Origin of Activity and Stability Enhancement for Ag3PO4 Photocatalyst after Calcination
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis
2.2. Characterization
2.3. Photocatalytic Tests
3. Results and Discussion
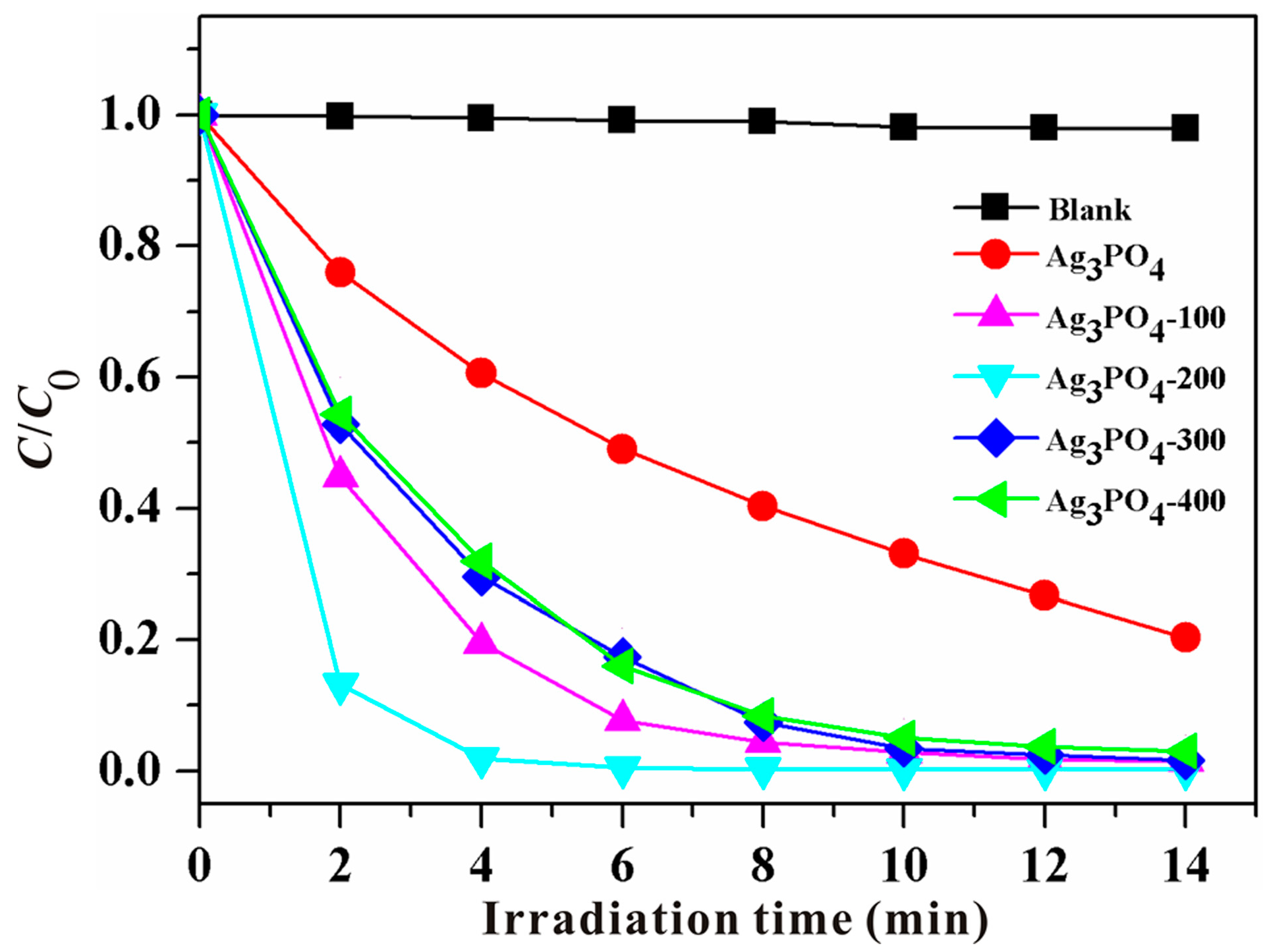
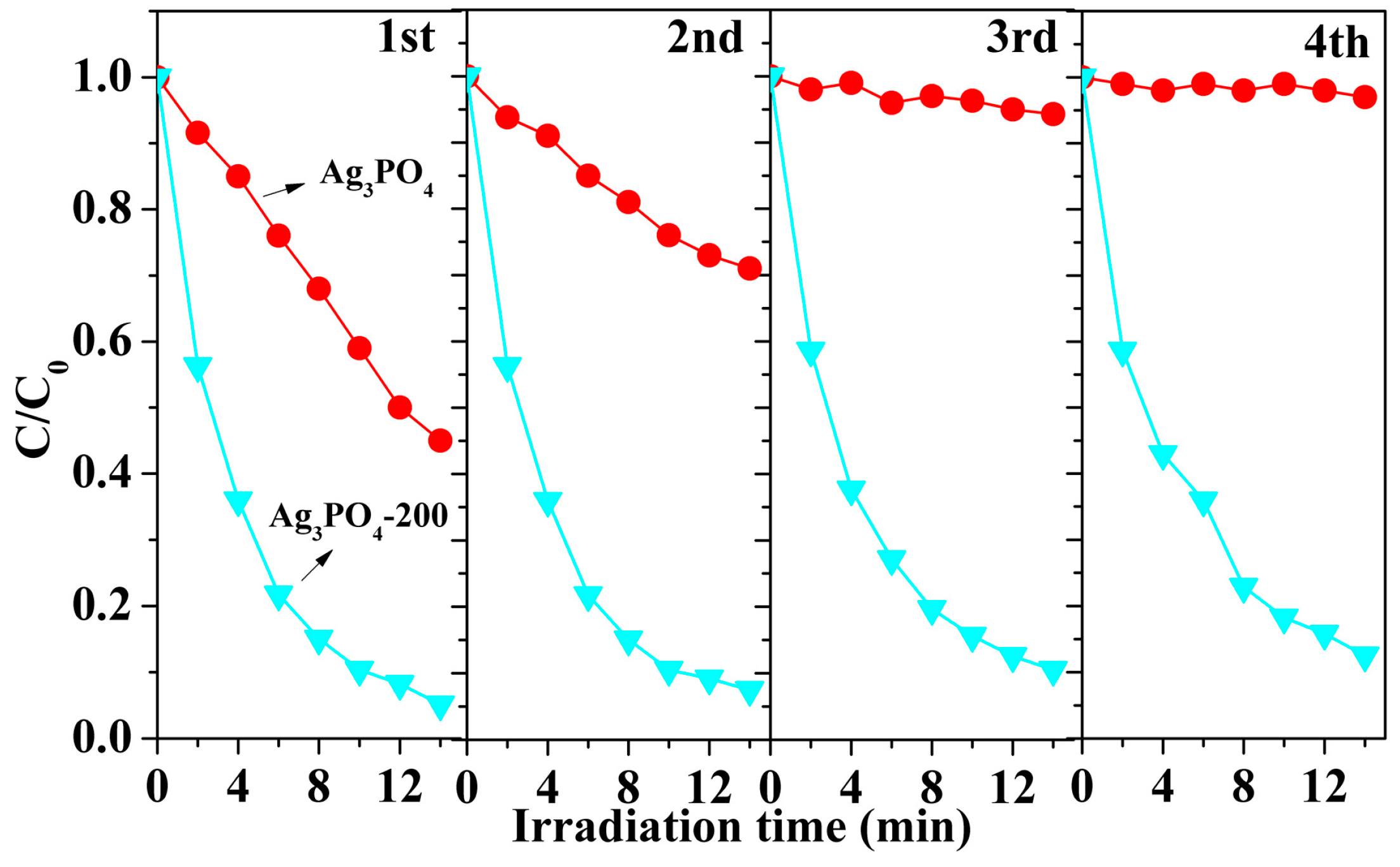
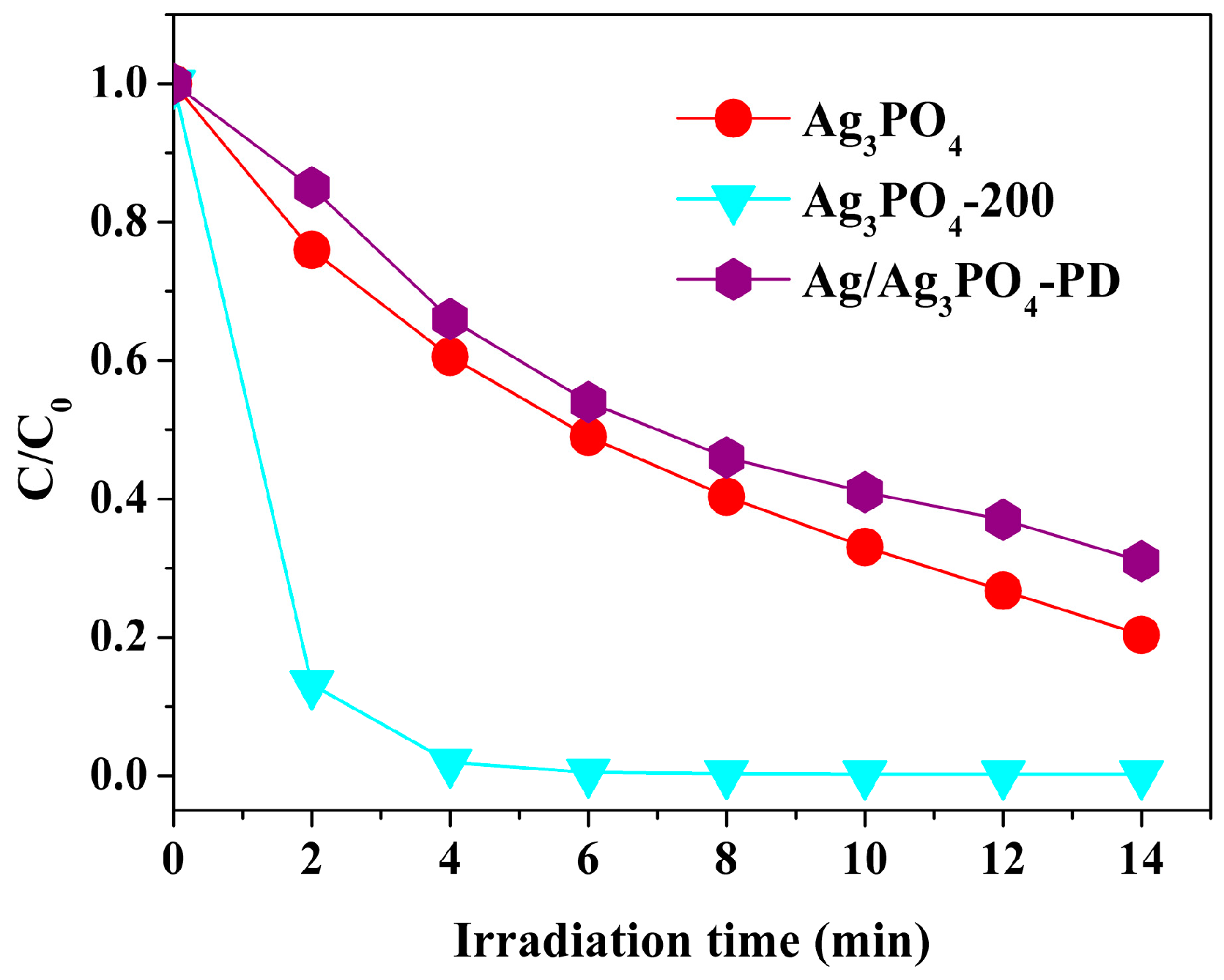
3.1. Phenomenon of the Enhanced Photocatalytic Activity and Stability
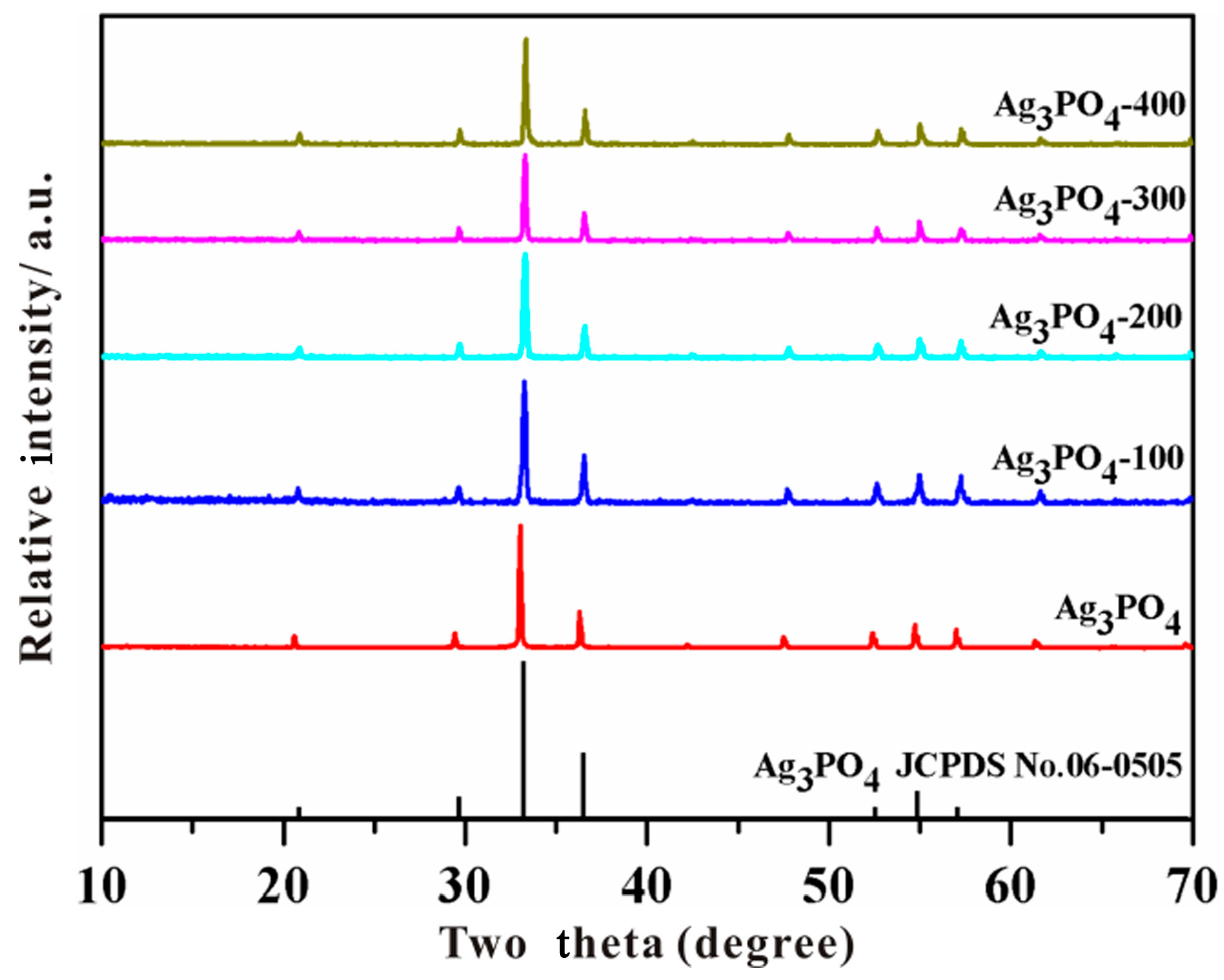
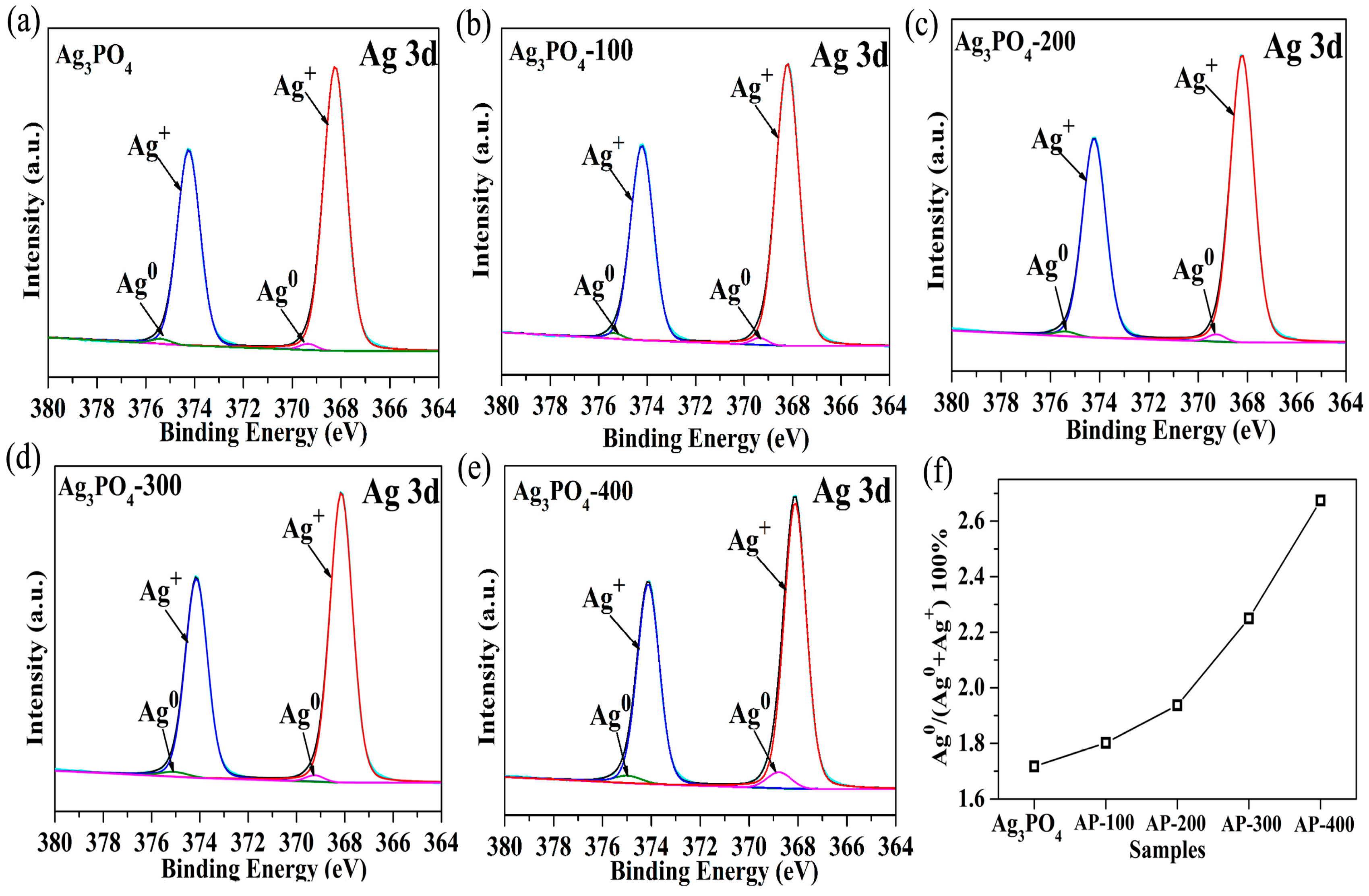
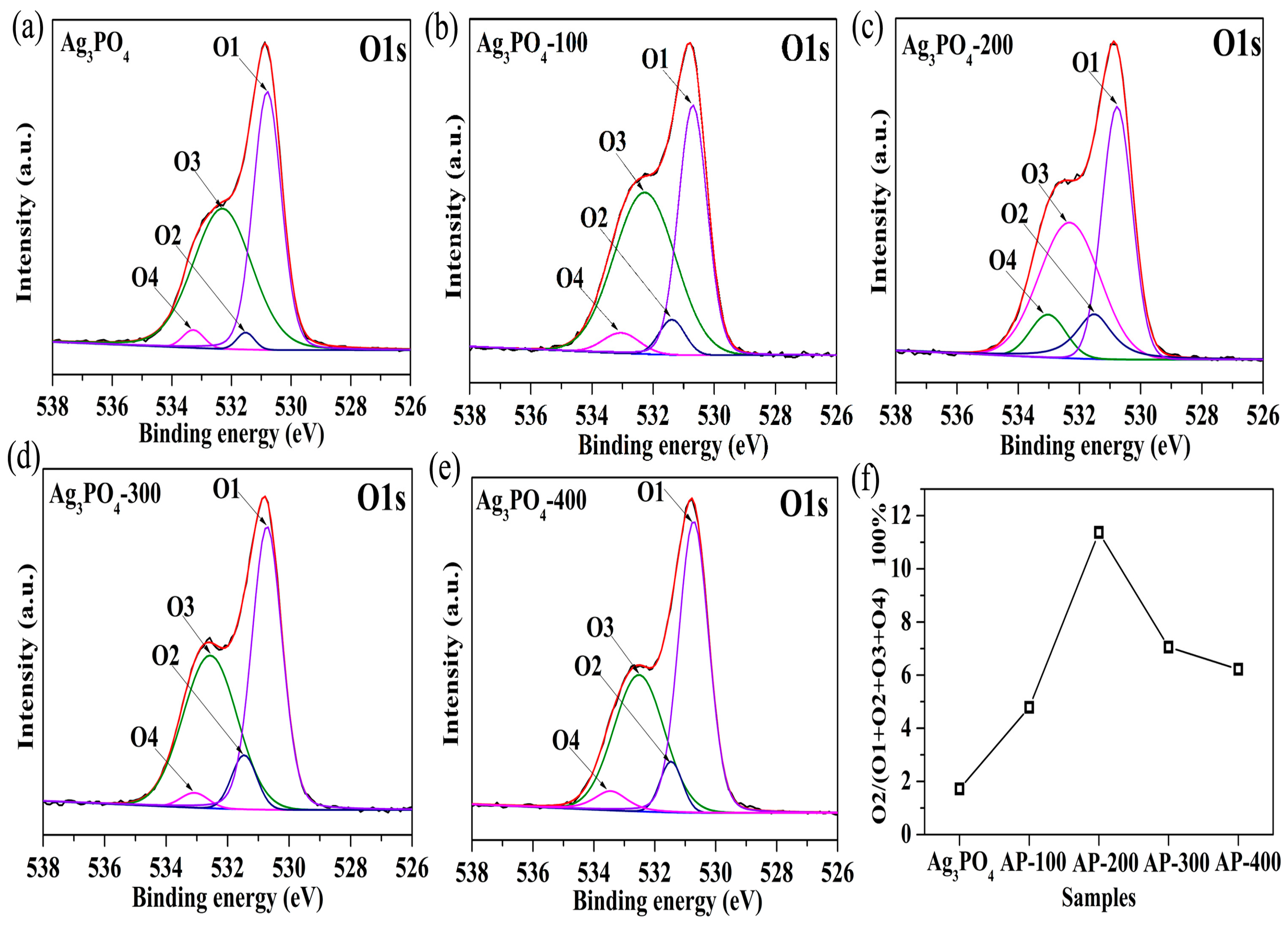
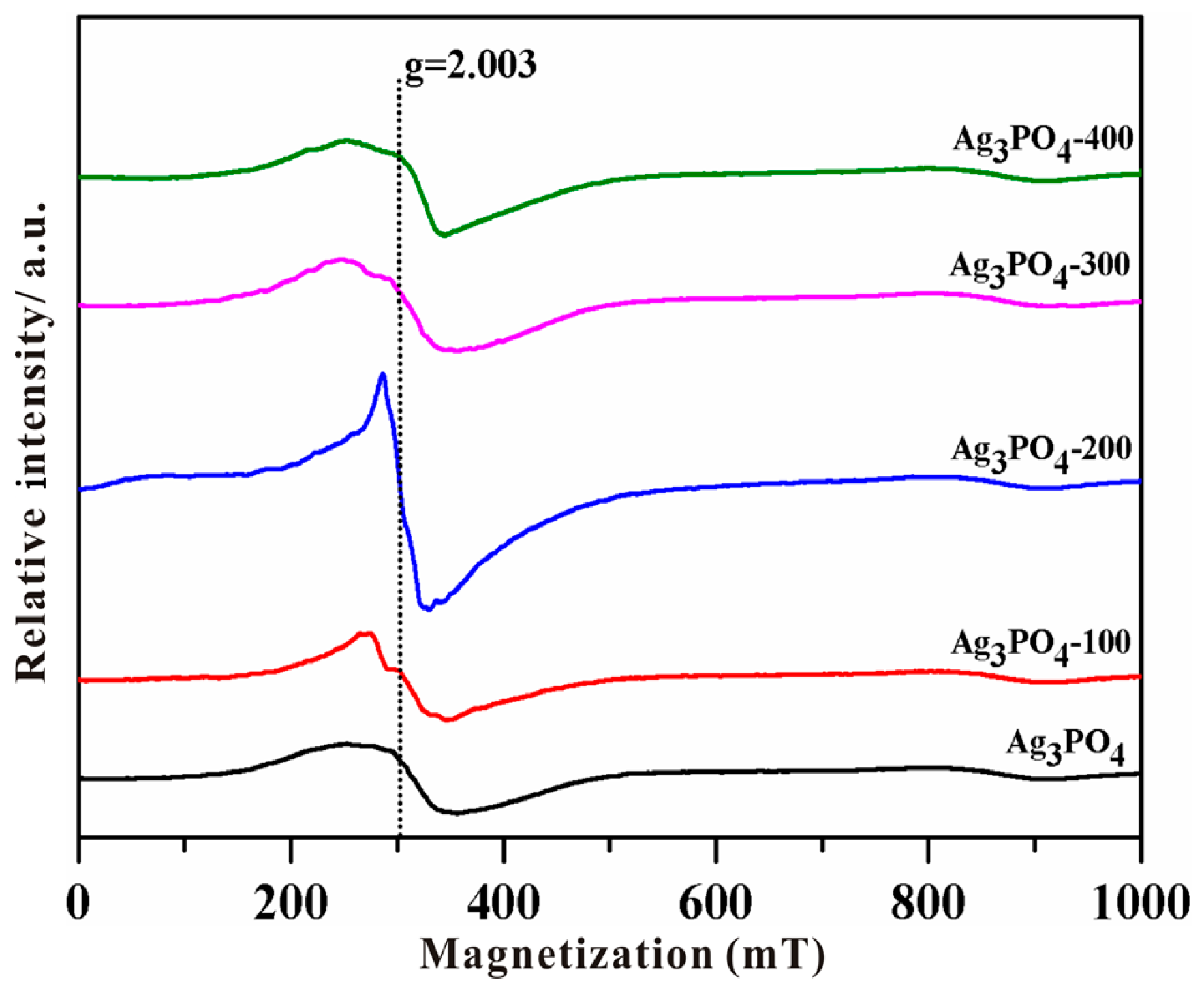
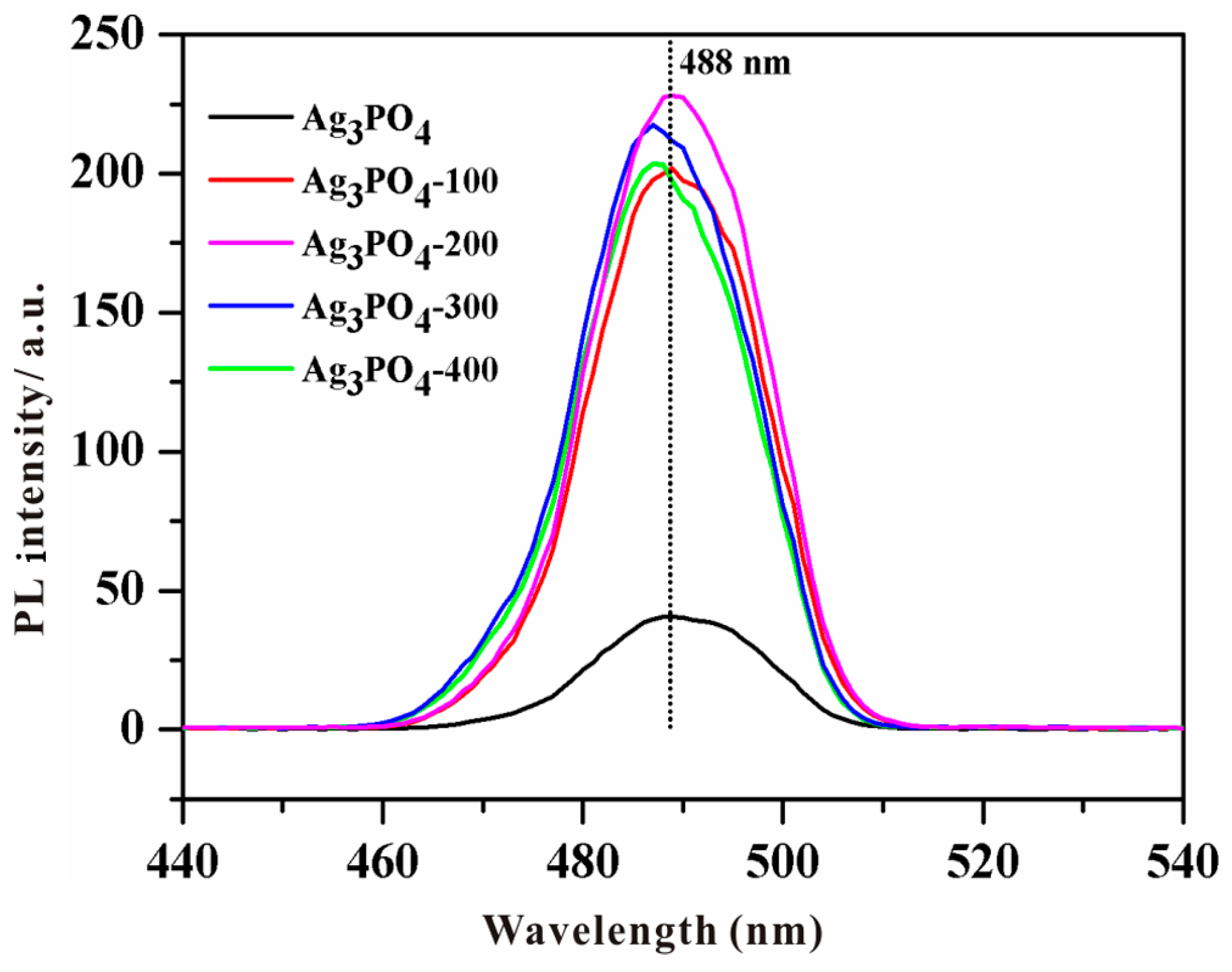
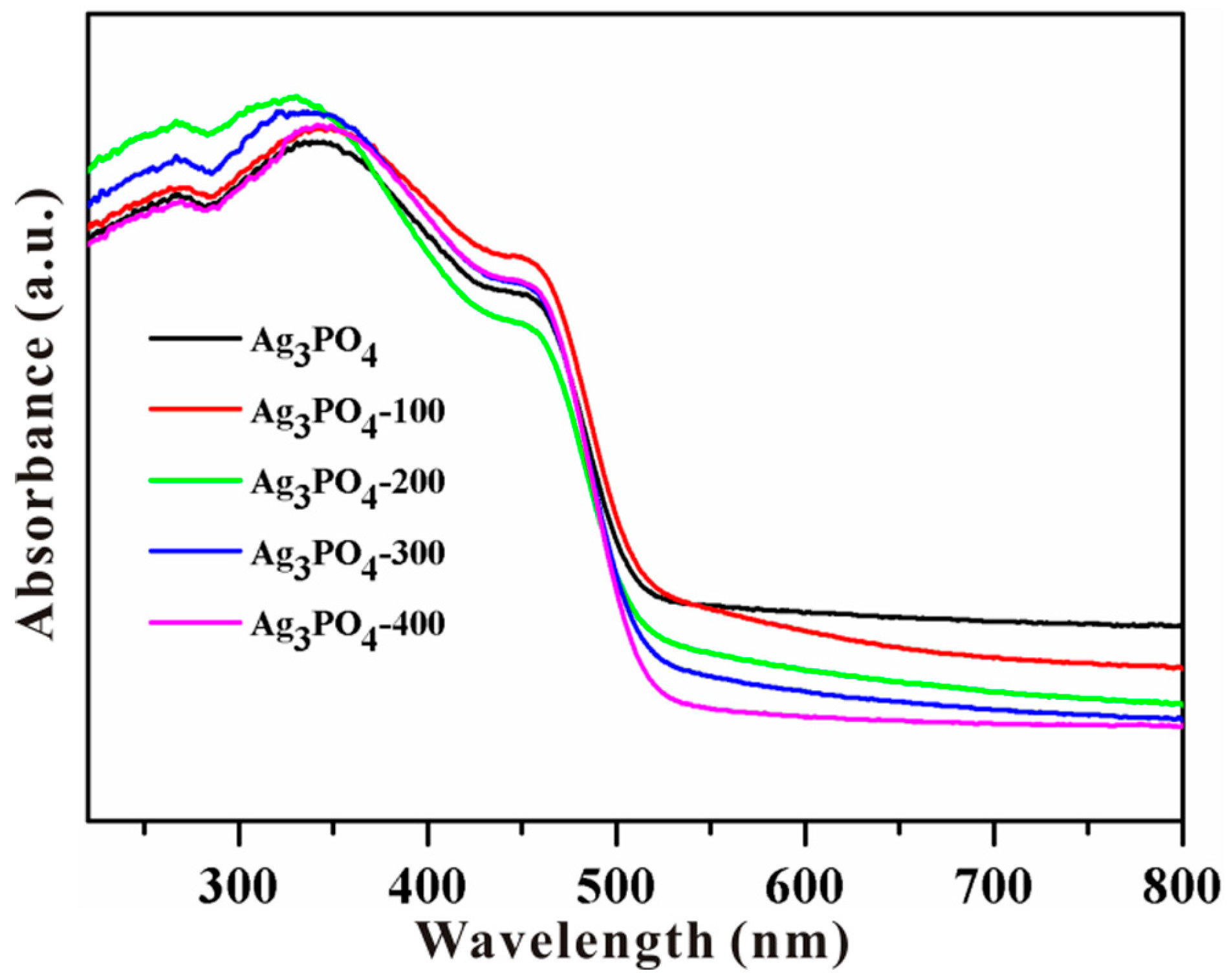
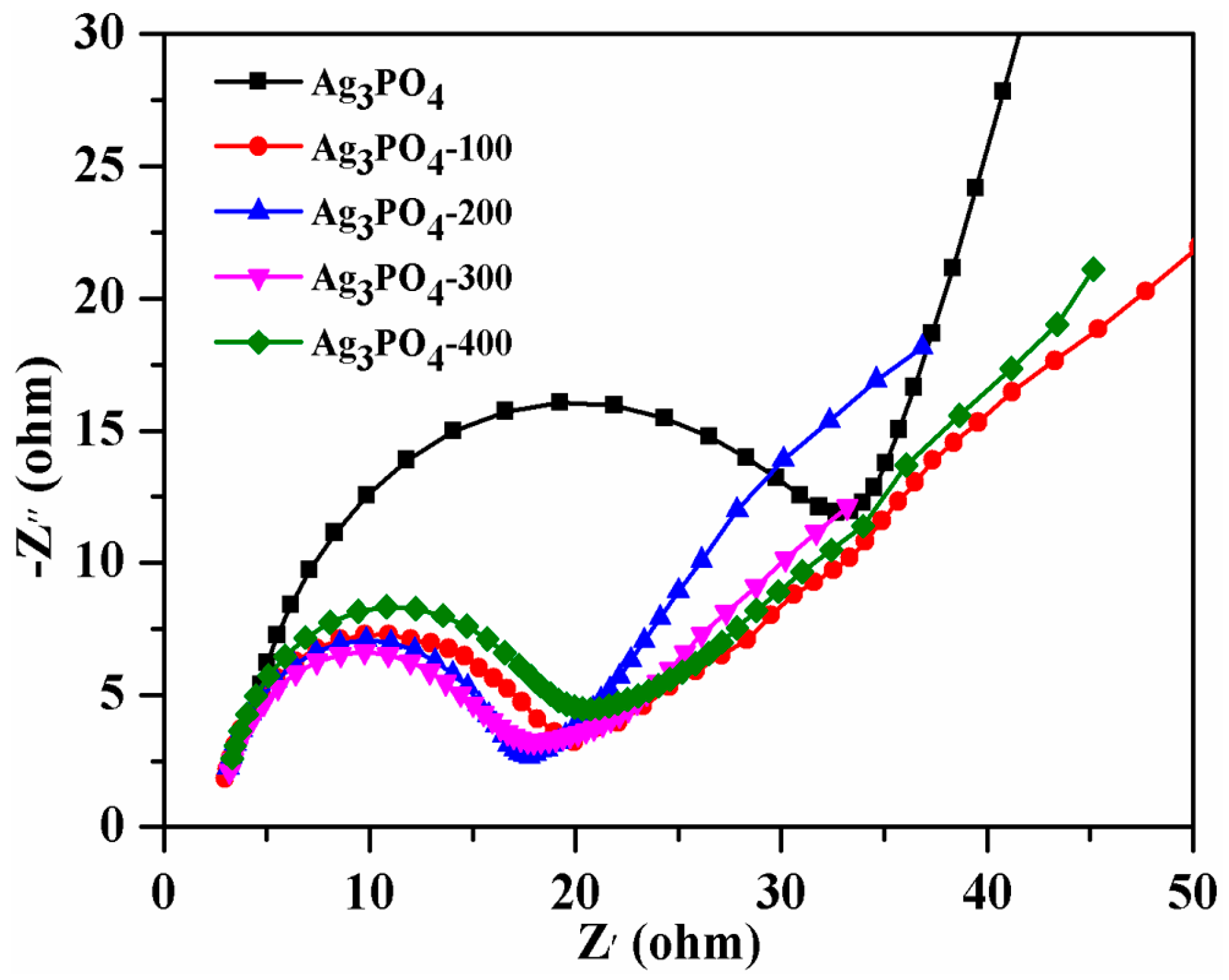
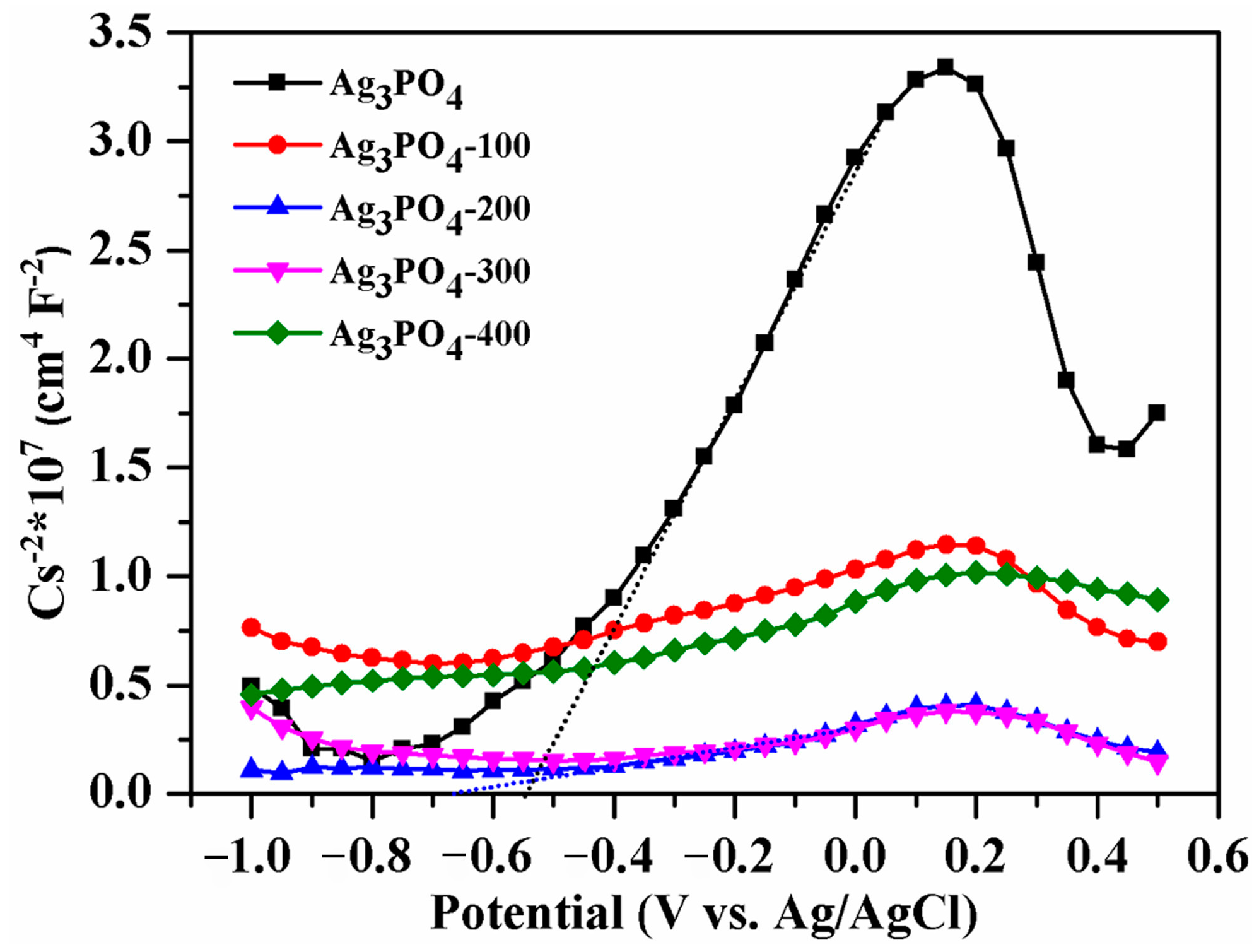
3.2. Investigation of Substantial Changes
