1. Introduction
The incineration of municipal solid wastes has relevant social, economic and environmental impacts. This process generates several gaseous effluents and produces both solid and liquid residues. The latter corresponds to 10% of initial waste volume. Hence, the proper management of these residues, particularly bottom and fly ash, is of crucial importance, and techniques for their stabilization/solidification need further optimization [
1,
2,
3].
The aforementioned issue can find an answer in eco-design approaches which aim for material recovery to reduce the consumption of natural raw materials in the field of cement-based materials manufacturing. The unreactive stabilized waste can be employed together with solid wastes produced by other industrial processes. In fact, resource optimization implies significant advantages in terms of economic, energetic and environmental parameters of concrete industry (e.g., LEED—Leadership in Energy and Environmental Design Indicators). This industry is able to recycle and stabilize many kinds of solid wastes both in binder and artificial aggregates production, in order to achieve sustainability objectives [
4,
5,
6,
7,
8,
9,
10,
11,
12].
Fly ash from municipal solid waste incinerators (MSWI-FA) are classified as hazardous in the European Union. Therefore, prior to a proper stabilization process, their contaminant release has to be evaluated. From this point of view, in addition to heavy metals, chlorides and sulfates pose major issues. In fact, untreated MSWI-FA release very high amounts of these pollutants when they are submitted to the leaching test UNI 10802-2004 [
13] that derives from test EN 12457-2: 2002 [
14]. These release values are always higher than the limits required for both hazardous and non-hazardous waste landfilling. Furthermore, any effective stabilization process is not economically sound if MSWI-FA are stabilized without a proper washing pretreatment. In this regard, attempts to optimize the solid/liquid ratio, with consequent water consumption reduction, can be found in the literature [
15,
16,
17,
18]. More specifically, Colangelo
et al. [
17] have applied one-step and two-step washing pretreatments on three different fly ash samples proving that the use of a very limited 2:1 overall liquid to solid ratio is possible. Thereby, the pre-washed MSWI-FA have been proposed as cement bound granular material in the manufacture of sub-base layer for road construction. Furthermore, a cost analysis of the complete process has been made too. Specifically, this cost analysis was carried out taking into account the charges for cement-stabilization, washing pretreatment and washing salt disposal less the benefits from material reuse and comparing this with the charge for untreated MSWI-FA simple disposal. The results have demonstrated that the proposed process is economically sound.
As far as the stabilizing matrices are considered, it is well known that cementitious ones based on cements, pozzolans, blast furnace slag and lime are often not suitable to reduce the very high mobility of chlorides and sulfates down to the imposed regulation limits. The reason for this is that high chloride and sulfate concentration has a strong negative effect on their efficiency [
19,
20]. Alternative matrices, such as those based on alkali-activated aluminosilicate binders, including the geopolymers, are worthy of consideration because excellent mechanical properties, durability, resistance to acid attack and thermal stability can be achieved. The synthesis of geopolymers takes place by polycondensation and can start from silicoaluminate and aluminosilicate materials. When they are in contact with the high pH of alkaline solution, raw materials dissolve and the inorganic polymers precipitate [
21,
22,
23,
24,
25]. Recently, the applications of this broad class of materials in several fields of engineering have been deeply discussed by many authors, revealing a great number of possible technological solutions [
26,
27,
28,
29,
30,
31,
32,
33]. Great interest also derives from the possibility of employing naturally occurring silicoaluminate and aluminosilicate industrial wastes, such as coal fly ash, blast furnace slag, clay sediment,
etc., thus decreasing the environmental impact for the manufacturing of new materials based on geopolymers. In addition, the synthesis of neo-formed phases takes place at low temperatures, not higher than 60 °C [
21,
22,
23,
24,
25,
34,
35,
36,
37]. All these considerations imply, in comparison to traditional cement-based materials, a reduction of natural raw materials consumption and greenhouse gases emission, particularly CO
2.
In the field of hazardous solid waste treatment, the above polycondensation phases can favour the entrapment of contaminants, by means of both physical and chemical mechanisms, when geopolymers are employed as stabilizing matrices. Particularly, the stabilization/solidification of MSWI-FA in geopolymers has been already discussed by several authors in recent years [
38,
39,
40,
41,
42,
43]. Even if geopolymeric matrices setting and hardening are based on a different chemistry, as for the cementitious systems, the negative effect of the presence of chlorides and sulfates on the polycondensation kinetic was observed [
44,
45].
