The Influence of Alkoxy Substitutions on the Properties of Diketopyrrolopyrrole-Phenyl Copolymers for Solar Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Physical Properties of Oligomers and Polymers

| Material | Mn (kg/mole) a | PDI | TGA (°C) b | Tm (°C) | Tc (°C) |
|---|---|---|---|---|---|
| 1% wt. loss | |||||
| P1 | 15 | 1.5 | 415 | >350 | >350 |
| P2 | 12 | 2.7 | 336 | >300 | 260 |
| P3 | 29 | 2.3 | 347 | 240 | 190 |
| O1 | – | – | 257 | 153 | 110 |
| O2 | – | – | 341 | 181 | 148 |
2.2. Electrochemical and Optical Properties
| Polymer | λmax (nm) | λonset (nm) | Eg, onset (eV) | HOMO a (eV) | LUMO (eV) |
|---|---|---|---|---|---|
| P1 | 751 | 816 | 1.52 | −5.10 | −3.58 |
| P2 | 718 | 925 | 1.34 | −4.89 | −3.55 |
| P3 | 773 | 943 | 1.32 | −4.88 | −3.56 |

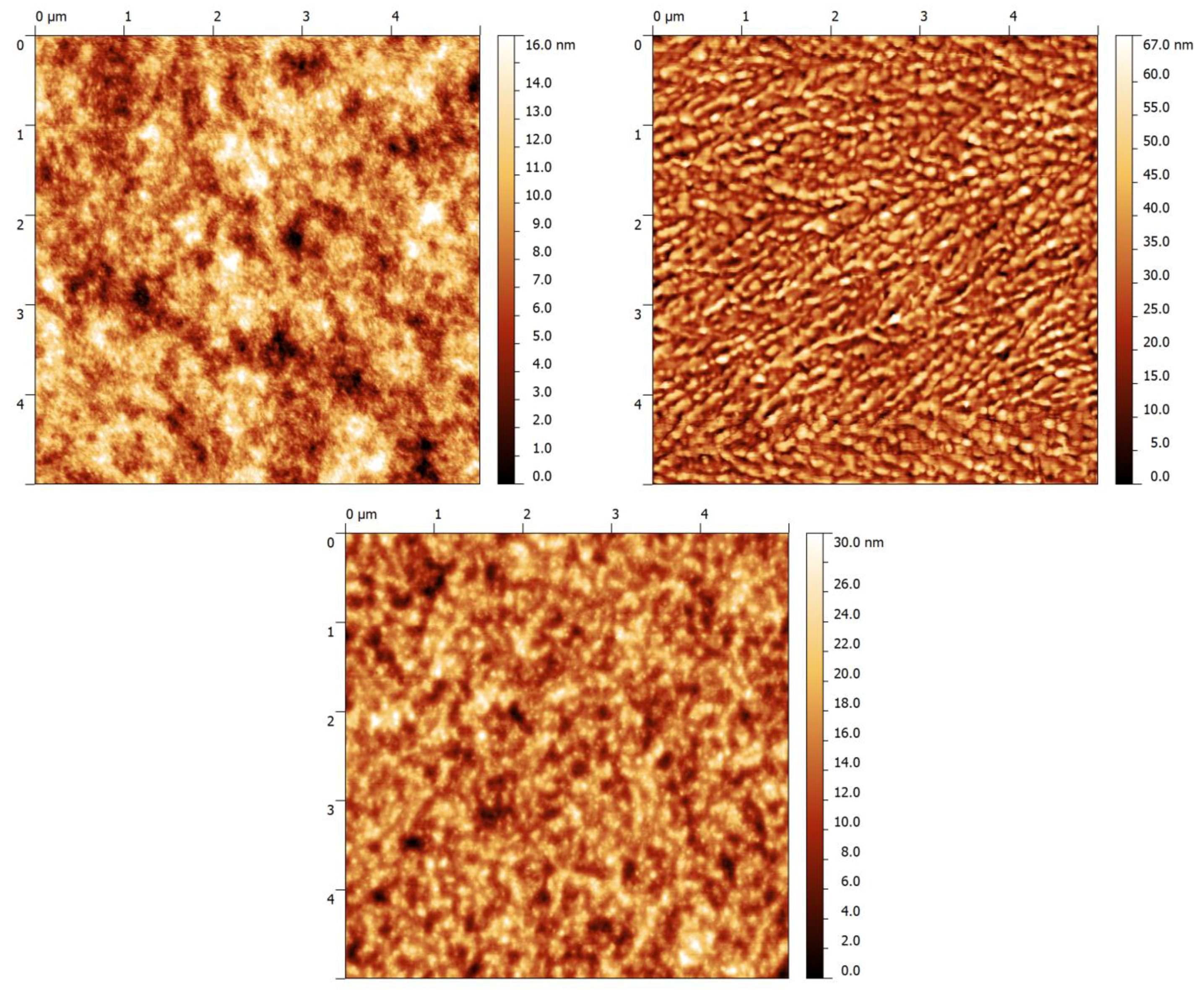
2.3. Device Performance and Atomic Force Microscope (AFM)
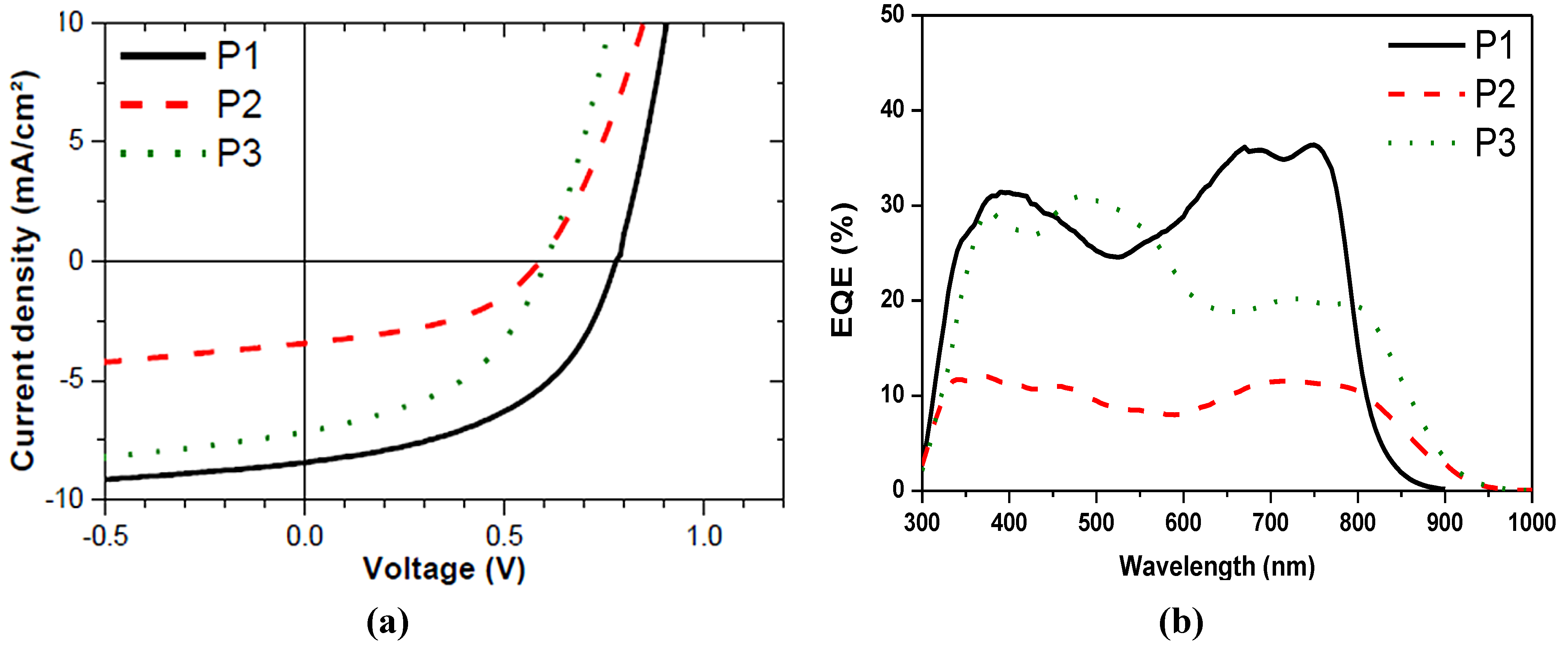
| Material | Polymer:PC71BM (w:w) | Thickness (nm) | RMS blend (nm) | Jsc (Ma/cm2) | Voc (mV) | FF | η (%) |
|---|---|---|---|---|---|---|---|
| P1 | 1:2 | 125 | 2.77 | 8.4 | 780 | 49 | 3.2 |
| P2 | 1:2 | 80 | 10.9 | 3.4 | 590 | 45 | 0.9 |
| P3 | 1:2 | 77 | 4.27 | 7.1 | 600 | 46 | 2.0 |
3. Experimental Section
3.1. Experimental Details
3.2. Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Conflict of Interest
References and Notes
- Shaheen, S.E.; Brabec, C.J.; Sariciftci, N.S.; Padinger, F.; Fromherz, T.; Hummelen, J.C. 2.5% efficient organic plastic solar cells. Appl. Phys. Lett. 2001, 78, 841–843. [Google Scholar] [CrossRef]
- Peumans, P.; Uchida, S.; Forrest, S.R. Efficient bulk heterojunction photovoltaic cells using small-molecular-weight organic thin films. Nature 2003, 425, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Spanggaard, H.; Krebs, F.C. A brief history of the development of organic and polymeric photovoltaics. Solar Energy Mater. Solar Cells 2004, 83, 125–146. [Google Scholar] [CrossRef]
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polymer-fullerene bulk-heterojunction solar cells. Adv. Mater. 2009, 21, 1323–1338. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, Z.; Xia, J.; Tsai, S.-T.; Wu, Y.; Li, G.; Ray, C.; Yu, L. For the bright future—Bulk heterojunction polymer solar cells with power conversion efficiency of 7.4%. Adv. Mater. 2010, 22, E135–E138. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Hou, J.; Zhang, S.; Liang, Y.; Yang, G.; Yang, Y.; Yu, L.; Wu, Y.; Li, G. Polymer solar cells with enhanced open-circuit voltage and efficiency. Nat. Photonics 2009, 3, 649–653. [Google Scholar] [CrossRef]
