3200 | – | – | Normal “polymeric” OH stretch |
| O–H | 3550–3450 | – | – | Dimeric OH stretch | |
| O–H | 1410–1310 | 1401.68 | 1402.04 | Phenol or tertiary alcohol; OH bend | |
| N–H | 3400–3380 | 3414.62 | 3418.94 | Aliphatic primary amine; N–H stretch | |
| N–H | 3510–3460 | 3414.62 | 3418.94 | Aromatic primary amine; N–H stretch | |
| >N–H >C=O C–O C–H | 1650–1550 | 1638.44 | 1646.74; 1543.00 | Secondary amine; NH bend associated with proteins >C=O stretch; ether; carboxylic groups C–H bend from CH2; C–O bend from carboxylate ions C–O and C–O–C from polysaccharides | |
| Methyl (−CH3) | –CH | 2935–29152865–2845 | 2918.31 2851.23 | 2958.81; 2922.91; 2852.11; | Methylene C–H asym./sym. stretch |
| >CH– | 2900–2880 | – | – | Methyne C–H stretch (Methyne) | |
| Aromatic ring (aryl) | C=C–C | 1510–1450 | 1549.42 | 1543.00 | Aromatic ring stretch |
| Thiols and thio-substituted compounds | S–S | 620–600 | 622.69 | 621.56 | Disulfides (S–S stretch) |
| S–S | 500–430 | 475.37 | 471.23 | Aryl disulfides (S–S stretch) | |
| Bioflocculant Type | Element Line | Element wt % | wt % Error | atom % | atom % Error | Compound Formula | Compound wt % |
|---|---|---|---|---|---|---|---|
| CPC Purified Bioflocculant | C K | 26.44 | ±0.45 | 59.89 | ±1.03 | C | 26.44 |
| N K | 0.42 | ±0.42 | 0.82 | ±1.87 | N | 0.42 | |
| O K | 8.65 | ±0.34 | 14.71 | ±0.58 | O | 8.65 | |
| Na K | 4.40 | ±0.08 | 5.20 | ±0.10 | Na | 4.40 | |
| Al K | 0.19 | ±0.03 | 0.19 | ±0.03 | Al | 0.19 | |
| P K | 0.96 | ±0.11 | 0.84 | ±0.10 | P | 0.96 | |
| S K | 0.60 | ±0.10 | 0.51 | ±0.08 | S | 0.60 | |
| Cl K | 14.47 | ±0.16 | 11.10 | ±0.12 | Cl | 14.47 | |
| Cu K | 2.30 | ±0.27 | 0.99 | ±0.11 | Cu | 2.30 | |
| Au L | 41.56 | ±4.48 | 5.74 | ±0.62 | Au | 41.56 | |
| Partial Purified Bioflocculant | C K | 34.84 | ±0.45 | 62.06 | ±0.81 | C | 34.84 |
| N K | 3.30 | ±1.44 | 5.04 | ±2.19 | N | 3.30 | |
| O K | 17.57 | ±0.49 | 23.49 | ±0.66 | O | 17.57 | |
| Na K | 0.22 | ±0.06 | 0.20 | ±0.06 | Na | 0.22 | |
| Mg K | 0.47 | ±0.07 | 0.41 | ±0.06 | Mg | 0.47 | |
| Al K | 0.20 | ±0.04 | 0.16 | ±0.03 | Al | 0.20 | |
| P K | 4.05 | ±0.09 | 2.80 | ±0.06 | P | 4.05 | |
| S K | 1.14 | ±0.16 | 0.76 | ±0.11 | S | 1.14 | |
| Cl K | 0.22 | ±0.05 | 0.13 | ±0.03 | Cl | 0.22 | |
| K K | 0.50 | ±0.05 | 0.27 | ±0.03 | K | 0.50 | |
| Cu K | 2.65 | ±0.32 | 0.89 | ±0.11 | Cu | 2.65 | |
| Au L | 34.86 | ±3.42 | 3.79 | ±0.37 | Au | 34.86 |

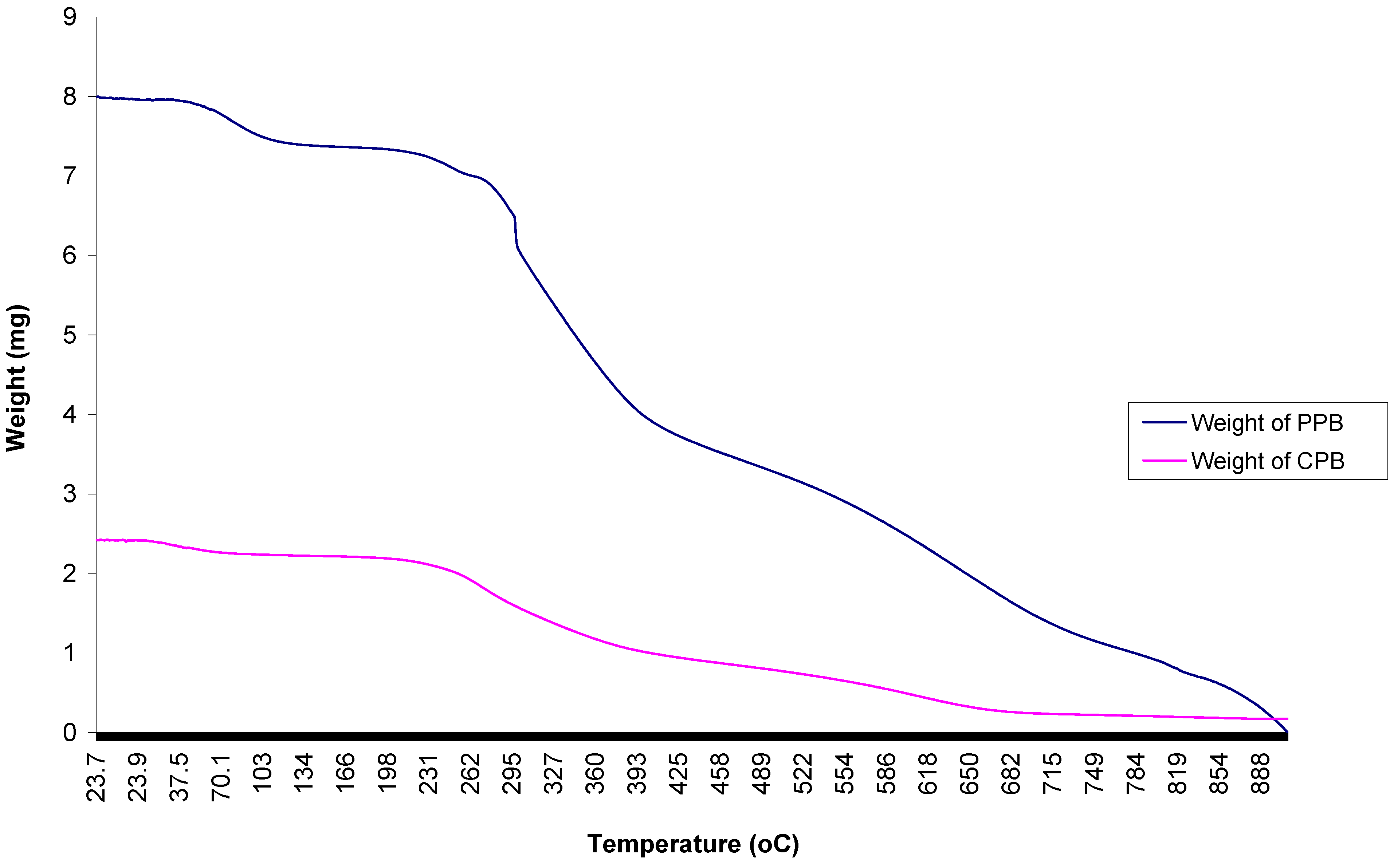
2.9. Thermal Analyses of Purified Bioflocculant
3. Experimental Section
3.1. Activation of Actinobacteria
3.2. Screening for Bioflocculant Production
3.3. Effect of Inoculum Cell Density on Bioflocculant Production
3.4. Measurement of Flocculation Activity
3.5. Assessment of Culture Conditions on Bioflocculant Production
