A Novel Biodegradable Polycaprolactone Fixator for Osteosynthesis Surgery of Rib Fracture: In Vitro and in Vivo Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of the Biodegradable PCL Fixator

2.2. In Vitro Biomechanical Study

2.3. Animal Model

2.4. Statistical Analysis
3. Results
3.1. Biomechanical Study
| Native Rib Parameters | |||
| Experimental results | Native ribs | ||
| Ti group (n = 10) | PCL group (n = 10) | p-value | |
| Mean load at failure (N) | 16.27 ± 4.13 | 18.59 ± 4.74 | 0.26 |
| Mean stress at failure (MPa) | 58.46 ± 8.33 | 64.2 ± 16.50 | 0.50 |
| Mean strain (mm) | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.11 |
| Comparison between Native Ribs and Ribs in Ti Group | |||
| Experimental results | Native ribs | Ti group | p-value |
| Mean load at failure (N) | 16.27 ± 4.13 | 17.12 ± 3.45 | 0.44 |
| Mean stress at failure (MPa) | 58.46 ± 8.33 | 70.82 ± 35.40 | 0.14 |
| Mean strain (mm) | 0.03 ± 0.01 | 0.12 ± 0.22 | 0.25 |
| Comparison between Native Ribs and Ribs in PCL Group | |||
| Experimental results | Native ribs | PCL group | p-value |
| Mean load at failure (N) | 18.59 ± 4.74 | 4.64 ± 2.75 | 0.005 |
| Mean stress at failure (MPa) | 64.2 ± 16.50 | 14.78 ± 13.16 | 0.005 |
| Mean strain (mm) | 0.03 ± 0.01 | 0.11 ± 0.03 | 0.005 |
| Comparison between Ribs in Ti Group and in PCL Group | |||
| Experimental results | Ti group | PCL group | p-value |
| Mean load at failure (N) | 17.12 ± 3.45 | 4,64 ± 2.75 | <0.001 |
| Mean stress at failure (MPa) | 70.82 ± 35.40 | 14.78 ± 13.16 | <0.001 |
| Mean strain (mm) | 0.12 ± 0.22 | 0.11 ± 0.03 | 0.005 |