- Chu, T.-Y.; Lu, J.; Beaupré, S.; Zhang, Y.; Pouliot, J.-R.M.; Wakim, S.; Zhou, J.; Leclerc, M.; Li, Z.; Ding, J.; Tao, Y. Bulk heterojunction solar cells using thieno[3,4-c]pyrrole-4,6-dione and dithieno[3,2-b:2′,3′-d]silole copolymer with a power conversion efficiency of 7.3%. J. Am. Chem. Soc. 2011, 133, 4250–4253. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; You, J.; Yang, J.; Chen, C.-C.; He, Y.; Murase, S.; Moriarty, T.; Emery, K.; Li, G.; Yang, Y. Tandem polymer solar cells featuring a spectrally matched low-bandgap polymer. Nat. Photonics 2012, 6, 180–185. [Google Scholar] [CrossRef]
- Heliatek sets new world record efficiency of 10.7% for its organic tandem cell. Available online: http://www.heliatek.com/wp-content/uploads/2012/09/120427_PI_Heliatek-world-record-10_7-percent-efficiency.pdf (accessed on 24 July 2012).
- Gendron, D.; Leclerc, M. New conjugated polymers for plastic solar cells. Energy Environ. Sci. 2011, 4, 1225–1237. [Google Scholar] [CrossRef]
- Bijleveld, J.C.; Verstrijden, R.A.M.; Wienk, M.M.; Janssen, R.A.J. Copolymers of diketopyrrolopyrrole and thienothiophene for photovoltaic cells. J. Mater. Chem. 2011, 21, 9224–9231. [Google Scholar] [CrossRef]
- Bijleveld, J.C.; Zoombelt, A.P.; Mathijssen, S.G.J.; Wienk, M.M.; Turbiez, M.; de Leeuw, D.M.; Janssen, R.A.J. Poly(diketopyrrolopyrrole−terthiophene) for ambipolar logic and photovoltaics. J. Am. Chem. Soc. 2009, 131, 16616–16617. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, H.; Chen, Z.; Ashraf, R.S.; Zhang, W.; Du, J.; Durrant, J.R.; Tuladhar, S.P.; Song, K.; Watkins, S.E.; Geerts, Y.; et al. Thieno[3,2-b]thiophene−Diketopyrrolopyrrole-containing polymers for high-performance organic field-effect transistors and organic photovoltaic devices. J. Am. Chem. Soc. 2011, 133, 3272–3275. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Graham, K.R.; Stalder, R.; Tiwari, S.P.; Cheun, H.; Shim, J.; Yoshio, M.; Nuckolls, C.; Kippelen, B.; Castellano, R.K.; Reynolds, J.R. Self-assembled amphiphilic diketopyrrolopyrrole-based oligothiophenes for field-effect transistors and solar cells. Chem. Mater. 2011, 23, 2285–2288. [Google Scholar] [CrossRef]