In order to optimize the entire cycle of MSWI-FA stabilization, water pre-washing can be applied for chlorides (and other soluble salts such as sulfates) removal. To this regards, Zheng
et al. [
46] investigated the effect of water-wash on geopolymerization. They concluded that a combined washing-stabilization process gave better immobilization efficiency of some heavy metals and higher early strength of hardened specimens. The drawbacks of this approach are the related water consumption for complete chlorides removal and the secondary pollution arising from the transfer of chlorides and other soluble salts from the ash to the washing water. So, the washing pretreatment must be optimized in relation to the minimum washing water requirement and maximum allowed residual amount of chlorides (and other soluble salts). Finally, an adequate binder to waste ratio is to be used in the stabilization process for the economically sound management of MSWI-FA landfilling/reuse.
In this work, coal fly ash has been used for the synthesis of geopolymeric matrices that can incorporate and stabilize three samples of fly ash from municipal solid wastes incinerators (MSWI). The different MSWI-FA samples have been used not only as received, but also after washing to reduce their chloride content. The products obtained under the different experimental conditions have been characterized from the qualitative point of view by means of Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD) and scanning electron microscopy (SEM) and from the quantitative point of view through the measurement of the amounts of silicate and water reacted upon polycondensation. Finally, density and compressive strength of hardened specimens have also been evaluated and the environmental and technological classification of the final materials has been assessed by means of leaching tests and management considerations. The main goal of this work is gathering experimental data useful for the MSWI-FA management in relation to final reuse/utilization options. From this point of view, it is clear that, due to the intrinsic characteristics of the materials employed, hi-tech solutions will be precluded and options such as abandoned quarry filling or low temperature setting, soft brick manufacturing will be more appropriate.
2. Materials and Methods
The three MSWI-FA samples come from plants located in southern, central and northern Italy and have been named A, B and C, respectively. These samples have been collected downstream of the air pollution control (APC) device and comprised MSWI fly ash plus APC residue. The three samples have been submitted to chemical analysis, as described extensively in a previous work [
17]. These three ash samples have been submitted to total acid digestion according to ASTM 5258-92 [
47] and subsequent chemical analysis through inductively coupled plasma atomic emission spectrometry (ICP-AES) technique for the determination of metal contents. Chloride and sulfate content has been determined by means of the Mohr method and ionic liquid chromatography, respectively. All the measurements have been replicated nine times, and when reporting the data, mean values and standard deviations have been shown.
The three MSWI-FA have been characterized in terms of heavy metal, chloride and sulfate release by means of UNI 10802 [
13] leaching tests. This is a test that makes use of deionized water with a liquid to solid ratio of 10.l/kg and, in case of granular wastes (size < 4 mm), has a duration of 24 h without leachant renewal. The release results have been previously reported together with the compulsory limits for landfilling of both hazardous and non-hazardous wastes (D.M. 27/09/2010) [
48]. The authors reported that MSWI-FA must be stabilized in order to reduce the release of some heavy metals and, in addition, of chlorides. In fact, even if the final option is landfilling, the disposal is not allowed because sometimes both the hazardous and non-hazardous limits are exceeded. Specifically, the release of cadmium exceeds the two limits in the case of ash A and B. Chromium release exceeds the landfill disposal limit for non-hazardous wastes in the cases of all the ash, while lead release exceeds both the limits in the case of ash B and only the limit for non-hazardous wastes disposal in the cases of the other two ash. All of the three ash showed values of zinc release slightly higher than the two limits. In the case of chloride release, the values were always much higher than the two limits, while only in the case of ash B, the sulfate release slightly exceeded the limits.
Furthermore, the need for an optimized washing pretreatment of ash must also be explored to improve stabilization/solidification (S/S) process efficiency. In fact, to address an economically sound proposal, the waste amounts have to be maximized and, as a consequence, a preliminary washing treatment of ash could be worthy of consideration. Following the results reported by Colangelo
et al. [
17] in the above cited experimentation, a double step washing treatment with a water to ash ratio of 2:1 has been applied to the present work where a geopolymeric stabilizing system is studied. This kind of process, although more complex, minimizes water consumption, and therefore, contributing to the economy of the whole process. In fact, each ash sample has been divided into equal parts, and in the first step, one of them has been washed with a water to solid ratio of 4:1. During the second step, the solution coming from the first step is contacted with the other part of the ash. In this way, each part of ash is in contact with an amount of water useful for better handling of the liquid/solid suspension. Moreover, the overall liquid/solid ratio is 2:1.
