Mechanical Properties and In Vitro Degradation of Sputtered Biodegradable Fe-Au Foils
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Samples
2.2. Corrosion Measurements
2.3. Mechanical Properties
3. Results and Discussion
3.1. Mechanical Properties
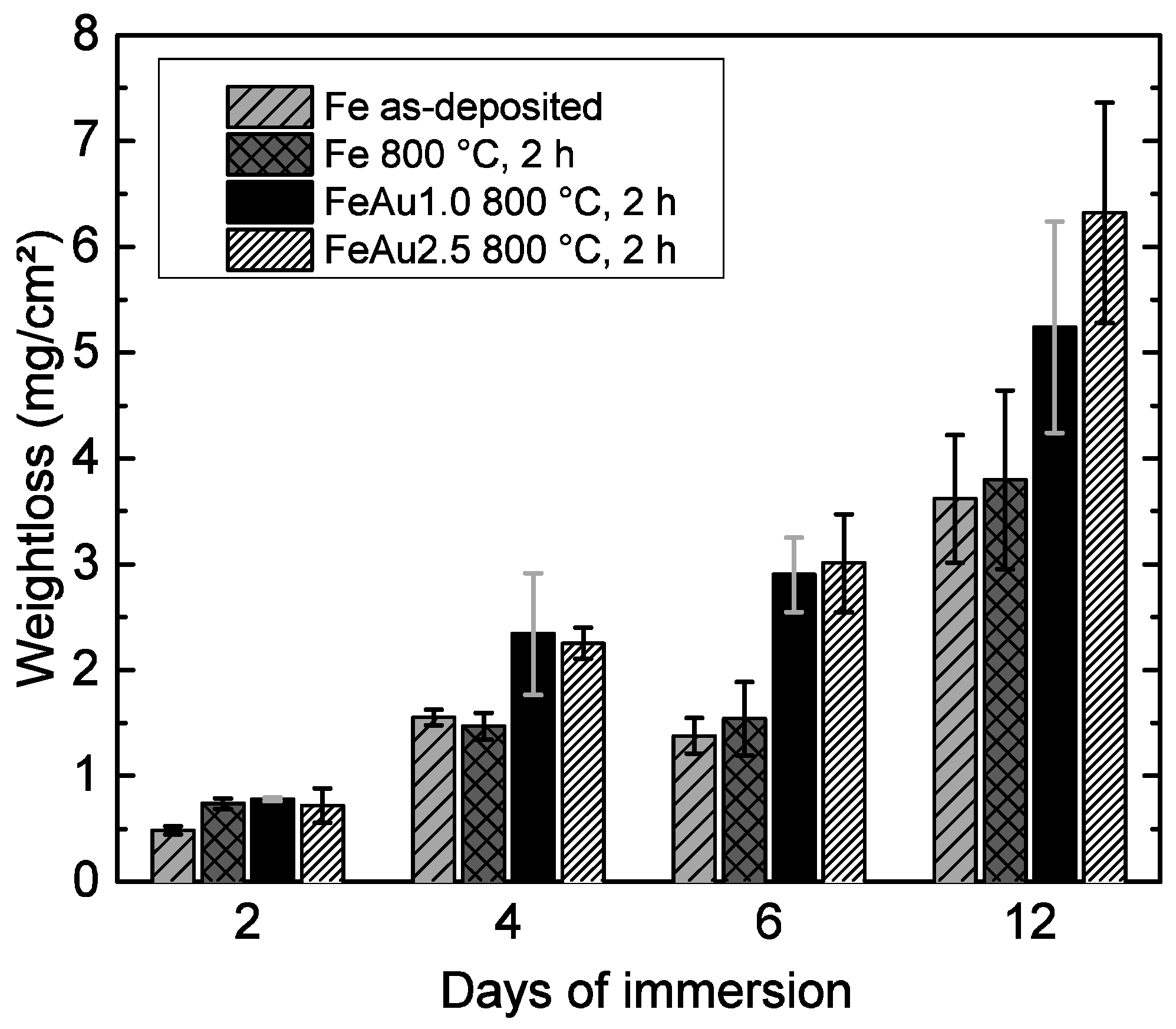
3.2. Corrosion Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Colombo, A.; Karvouni, E. Biodegradable stents “fulfilling the mission and stepping away”. Circulation 2000, 102, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Farb, A.; Guagliumi, G.; Kolodgie, F.D. Drug-eluting stents: Caution and concerns for long-term outcome. Coron. Artery Dis. 2004, 15, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, B.; Wu, Y.H.; Zheng, Y.F. Comparative in vitro study on pure metals (Fe, Mn, Mg, Zn and W) as biodegradable metals. J. Mater. Sci. Technol. 2013, 29, 619–627. [Google Scholar] [CrossRef]
- Peuster, M.; Wohlsein, M.; Brügmann, M.; Ehlerding, M.; Seidler, K.; Fink, C.; Brauer, H.; Fischer, A.; Hausdorf, G. A novel approach to temporary stenting: Degradable cardiovascular stents produced from corrodible metal–results 6–18 months after implantation into New Zealand white rabbits. Heart 2001, 86, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Peuster, M.; Hessea, C.; Schloo, T.; Fink, C.; Beerbaum, P.; von Schnakenburg, C. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials 2006, 12, 4955–4962. [Google Scholar] [CrossRef] [PubMed]
- Moravej, M.; Prima, F.; Fiset, M.; Mantovani, D. Electroformed iron as new biomaterial for degradable stents: Development process and structure-properties relationship. Acta Biomater. 