- Egbe, D.A.M.; Türk, S.; Rathgeber, S.; Kühnlenz, F.; Jadhav, R.; Wild, A.; Birckner, E.; Adam, G.; Pivrikas, A.; Cimrova, V. Anthracene based conjugated polymers: Correlation between π–π-stacking ability, photophysical properties, charge carrier mobility, and photovoltaic performance. Macromolecules 2010, 43, 1261–1269. [Google Scholar] [CrossRef]
- Falzon, M.-F.; Zoombelt, A.P.; Wienk, M.M.; Janssen, R.A.J. Diketopyrrolopyrrole-based acceptor polymers for photovoltaic application. Phys. Chem. Chem. Phys. 2011, 13, 8931–8939. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Yamakawa, S.; Tajima, K.; Yang, C.; Hashimoto, K. Synthesis and photovoltaic properties of diketopyrrolopyrrole-based donor−acceptor copolymers. Chem. Mater. 2009, 21, 4055–4061. [Google Scholar] [CrossRef]
- Beyerlein, T.; Tieke, B. New photoluminescent conjugated polymers with 1,4-dioxo-3,6-diphenylpyrrolo[3,4-c]pyrrole (DPP) and 1,4-phenylene units in the main chain. Macromol. Rapid Commun. 2000, 21, 182–189. [Google Scholar] [CrossRef]
- Bijleveld, J.C.; Gevaerts, V.S.; Nuzzo, D.D.; Turbiez, M.; Mathijssen, S.G.J.; de Leeuw, D.M.; Wienk, M.M.; Janssen, R.A.J. Efficient solar cells based on an easily accessible diketopyrrolopyrrole polymer. Adv. Mater. 2010, 22, E242–E246. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Feng, D.; Wu, Y.; Tsai, S.-T.; Li, G.; Ray, C.; Yu, L. Highly efficient solar cell polymers developed via fine-tuning of structural and electronic properties. J. Am. Chem. Soc. 2009, 131, 7792–7799. [Google Scholar] [CrossRef] [PubMed]
- Van Mullekom, H.A.M.; Vekemans, J.A.J.M.; Havinga, E.E.; Meijer, E.W. Developments in the chemistry and band gap engineering of donor-acceptor substituted conjugated polymers. Mater. Sci. R Rep. 2001, 32, 1–40. [Google Scholar]
- Scharber, M.C.; Mühlbacher, D.; Koppe, M.; Denk, P.; Waldauf, C.; Heeger, A.J.; Brabec, C.J. Design rules for donors in bulk-heterojunction solar cells—Towards 10% energy-conversion efficiency. Adv. Mater. 2006, 18, 789–794. [Google Scholar] [CrossRef]
- Wienk, M.M.; Turbiez, M.; Gilot, J.; Janssen, R.A.J. Narrow-bandgap diketo-pyrrolo-pyrrole polymer solar cells: The effect of processing on the performance. Adv. Mater. 2008, 20, 2556–2560. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Tsang, S.-W.; Du, X.; Zhou, J.; Tao, Y.; Ding, J. Alkyl side chain impact on the charge transport and photovoltaic properties of benzodithiophene and diketopyrrolopyrrole-based copolymers. J. Phys. Chem. C 2011, 115, 18002–18009. [Google Scholar] [CrossRef]