3.2. Animal Study
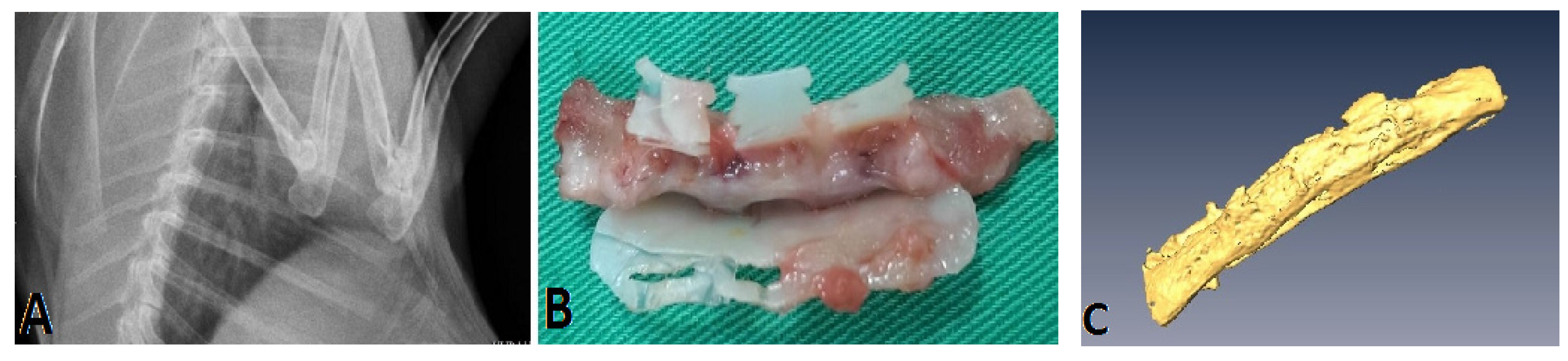
3.3. Histological Examination

4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vu, K.C.; Skourtis, M.E.; Gong, X.; Zhou, M.; Ozaki, W.; Winn, S.R. Reduction of rib fractures with a bioresorbable plating system: Preliminary observations. J. Trauma Acute Care Surg. 2008, 64, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Mayberry, J.C.; Terhes, J.T.; Ellis, T.J.; Waneks, S.; Mullins, R.J. Absorbable plates for rib fracture repair: Preliminary experience. J. Trauma Acute Care Surg. 2003, 55, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Civil, I. An Australasian perspective of chest trauma. Aust. N. Z. J. Surg. 1999, 69, 576–577. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Mohyunddin, Z. Management of flail chest injury internal fixation versus endotracheal intubation and ventilation. J. Thorac. Cardiovasc. Surg. 1995, 110, 1676–1680. [Google Scholar] [CrossRef]
- Nirula, R.; Mayberry, J.C. Rib fracture fixation: Controversies and technical challenges. Am. Surg. 2010, 76, 793–802. [Google Scholar] [PubMed]
- Engel, C.; Krieg, J.C.; Madey, S.M.; Long, W.B.; Bottlang, M. Operative chest wall fixation with osteosynthesis plates. J. Trauma Acute Care Surg. 2005, 58, 181–186. [Google Scholar] [CrossRef]
- Campbell, N.; Richardson, M.; Antippa, P. Biomechanical testing of two devices for internal fixation of fractured ribs. . J. Trauma Acute Care Surg. 2010, 68, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Marasco, S.F.; Liovic, P.; Šutalo, I.D. Structural integrity of intramedullary rib fixation using a single bioresorbable screw. J. Trauma Acute Care Surg. 2012, 73, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Chao, Y.K.; Liu, K.S.; Wang, Y.C.; Huang, Y.L.; Liu, S.J. Biodegradable cisplatin-eluting tracheal stent for malignant airway obstruction: In vivo and in vitro studies. Chest J. 2013, 144, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Pitt, C.G.; Gratzl, M.M.; Kimmel, G.L.; Surles, J.; Schindler, A. Aliphatic polyesters II. The degradation of poly(DL-lactide), poly(ε-caprolactone) and their copolymers in vivo. Biomaterials 1981, 2, 215–220. [Google Scholar] [CrossRef]
- Porter, J.R.; Henson, A.; Popat, K.C. Biodegradable poly(ε-caprolactone) nanowires for bone tissue engineering applications. Biomaterials 2009, 30, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Rokkanen, P.U.; Böstman, O.; Hirvensalo, E.; Mäkelä, E.A.; Partio, E.K.; Pätiälä, H.; Vainionpää, S.I.; Vihtonen, K.; Törmälä, P. Bioabsorable fixation in orthopaedic surgery and traumatomogy. Biomaterials 2000, 21, 2607–2613. [Google Scholar] [CrossRef]
- Daniels, A.U.; Chang, M.K.; Andriano, K.P.; Heller, J. Mechanical properties of biodegradable polymers and composites proposed for internal fixation of bone. J. Appl. Biomater. 1990, 1, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur. Cells Mater. 2003, 5, 1–16. [Google Scholar]
- Liu, K.S.; Liu, Y.H.; Peng, Y.J.; Liu, S.J. Experimental absorbable stent permits airway remodeling. J. Thorac. Cardiovasc. Surg. 2011, 141, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Brandao-Burch, A.; Meghji, S.; Arnett, T.R. Acidosis strongly upregulates mRNA for cathepsin K, TRAP and TRAF-6 in bone. Calcif. Tissue Int. 2003, 72, 364. [Google Scholar]
- Bushinsky, D.A. Simulated osteoclastic and suppressed osteoblastic activity in metabolic but not respiratory acidosis. Am. J. Physiol. Cell Physiol. 1995, 268, C80–C88. [Google Scholar]
- Brandao-Burch, A.; Utting, J.C.; Orriss, I.R.; Arnett, T.R. Acidosis inhibits bone formation by osteoblasts in vitro by preventing mineralization. Calcif. Tissue Int. 2005, 77, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Arnett, T.R. Acid-base regulation of bone metabolism. Int. Congr. Ser. 2007, 1297, 255–267. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C.M.L. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- Obarzanek-Fojt, M.; Elbs-Glatz, Y.; Lizundia, E.; Diener, L.; Sarasua, J.R.; Bruinink, A. From implantation to degradation—Are poly(L-lactide)/multiwall carbon nanotube composite materials really cytocompatible? Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.; Conaglen, P.; Martin, K.; Antippa, P. Surgical stabilization of rib fractures using Inion OTPS wraps-techniques and quality of life follow-up. J. Trauma Acute Care Surg. 2009, 67, 596–601. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.-H.; Fan, C.-L.; Hsu, Y.-H.; Chou, Y.-C.; Ueng, S.W.N.; Liu, S.-J. A Novel Biodegradable Polycaprolactone Fixator for Osteosynthesis Surgery of Rib Fracture: In Vitro and in Vivo Study. Materials 2015, 8, 7714-7722. https://doi.org/10.3390/ma8115415
Yu Y-H, Fan C-L, Hsu Y-H, Chou Y-C, Ueng SWN, Liu S-J. A Novel Biodegradable Polycaprolactone Fixator for Osteosynthesis Surgery of Rib Fracture: In Vitro and in Vivo Study. Materials. 2015; 8(11):7714-7722. https://doi.org/10.3390/ma8115415
Chicago/Turabian StyleYu, Yi-Hsun, Chin-Lung Fan, Yung-Heng Hsu, Ying-Chao Chou, Steve W. N. Ueng, and Shih-Jung Liu. 2015. "A Novel Biodegradable Polycaprolactone Fixator for Osteosynthesis Surgery of Rib Fracture: In Vitro and in Vivo Study" Materials 8, no. 11: 7714-7722. https://doi.org/10.3390/ma8115415