2010, 6, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Schinhammer, M.; Steiger, P.; Moszner, F.; Löffler, J.F.; Uggowitzer, P.J. Degradation performance of biodegradable FeMnC(Pd) alloys. Mater. Sci. Eng. 2013, 33, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Cheng, J.; Zheng, Y.F. In vitro degradation and biocompatibility of Fe-Pd and Fe-Pt composites fabricated by spark plasma sintering. Mater. Sci. Eng. 2014, 35, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Zamponi, C.; Schürmann, U.; Jurgeleit, T.; Kienle, L.; Quandt, E. Microstructures of magnetron sputtered Fe Au thin films. Int. J. Mater. Res. 2015, 106, 103–107. [Google Scholar] [CrossRef]
- Huang, T.; Cheng, J.; Bian, D.; Zheng, Y.F. Fe-au and Fe-Ag composites as candidates for biodegradable stent materials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015. [Google Scholar] [CrossRef] [PubMed]
- Zamponi, C.; Rumpf, H.; Schmutz, C.; Quandt, E. Structuring of sputtered superelastic niti thin films by photolithography and etching. Mater. Sci. Eng. 2008, 481, 623–625. [Google Scholar] [CrossRef]
- Lima de Miranda, R.; Zamponi, C.; Quandt, E. Fabrication of tini thin film stents. Smart Mater. Struct. 2009, 18, 103–107. [Google Scholar] [CrossRef]
- Lima de Miranda, R.; Zamponi, C.; Quandt, E. Micropatterned Freestanding Superelastic TiNi Films. Adv. Eng. Mater. 2013, 15, 66–69. [Google Scholar] [CrossRef]
- Siekmeyer, G.; Schüßler, A.; Lima de Miranda, R.; Quandt, E. Comparison of the fatigue performance of commercially produced nitinol samples versus sputter-deposited nitinol. J. Mater. Eng. Perform. 2014, 23, 2437–2445. [Google Scholar] [CrossRef]
- Schlüter, K.; Shi, Z.; Zamponi, C.; Cao, F.; Quandt, E.; Atrens, A. Corrosion performance and mechanical properties of sputter-deposited MgY and MgGd alloys. Corros. Sci. 2010, 78, 43–54. [Google Scholar] [CrossRef]