- Cho, C.-H.; Kim, H.J.; Kang, H.; Shin, T.J.; Kim, B.J. The effect of side-chain length on regioregular poly[3-(4-n-alkyl)phenylthiophene]/PCBM and ICBA polymer solar cells. J. Mater. Chem. 2012, 22, 14236–14245. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Liu, X.; Wang, Z.; Yang, X.; Tu, Y.; Zhu, X. Controlling blend film morphology by varying alkyl side chain in highly coplanar donor–acceptor copolymers for photovoltaic application. Macromolecules 2011, 44, 6370–6381. [Google Scholar] [CrossRef]
- Egbe, D.A.M.; Nguyen, L.H.; Hoppe, H.; Mühlbacher, D.; Sariciftci, N.S. Side chain influence on electrochemical and photovoltaic properties of yne-containing poly(phenylene vinylene)s. Macromol. Rapid Commun. 2005, 26, 1389–1394. [Google Scholar] [CrossRef]
- Schubert, M.; Dolfen, D.; Frisch, J.; Roland, S.; Steyrleuthner, R.; Stiller, B.; Chen, Z.; Scherf, U.; Koch, N.; Facchetti, A.; et al. Influence of aggregation on the performance of all-polymer solar cells containing low-bandgap naphthalenediimide copolymers. Adv. Energy Mater. 2012, 2, 369–380. [Google Scholar] [CrossRef]
- Traiphol, R.; Potai, R.; Charoenthai, N.; Srikhirin, T.; Kerdcharoen, T.; Osotchan, T. Effects of chain conformation and chain length on degree of aggregation in assembled particles of conjugated polymer in solvents–nonsolvent: A spectroscopic study. J. Poly. Sci. Part B. Poly. Phys. 2010, 48, 894–904. [Google Scholar] [CrossRef]
- Donald, A.; Windle, A.; Hanna, S. Liquid Crystalline Polymers, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006; p. 69. [Google Scholar]
- Ballauff, M. Stiff-chain polymers—Structure, phase behavior, and properties. Angew. Chem. Inter. Ed. Engl. 1989, 28, 253–267. [Google Scholar] [CrossRef]
- Panda, P.; Veldman, D.; Jörgen, S.; Jolanda, J.A.M.B.; Langeveld-Voss, B.M.W.; Stefan, C.J.M. Charge transfer absorption for π-conjugated polymers and oligomers mixed with electron acceptors. J. Phys. Chem. B 2007, 111, 5076–5081. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Amb, C.M.; Reynolds, J.R. Spectral engineering in π-conjugated polymers with intramolecular donor−acceptor interactions. Acc. Chem. Res. 2010, 43, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Aubert, P.