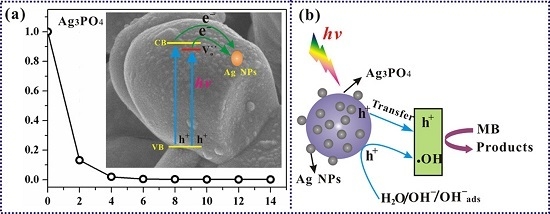
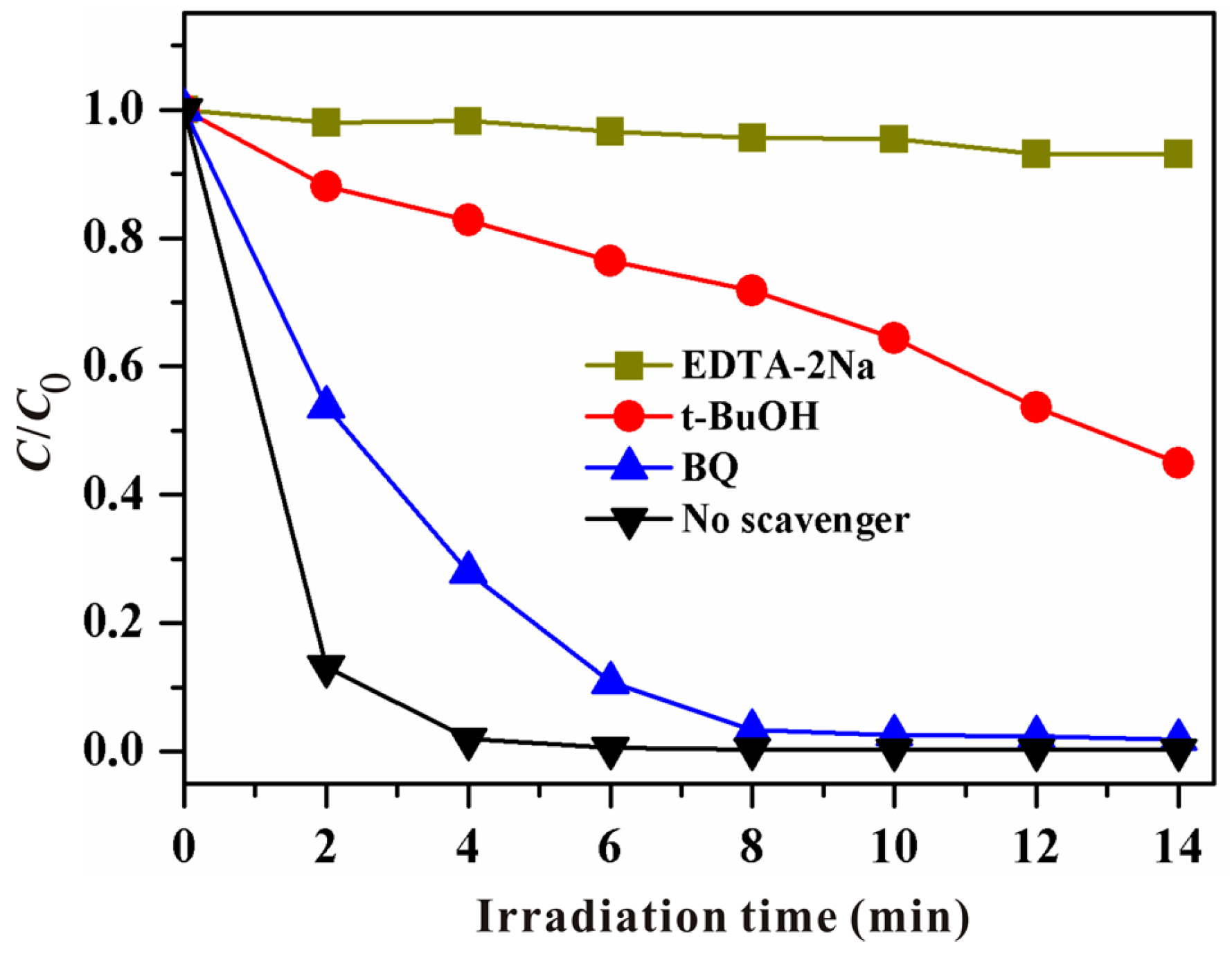
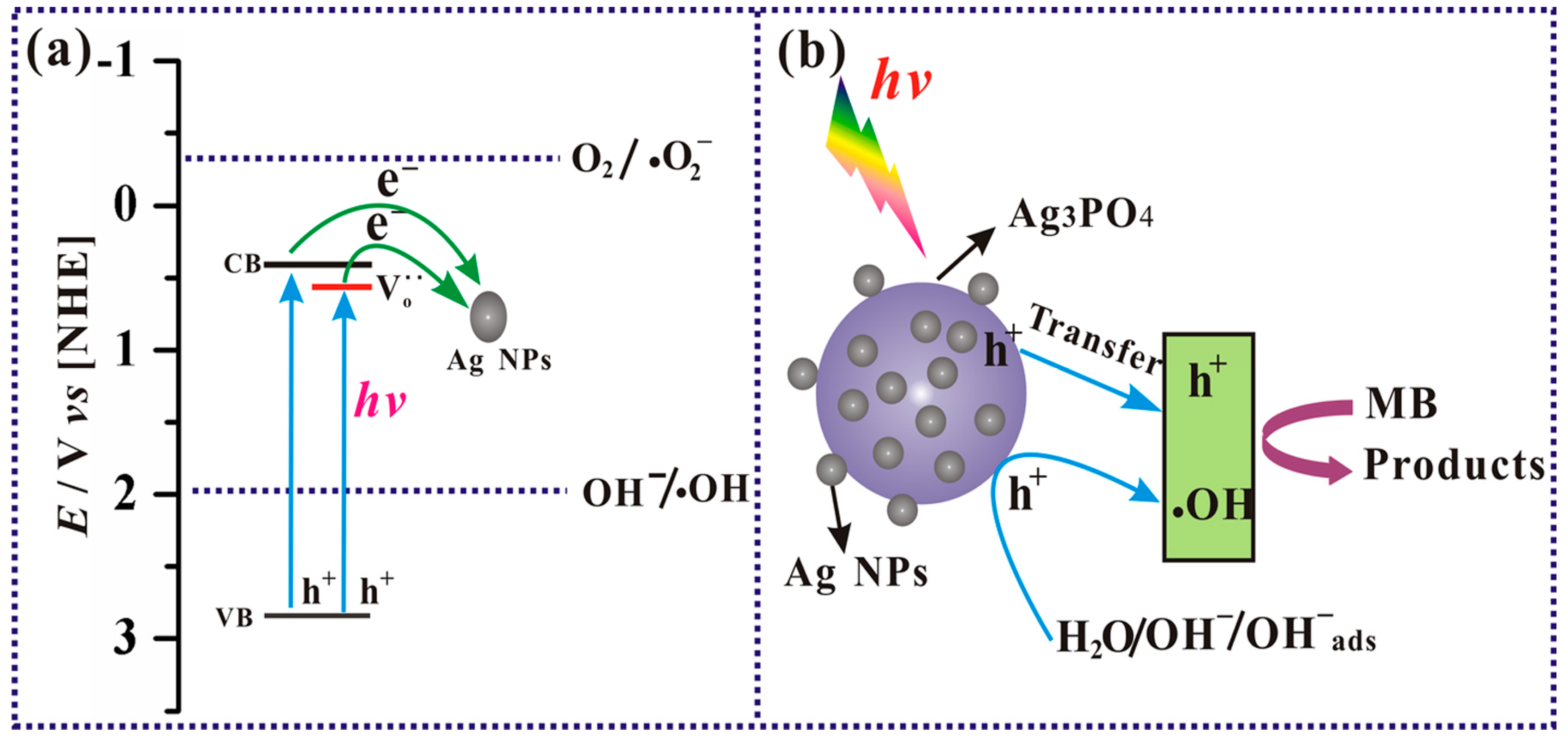
3.3. Mechanism of the Photocatalytic Reaction
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fujishima, A.; Zhang, X.; Tryk, D.A. Heterogeneous photocatalysis: From water photolysis to applications in environmental cleanup. Int. J. Hydrogen Energy 2007, 32, 2664–2672. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Joo, J.-B.; Lu, Z.; Dahl, M.; Oliveira, D.L.; Ye, M.; Yin, Y. Self-assembly and photocatalysis of mesoporous TiO2 nanocrystal clusters. Nano Res. 2011, 4, 103–114. [Google Scholar] [CrossRef]