- Schlüter, K.; Zamponi, C.; Hort, N.; Kainer, K.U.; Quandt, E. Polycrystalline and amorphous MgZnCa thin films. Corros. Sci. 2012, 63, 234–238. [Google Scholar] [CrossRef]
- Schlüter, K.; Zamponi, C.; Piorra, A.; Quandt, E. Comparison of the corrosion behavior of bulk and thin film magnesium alloys. Corros. Sci. 2010, 52, 3973–3977. [Google Scholar] [CrossRef]
- Jurgeleit, T.; Quandt, E.; Zamponi, C. Magnetron sputtering a new fabrication method of iron based biodegradable implant materials. Adv. Mater. Sci. Eng. 2015, 2015, 294686. Available online: http://dx.doi.org/10.1155/2015/294686 (accessed on 18 August 2016). [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical polarization i. a theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Jones, D.A. Electrochemical Kinetics of Corrosion: Polarization Methods to Measure Corrosion Rate. In Principles and Prevention of Corrosion, 2nd ed.; Stenquist, B., Kernan, R., Daly, P., Eds.; Pearson-Prentice Hall International: London, UK, 1996; pp. 75–162. [Google Scholar]
- Zhu, S.; Huang, N.; Xu, L.; Zhang, Y.; Liu, H.; Sun, H.; Leng, Y. Biocompatibility of pure iron: In vitro assessment of degradation kinetics and cytotoxicity on endothelial cells. Mater. Sci. Eng. 2009, 5, 1589–1592. [Google Scholar] [CrossRef]
- Predel, B.; Madelung, O. (Eds.) Landolt-Börnstein, Group IV–Physical Chemistry; Springer: Berlin, Germany, 1998; Volume 5a, pp. 362–365.
- Hall, E.O. The deformation and ageing of mild steel: II characteristics of the Lüders deformation. Proc. Phys. Soc. Sect. 1951, 64, 742–747. [Google Scholar] [CrossRef]