3.6. Evaluation of Carbon, Nitrogen and Cation Sources on Bioflocculant Production
3.7. Bioflocculant Production Time Course
3.8. Purification of Bioflocculant
3.9. Effect of pH and Cations on Flocculation Activity
3.10. Compositional Analyses of Bioflocculants
3.11. SEM Imaging, Elemental Analysis and FTIR Spectroscopy of Purified Bioflocculant
3.12. Thermal Studies of Purified Bioflocculant
3.13. Identification of the Test Actinobacteria
4. Conclusions
Acknowledgments
References
- Kanungo, S.B. Effects of some commercial flocculating agents in settling and filtration rates of low grade, fragile manganese ores of Andhra Pradesh. Indian J. Chem. Technol. 2005, 12, 550–558. [Google Scholar]
- Salipira, K.L.; Mamba, B.B.; Krause, R.W.; Malefetse, T.J.; Durbach, S.H. Cyclodextrin polyurethanes polymerised with carbon nanotubes for the removal of organic pollutants. Water SA 2008, 34, 113–118. [Google Scholar]
- Liu, W.; Yuan, H.L.; Yang, J.S.; Li, B.Z. Characterization of bioflocculants from biologically aerated filter backwashed sludge and its application in dying wastewater treatment. Bioresour. Technol. 2009, 100, 2629–2632. [Google Scholar] [CrossRef] [PubMed]
- Mamba, B.B.; Krause, R.W.; Matsebula, B.; Haarhoff, J. Monitoring natural organic matter and disinfection by-products at different stages in two South African water treatment plants. Water SA 2009, 35, 121–127. [Google Scholar]
- Qiang, L.I.; Hong, L.L.; Qing, S.Q.; Feng, S.W.; Yu, Z.Z. Isolation and characterization of temperature and alkaline stable bioflocculants from agrobacteriuu sp. M-503. New Biotechnol. 2010, 27, 789–794. [Google Scholar] [CrossRef]
- Jang, J.H.; Ike, M.; Kim, S.M.; Fujita, M. Production of a Novel bioflocculant by fed-batch culture of Citrobacter sp. Biotechnol. Lett. 2001, 23, 593–597. [Google Scholar] [CrossRef]
- Wu, H.; Li, Q.; Lu, R.; Wang, Y.; Zhuang, X.; He, N. Fed-batch production of a bioflocculant from Corynebacterium glutamicum. J. Ind. Microbiol. Biotechnol. 2010, 37, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Mabinya, V.L.; Cosa, S.; Nwodo, U.U.; Okoh, A.I. Studies on bioflocculant production by Arthrobacter sp. raats, a freshwater bacteria isolated from tyume river, South Africa. Int. J. Mol. Sci. 2012, 13, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, H.; Natsuda, O.; Sachio, J.H.; Takasaki, Y. Characteristics of a biopolymer flocculant produced by Bacillus sp. PY-90. J. Ferment. Bioeng. 1995, 79, 378–380. [Google Scholar] [CrossRef]
- Salehizadeh, H.; Shojaosadati, S.A. Extracellular biopolymeric flocculants recent trends and biotechnological importance. Biotechnol. Adv. 2001, 19, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Dearfield, K.L.; Albernathy, C.O.; Ottley, M.S.; Brantner, J.H.; Hayes, P.F. Acrylamide: Its metabolism, developmental and reproductive effects, genotoxicity, and carcinogenicity. Mut. Res. 1988, 195, 45–77. [Google Scholar] [CrossRef]