- Yi, Z.; Ye, J.; Kikugawa, N.; Kako, T.; Ouyang, S.; Stuart-Williams, H.; Yang, H.; Cao, J.; Luo, W.; Li, Z. An orthophosphate semiconductor with photooxidation properties under visible-lightirradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Ouyang, S.; Umezawa, N.; Cao, J.; Ye, J. Facet Effect of Single-Crystalline Ag3PO4 Sub-microcrystals on Photocatalytic Properties. J. Am. Chem. Soc. 2011, 133, 6490–6492. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lu, B.; Li, D.; Shi, R.; Pan, C.; Zhu, Y. Origin of Photocatalytic Activation of Silver Orthophosphate from First-Principles. J. Phys. Chem. C 2011, 115, 4680–4687. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, B.; Huang, C.; Ma, C.; Song, X.; Xu, Q. Synthesis and characterization of high efficiency and stable Ag3PO4/TiO2 visible light photocatalyst for the degradation of methylene blue and rhodamine B solutions. J. Mater. Chem. 2012, 22, 4050–4055. [Google Scholar] [CrossRef]
- Teng, W.; Li, X.; Zhao, Q.; Chen, G. Fabrication of Ag/Ag3PO4/TiO2 heterostructure photoelectrodes for efficient decomposition of 2-chlorophenol under visible light irradiation. J. Mater. Chem. A 2013, 1, 9060–9068. [Google Scholar] [CrossRef]
- Rawal, S.B.; Sung, S.D.; Lee, W.I. Novel Ag3PO4/TiO2 composites for efficient decomposition of gaseous 2-propanol under visible-light irradiation. Catal. Commun. 2011, 17, 131–135. [Google Scholar] [CrossRef]
- Bi, Y.; Ouyang, S.; Cao, J.; Ye, J. Facile synthesis of rhombic dodecahedral AgX/Ag3PO4 (X = Cl, Br, I) heterocrystals with enhanced photocatalytic properties and stabilities. Phys. Chem. Chem. Phys. 2011, 13, 10071–10075. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, L.; Lu, H.; Liu, L.; Wang, H.; Hu, C. Highly efficient and stable Ag/Ag3PO4 plasmonic photocatalyst in visible light. Catal. Commun. 2011, 17, 200–204. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, L.; Lu, H.; Li, Y.; Hu, C.; Yu, H. One-pot pyridine-assisted synthesis of visible-light-driven photocatalyst Ag/Ag3PO4. Appl. Catal. B 2012, 115, 245–252. [Google Scholar] [CrossRef]
- Bi, Y.; Hu, H.; Ouyang, S.; Jiao, Z.; Lu, G.; Ye, J. Selective growth of Ag3PO4 submicro-cubes on Ag nanowires to fabricate necklace-like heterostructures for photocatalytic applications. J. Mater. Chem. 2012, 22, 14847–14850. [Google Scholar] [CrossRef]
- He, P.; Song, L.; Zhang, S.; Wu, X.; Wei, Q. Synthesis of g-C3N4/Ag3PO4 heterojunction with enhanced photocatalytic performance. Mater. Res. Bull. 2014, 51, 432–437. [Google Scholar]
- Katsumata, H.; Sakai, T.; Suzuki, T.; Kaneco, S. Highly Efficient Photocatalytic Activity of g-C3N4/Ag3PO4 Hybrid Photocatalysts through Z-Scheme Photocatalytic Mechanism under Visible Light. Ind. Eng. Chem. Res. 2014, 53, 8018–8025. [Google Scholar]
- Dong, P.; Wang, Y.; Cao, B.; Xin, S.; Guo, L.; Zhang, J.; Li, F. Ag3PO4/reduced graphite oxide sheets nanocomposites with highly enhanced visible light photocatalytic activity and stability. Appl. Catal. B 2013, 132, 45–53. [Google Scholar]
- Yang, X.; Cui, H.; Li, Y.; Qin, J.; Zhang, R.; Tang, H. Fabrication of Ag3PO4-Graphene Composites with Highly Efficient and Stable Visible Light Photocatalytic Performance. ACS Catal. 