-H.; Knipper, M.; Groenendaal, L.; Lutsen, L.; Manca, J.; Vanderzande, D. Copolymers of 3,4-ethylenedioxythiophene and of pyridine alternated with fluorene or phenylene units: Synthesis, optical properties, and devices. Macromolecules 2004, 37, 4087–4098. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, C.; Lin, J.; Nguyen, T.-Q. Solution-processed ambipolar field-effect transistor based on diketopyrrolopyrrole functionalized with benzothiadiazole. Adv. Funct. Mater. 2011, 22, 97–105. [Google Scholar] [CrossRef]
- Pavlishchuk, V.V.; Addison, A.W. Conversion constants for redox potentials measured versus different reference electrodes in acetonitrile solutions at 25 °C. Inorg. Chim. Acta 2000, 298, 97–102. [Google Scholar] [CrossRef]
- Bard, A.J.; Fualkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Gaussian 09; Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2010.
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, W.Y.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self—Consistent molecular orbital methods. XII. Further extensions of gaussian—Type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
George, Z.; Kroon, R.; Gehlhaar, R.; Gbabode, G.; Lundin, A.; Hellström, S.; Müller, C.; Geerts, Y.; Heremans, P.; Andersson, M.R. The Influence of Alkoxy Substitutions on the Properties of Diketopyrrolopyrrole-Phenyl Copolymers for Solar Cells. Materials 2013, 6, 3022-3034. https://doi.org/10.3390/ma6073022
George Z, Kroon R, Gehlhaar R, Gbabode G, Lundin A, Hellström S, Müller C, Geerts Y, Heremans P, Andersson MR. The Influence of Alkoxy Substitutions on the Properties of Diketopyrrolopyrrole-Phenyl Copolymers for Solar Cells. Materials. 2013; 6(7):3022-3034. https://doi.org/10.3390/ma6073022
Chicago/Turabian StyleGeorge, Zandra, Renee Kroon, Robert Gehlhaar, Gabin Gbabode, Angelica Lundin, Stefan Hellström, Christian Müller, Yves Geerts, Paul Heremans, and Mats R. Andersson. 2013. "The Influence of Alkoxy Substitutions on the Properties of Diketopyrrolopyrrole-Phenyl Copolymers for Solar Cells" Materials 6, no. 7: 3022-3034. https://doi.org/10.3390/ma6073022
APA StyleGeorge, Z., Kroon, R., Gehlhaar, R., Gbabode, G., Lundin, A., Hellström, S., Müller, C., Geerts, Y., Heremans, P., & Andersson, M. R. (2013). The Influence of Alkoxy Substitutions on the Properties of Diketopyrrolopyrrole-Phenyl Copolymers for Solar Cells. Materials, 6(7), 3022-3034. https://doi.org/10.3390/ma6073022