- Smith, M.A.; Perry, G.; Sayre, L.M.; Anderson, V.E.; Beal, M.F.; Kowall, N. Oxidative damage in Alzheimer’s. Nature 1996, 382, 120–121. [Google Scholar] [CrossRef] [PubMed]
- Stephens, C. Microbiology: Breaking down biofilms. Curr. Biol. 2002, 12, 132–134. [Google Scholar] [CrossRef]
- Rudén, C. Acrylamide and cancer risk-expert risk assessments and the public debate. Food Chem. Toxicol. 2004, 42, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Cosa, S.; Mabinya, L.V.; Olaniran, O.A.; Okoh, O.O.; Bernard, K.; Deyzel, S.; Okoh, A.I. Bioflocculant production by Virgibacillus sp. rob isolated from the bottom sediment of algoa bay in the eastern cape, South Africa. Molecules 2011, 16, 2431–2442. [Google Scholar] [CrossRef] [PubMed]
- Piyo, N.; Cosa, S.; Mabinya, V.L.; Okoh, A.I. Assessment of bioflocculant production by Bacillus sp. gilbert, a marine bacterium isolated from the bottom sediment of algoa bay. Mar. Drugs 2011, 9, 1232–1242. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Li, Y.; Chen, J. Production of a novel polygalacturonic acid bioflocculant REA-11 by Corynebacterium glutamicum. Bioresour. Technol. 2004, 94, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; Lin, B.; Xia, S.Q.; Wang, X.J.; Yang, A.M. Production and application of a bioflocculant by multiple-microorganism consortia using brewery wastewater as carbon source. J. Environ. Sci. 2007, 19, 660–666. [Google Scholar]
- Mullins, J.T.; NeSmith, C. Integrated ethanol production and utilization system for small farms. Biomass 1984, 6, 155–166. [Google Scholar] [CrossRef]
- Gibbons, W.R.; Westby, C.A. Effects of inoculum size on solid-phase fermentation of fodder beets for fuel ethanol production. Appl. Environ. Microbiol. 1986, 52, 960–962. [Google Scholar] [PubMed]
- Lu, W.; Zhang, T.; Zhang, D.; Li, C.; Wen, J.; Du, L. A novel bioflocculant produced by Enterobacter aerogenes and its use in defecating the trona suspension. Biochem. Eng. J. 2005, 27, 1–7. [Google Scholar] [CrossRef]
- He, N.; Li, Y.; Chen, J.; Lun, S.Y. Identification of a novel bioflocculant from a newly isolated Corynebacterium glutamicum. Biochem. Eng. J. 2002, 11, 137–148. [Google Scholar] [CrossRef]
- Li, J.; Kisara, K.; Danielsson, S.; Lindstrom, M.E.; Gellerstedt, G. An improved methodology for the quantification of uronic acid units in xylans and other polysaccharides. Carbohydr. Res. 2007, 342, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Flemming, H. FTIR-spectroscopy in microbial and material analysis. Int. Biodeterior. Biodegrad. 1998, 41, 1–11. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2000. [Google Scholar]