Geopolymer systems produced from coal fly ash have been used as stabilizing matrices of the three different MSWI-FA. The coal fly ash used for the synthesis of the geopolymers has been supplied by the Italian electricity board (ENEL S.p.A., Rome, Italy) and comes from a power plant located in Brindisi (Southern Italy). It is the same as that used in a previous work [
21] and its characterization, made by means of the same chemical analytical techniques as reported for MSWI-FAs, has given the following chemical composition: SiO
2, 44.3% (2060 mg,
Si/kg,
FA) Al
2O
3, 20.2% (1070 mg/kg); Fe
2O
3, 10.5% (734 mg/kg); K
2O, 8.1% (737 mg/kg); CaO, 0.5% (36 mg/kg); Na
2O, 0.3% (22 mg/kg); loss on ignition at 1050 °C, 11.3%. Alkali activation, necessary to promote polycondensation, has been carried out by adding NaOH and sodium silicate solutions of proper concentration.
The three samples of MSWI-FA have been submitted to the stabilization treatment both as received, and after partial soluble salt removal (mainly chlorides and sulfates) carried out by the double step water washing previously described.
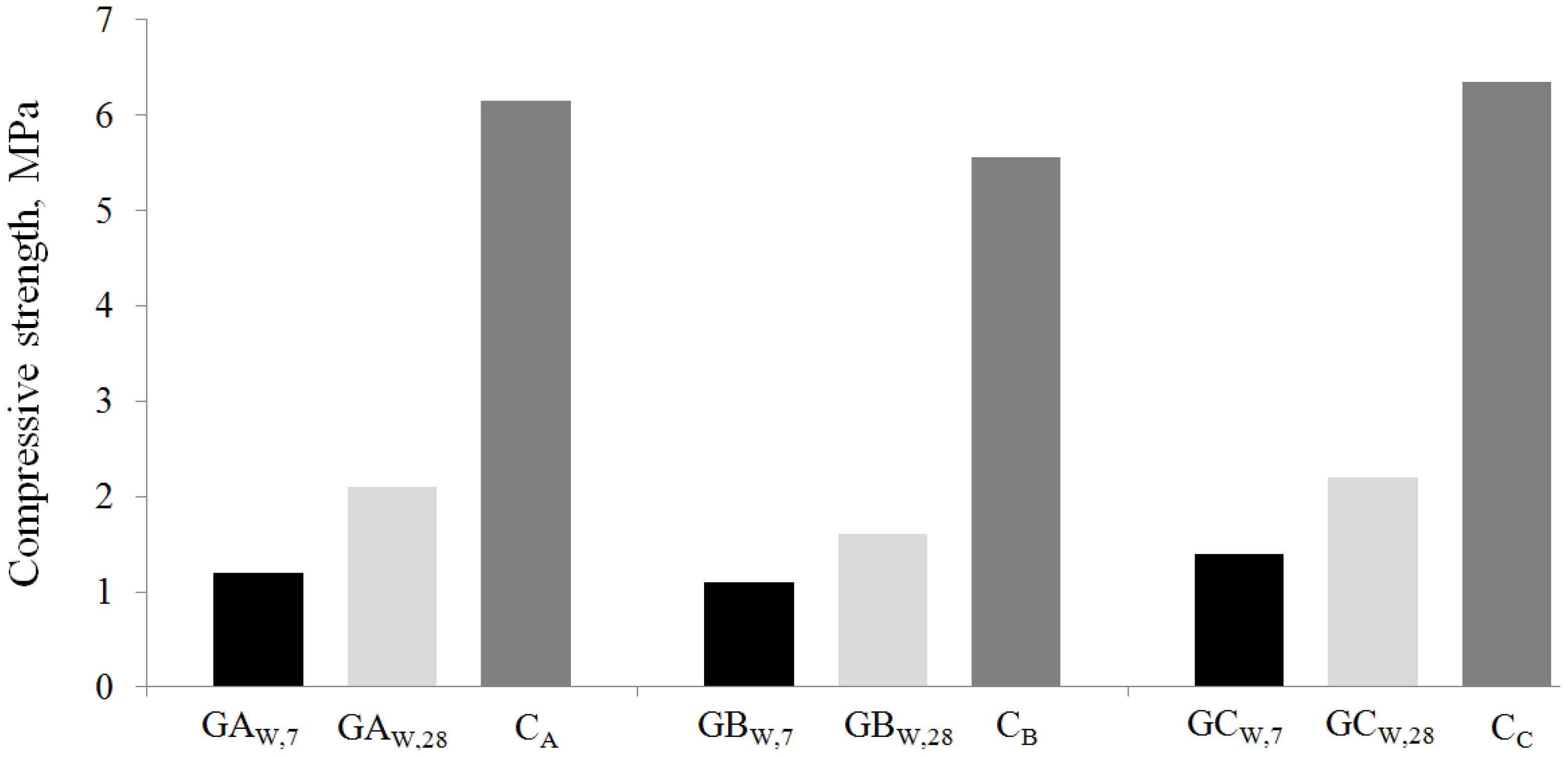
The compositions of the systems tested are reported in
Table 2 and have been designed by fixing at 75/25 the MSWI-FA/coal fly ash ratio. Cylindrical samples (diameter 3 cm, height 6 cm) have been prepared by pouring each mixture into polyethylene moulds. Three samples have been cured for three days at 60 °C in oven under 100% relative humidity (RH) conditions. Afterwards, the specimens have been extracted from the moulds and subjected to Unconfined Compressive Strength (UCS) determination by using a 100 kN capacity Controls
® MCC8 testing machine.
This mechanical evaluation is significant because it is well known that rapid strength development is a peculiar feature of geopolymerization.
It is important to underline that in previous works the MSWI-FA/solid precursor ratios were much lower than 75/25. Specifically, Lancellotti
et al. [
40] employed systems based on metakaolin/MSWI-FA mixtures with about 17% ash, while Luna Galiano
et al. [