- Hall, E.O. The deformation and ageing of mild steel: III discussion of results. Proc. Phys. Soc. Sect. 1951, 64, 747–752. [Google Scholar] [CrossRef]
- Heiden, M.; Walker, E.; Stanciu, L. Magnesium, Iron and Zinc Alloys, the Trifecta of Bioresorbable Orthopaedic and Vascular Implantation-A Review. J. Biotechnol. Biomater. 2015, 5, 1. [Google Scholar]
- Ralston, K.D.; Birbilis, N.; Davies, C.H.J. Revealing the relationship between grain size and corrosion rate of metals. Scr. Mater. 2010, 12, 1201–1204. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 90th ed.; (Internet Version 2010); CRC PressPress/Taylor and Francis: Boca Raton, FL, USA, 2010. [Google Scholar]


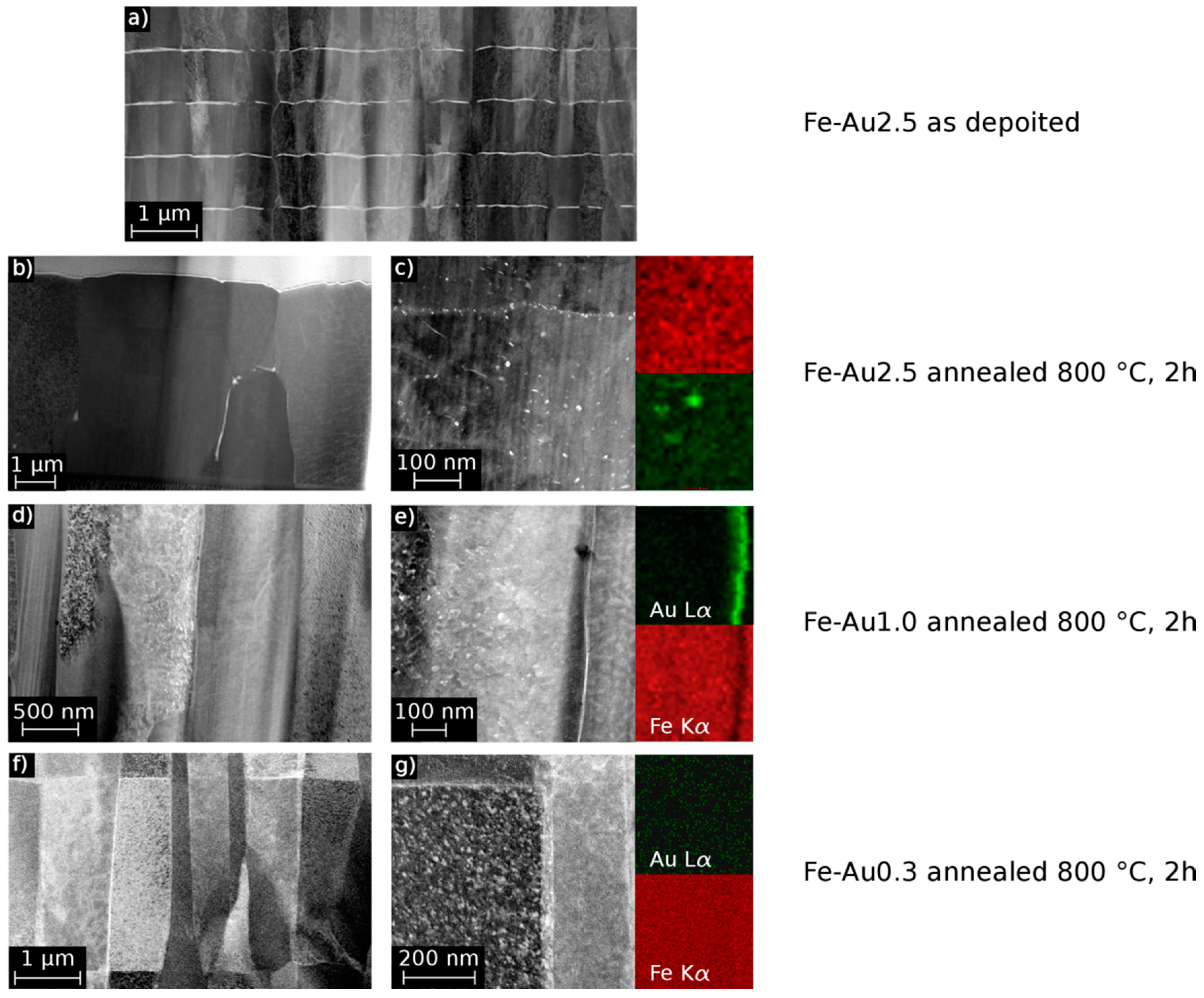


| Element | Power (W) | Pressure (mbar) | Ar Gas Flow (sccm) | Sputtering Rate (nm/s) |
|---|---|---|---|---|
| Cu | 1000 (DC *) | 2.3 × 10−3 | 25 | 4.1 |
| Fe | 600 (RF #) | 2.3 × 10−3 | 35 | 0.6 |
| Au | 200 (DC *) | 2.3 × 10−3 | 35 | 1.0 |
| Sample | Average Grain Size (µm) |
|---|---|
| Fe [21] | 3.01 ± 1.14 |
| FeAu0.3 | 1.34 ± 0.91 |
| FeAu1.0 | 1.23 ± 0.82 |
| FeAu2.5 | 1.25 ± 0.74 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurgeleit, T.; Quandt, E.; Zamponi, C. Mechanical Properties and In Vitro Degradation of Sputtered Biodegradable Fe-Au Foils. Materials 2016, 9, 928. https://doi.org/10.3390/ma9110928
Jurgeleit T, Quandt E, Zamponi C. Mechanical Properties and In Vitro Degradation of Sputtered Biodegradable Fe-Au Foils. Materials. 2016; 9(11):928. https://doi.org/10.3390/ma9110928
Chicago/Turabian StyleJurgeleit, Till, Eckhard Quandt, and Christiane Zamponi. 2016. "Mechanical Properties and In Vitro Degradation of Sputtered Biodegradable Fe-Au Foils" Materials 9, no. 11: 928. https://doi.org/10.3390/ma9110928