- Wu, J.Y.; Ye, H.F. Characterization and flocculating properties of an extracellular biopolymer produced from a Bacillus subtilis DYU1 isolate. Process. Biochem. 2007, 42, 1114–1123. [Google Scholar] [CrossRef]
- Niu, S.; Han, K.; Zhou, F.; Lu, C. Thermogravimetric analysis of the decomposition characteristics of two kinds of calcium based organic compounds. Powder Technol. 2011, 209, 46–52. [Google Scholar] [CrossRef]
- Wang, L.; Ma, F.; Qu, Y.; Sun, D.; Li, A.; Guo, J.; Yu, B. Characterization of a compound bioflocculant produced by mixed culture of Rhizobium radiobacter F2 and Bacillus sphaeicus F6. World J. Microbiol. Biotechnol. 2011, 27, 2559–2565. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards. Methods for Dilution in Antimicrobial Susceptibility Tests: Approved Standard M2-A5; National Committee for Clinic Laboratory Standards: Villanova, PA, USA, 1993. [Google Scholar]
- Kumar, C.G.; Joo, H.S.; Choi, J.W.; Koo, Y.M.; Chang, C.S. Purification and characterization of an extracellular polysaccharide from haloalkalophilic Bacillus sp. I-450. Enzyme Microb. Technol. 2004, 34, 673–681. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin-phenol reagents. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Zhang, W. Biological-Chemical Analysis of Glycoconjugates, 2nd ed.; Zhejiang University Press: Zhejiang, China, 2003. [Google Scholar]
- Chaplin, M.F.; Kennedy, J.F. Carbohydrate Analysis, 2nd ed.; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Cook, A.E.; Meyers, P.R. Rapid identification of filamentous actinomycetes to the genus level using genus-specific 16S rRNA gene restriction fragment patterns. Int. J. Syst. Evol. Microbiol. 2003, 53, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nwodo, U.U.; Agunbiade, M.O.; Green, E.; Nwamadi, M.; Rumbold, K.; Okoh, A.I. Characterization of an Exopolymeric Flocculant Produced by a Brachybacterium sp. Materials 2013, 6, 1237-1254. https://doi.org/10.3390/ma6041237
Nwodo UU, Agunbiade MO, Green E, Nwamadi M, Rumbold K, Okoh AI. Characterization of an Exopolymeric Flocculant Produced by a Brachybacterium sp. Materials. 2013; 6(4):1237-1254. https://doi.org/10.3390/ma6041237
Chicago/Turabian StyleNwodo, Uchechukwu U., Mayowa O. Agunbiade, Ezekiel Green, Mutshinyalo Nwamadi, Karl Rumbold, and Anthony I. Okoh. 2013. "Characterization of an Exopolymeric Flocculant Produced by a Brachybacterium sp." Materials 6, no. 4: 1237-1254. https://doi.org/10.3390/ma6041237
APA StyleNwodo, U. U., Agunbiade, M. O., Green, E., Nwamadi, M., Rumbold, K., & Okoh, A. I. (2013). Characterization of an Exopolymeric Flocculant Produced by a Brachybacterium sp. Materials, 6(4), 1237-1254. https://doi.org/10.3390/ma6041237