41] used about 26% ash in respect to coal fly ash in geopolymeric systems based on coal fly ash/MSWI-FA, coal fly ash + blast furnace slag/MSWI-FA, coal fly ash + metakaolin/MSWI-FA and coal fly ash + kaolin/MSWI-FA.
The complete set of experimental compositions is reported in
Table 1. These compositions have been designed taking into account those studied in the previous work [
21] that gave good geopolymerization results and also by considering that a large portion of coal FA is replaced by MSWI-FA in this work. The components of all the systems listed in
Table 2 have been carefully mixed and the resulting mixtures have been kept in small polyethylene cylinders of size
d ×
h = 3 cm × 6 cm. The polycondensation reaction has been carried out at 25 °C for times equal to 1, 3, 7, 14 and 28 days.
Table 1.
Composition of the geopolymer materials, wt %.
Table 1.
Composition of the geopolymer materials, wt %.
| System | MSWI-FA | Coal fly ash | Sodium silicate solution (1.15 M) | NaOH solution |
|---|
| GAAR 1 | 48 | 16 | 18 | 18 (10 M) |
| GAW 2 | 51.5 | 16.5 | 16 | 16 (10 M) |
| GBAR | 57.5 | 18.5 | 12 | 12 (10 M) |
| GBW | 60 | 20 | 10.5 | 10.5 (10 M) |
| GCAR | 53 | 17 | 15 | 15 (17 M) |
| GCW | 55 | 18 | 13.5 | 13.5 (17 M) |
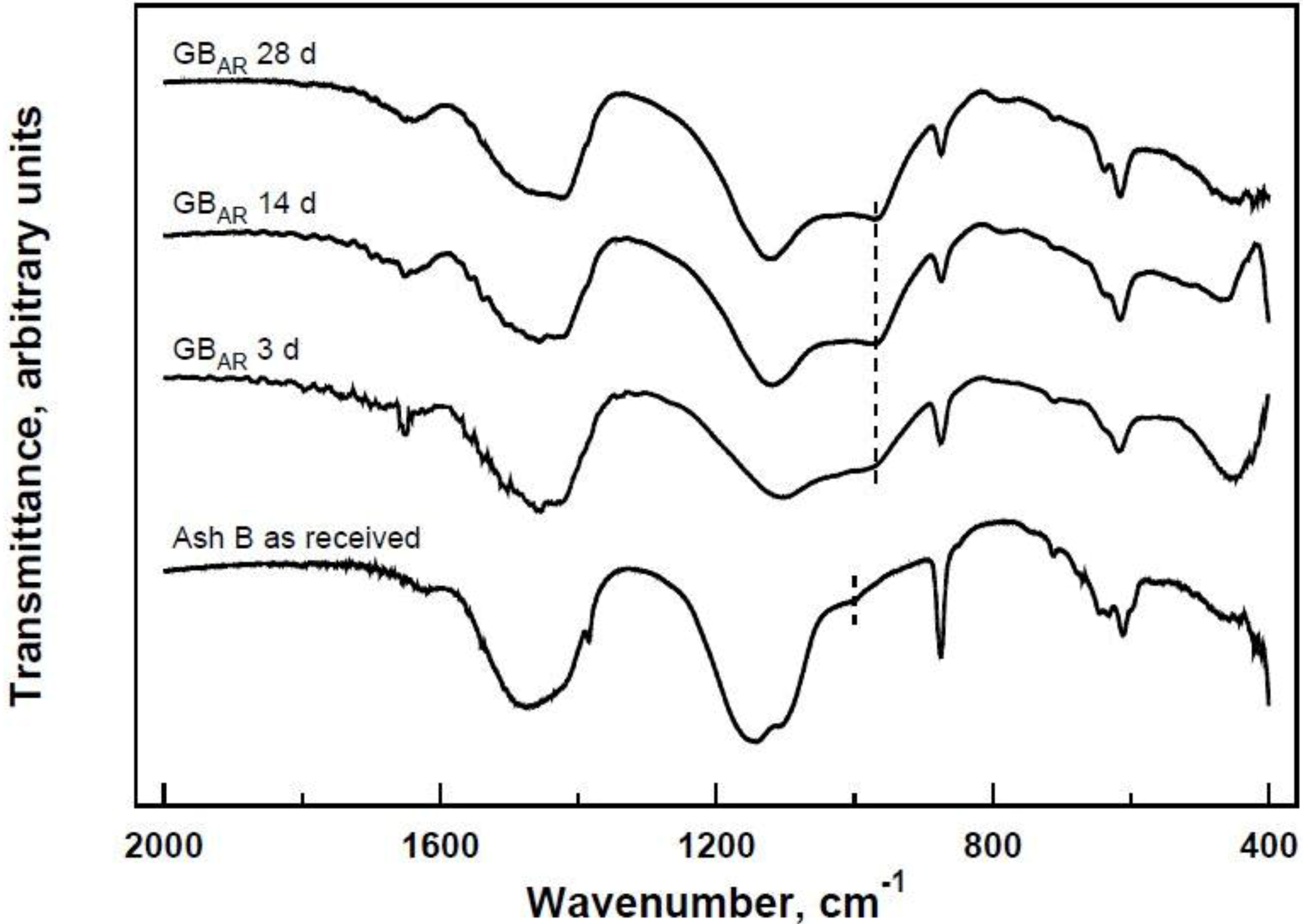
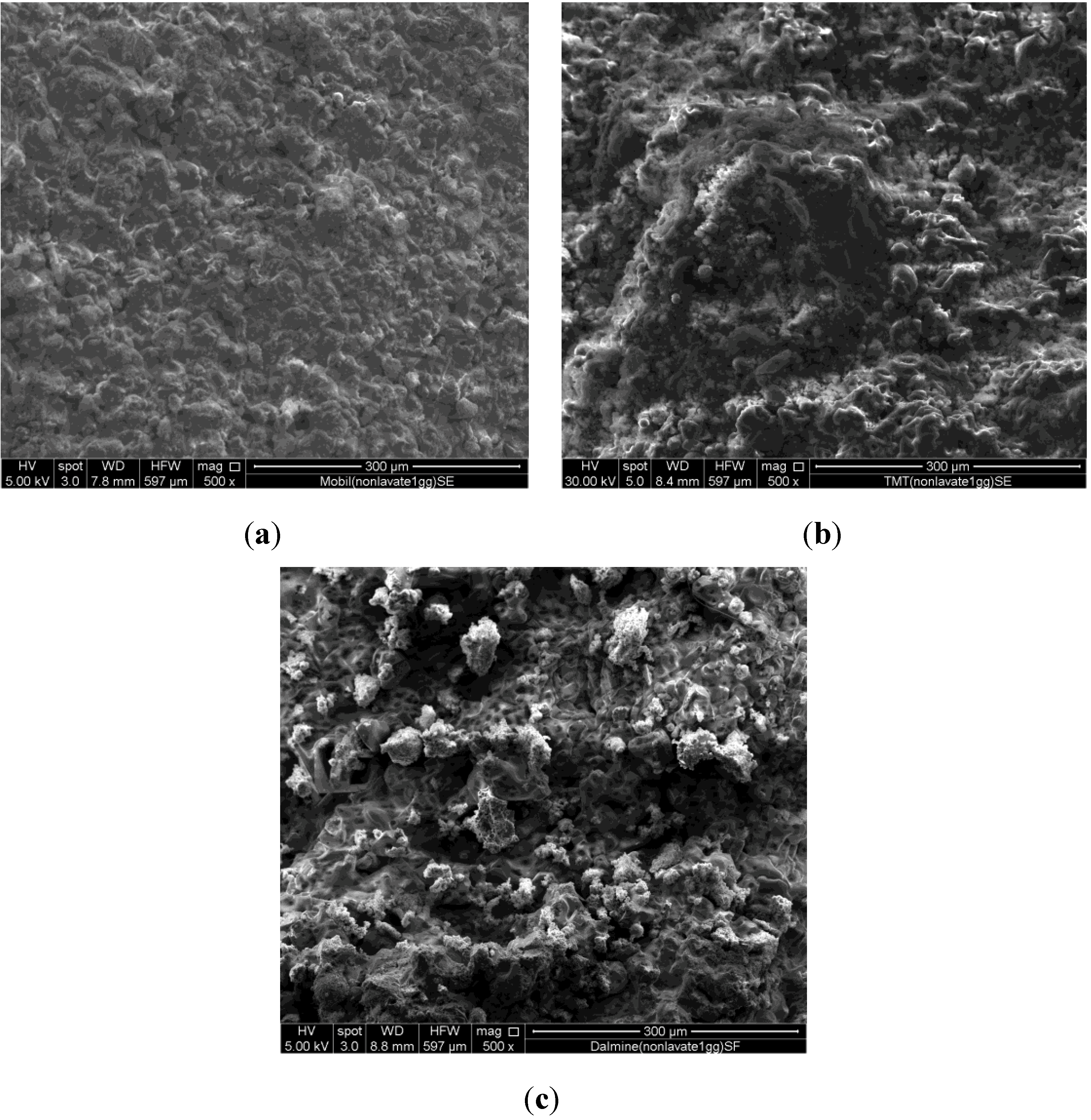
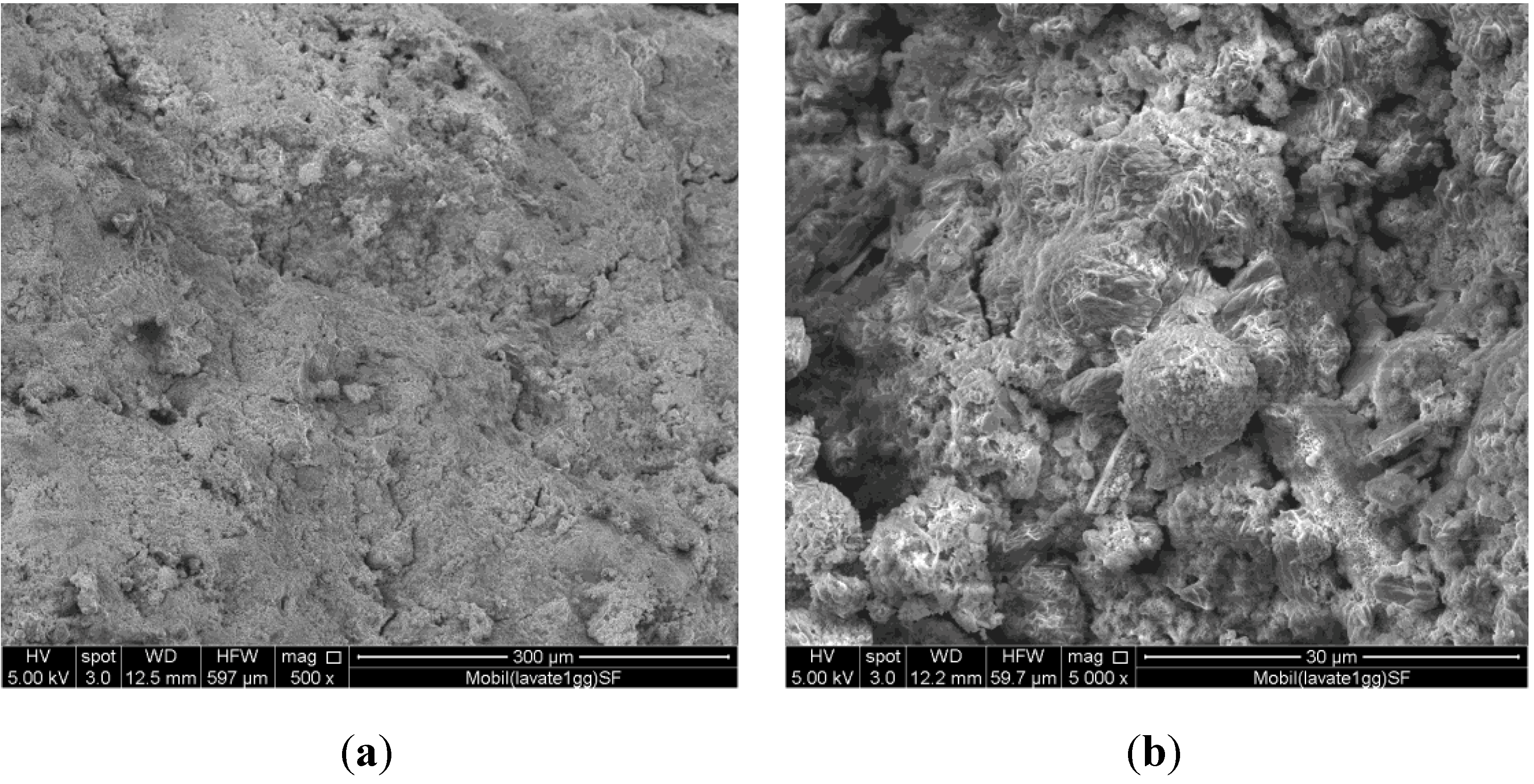
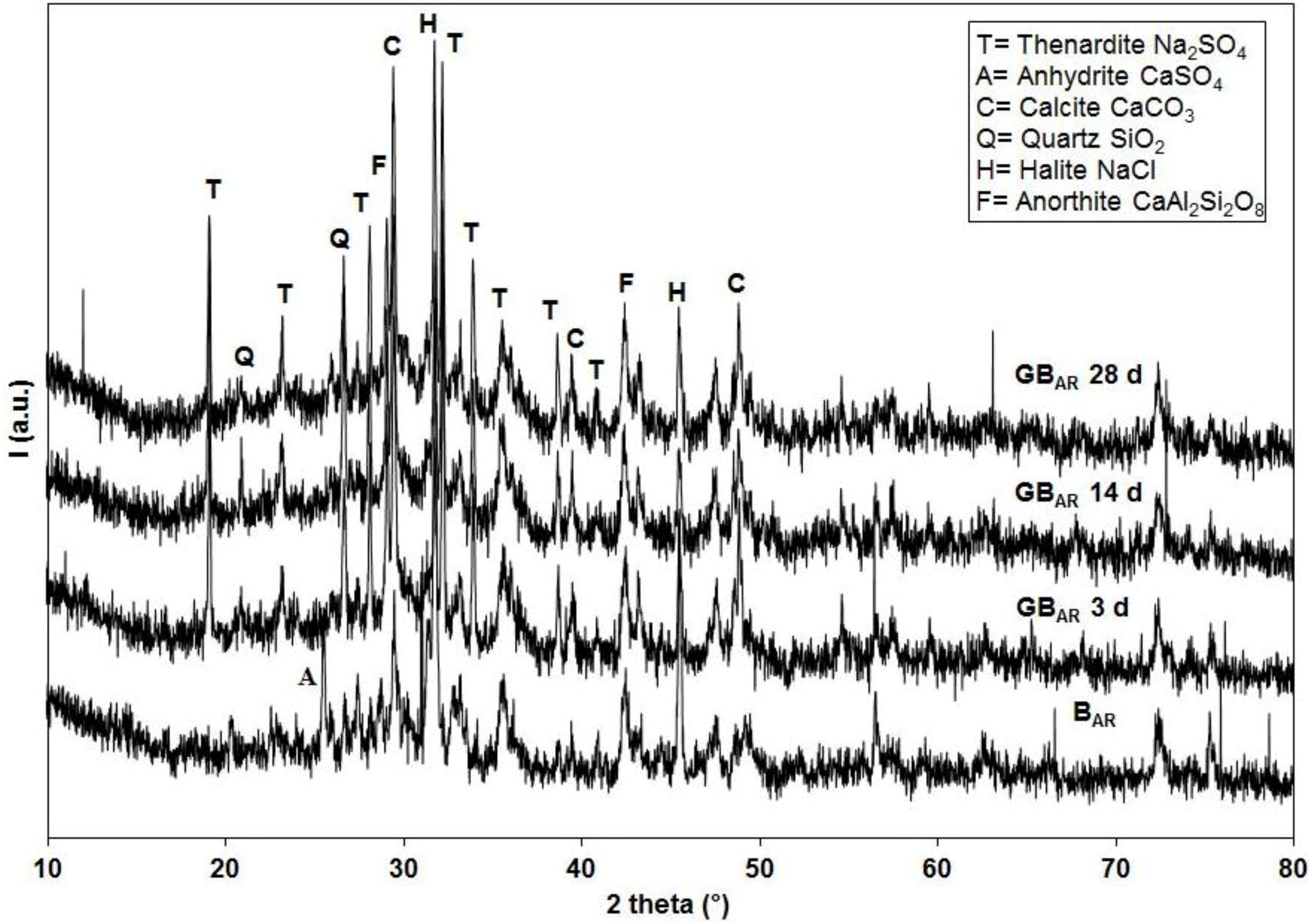
The specimens obtained at any prefixed polycondensation time have been characterized by means of a Thermo Scientific Nicolet Nexus FT-IR spectrometer (Thermo Scientific, Waltham, MA, USA) equipped with a DTGS KBr (deuterated triglycine sulfate with potassium bromide windows) detector. FT-IR absorption spectra have been recorded in the 4000–400 cm−1 range. A spectral resolution of 2 cm−1 has been chosen. 2.0 mg of each test sample has been mixed with 200 mg of KBr in an agate mortar, and then pressed into 200 mg pellets of 13 mm diameter. The spectrum of each sample represents an average of 32 scans. Furthermore, a Philips PW 1730 X-ray diffractometer (Philips, Eindhoven, The Netherlands) (CuKα radiation, 40 kV, 40 mA, 2θ range from 10° to 80°, equivalent step size 0.0179° 2θ, equivalent counting time 120 s per step) has been employed in order to obtain the mineralogical characterization of the same series of samples. Selected hardened samples have been also submitted to a microstructural characterization by means of a FEI Quanta 200 FEG scanning electron microscope (FEI, Hillsboro, OR, USA).
The same specimens have been used for the quantitative determination of water and sodium silicate consumed during the polycondensation reaction. The amounts of reacted sodium silicate and water at any polycondensation time have been determined as follows. Each specimen has been ground under acetone, filtered and washed with diethyl ether to remove all the residual aqueous phase. Finally, the samples have been heated in an oven up to 40 °C in order to ensure the loss of any residual fraction of the liquids previously used. The cumulative amount of reacted sodium silicate and water has been obtained by weight difference between the solid recovered after the above treatments and the ash initially employed. The amount of reacted water has been determined by the excess loss on ignition of the recovered solid over that of the initial ash. This method is extensively described in previous works [
21,
35].
The leaching behaviour of the stabilized systems has been assessed submitting cubic specimens of 4 cm in size to UNI 10802 test (UNI 10802, 2004) [
13]. This procedure follows the protocol for monolithic specimens, which imposes water renewals after 2 and 18 h, for a total duration of 48 h. The solid surface to liquid ratio has been fixed at 1:10. At the end of each test, the pH of leachate has been measured.
To evaluate the suitability of the stabilized/solidified geopolymeric systems containing pre-washed ash for material reuse, three series of cubic specimens of 4 cm in size have been cured for 28 days at room temperature and 100% RH. Then, they have been submitted to density evaluation and UCS measurements making use of the same testing machine described above.