2013, 3, 363–369. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Sun, D. Graphene Oxide Enwrapped Ag3PO4 Composite: Towards a Highly Efficient and Stable Visible-Light-Induced Photocatalyst for Water Purification. Catal. Sci. Technol. 2012, 2, 2525–2532. [Google Scholar] [CrossRef]
- Chai, B.; Li, J.; Xu, Q. Reduced Graphene Oxide Grafted Ag3PO4 Composites with Efficient Photocatalytic Activity under Visible-Light Irradiation. Ind. Eng. Chem. Res. 2014, 53, 8744–8752. [Google Scholar] [CrossRef]
- Dong, P.; Wang, Y.; Li, H.; Li, H.; Ma, X.; Han, L. Shape-controllable synthesis and morphology-dependent photocatalytic properties of Ag3PO4 crystals. J. Mater. Chem. A 2013, 1, 4651–4656. [Google Scholar] [CrossRef]
- Dong, P.; Yin, Y.; Xu, N.; Guan, R.; Hou, G.; Wang, Y. Facile synthesis of tetrahedral Ag3PO4 mesocrystals and its enhanced photocatalytic activity. Mater. Res. Bull. 2014, 60, 682–689. [Google Scholar] [CrossRef]
- Bi, Y.; Hu, H.; Ouyang, S.; Lu, G.; Cao, J.; Ye, J. Photocatalytic and photoelectric properties of cubic Ag3PO4 sub-microcrystals with sharp corners and edges. Chem. Commun. 2012, 48, 3748–3750. [Google Scholar] [CrossRef] [PubMed]
- Dinh, C.T.; Nguyen, T.D.; Kleitz, F.; Do, T.O. Large-scale synthesis of uniform silver orthophosphate colloidal nanocrystals exhibiting high visible light photocatalytic activity. Chem. Commun. 2011, 47, 7797–7799. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Ma, W.; Shi, Y.; Li, Z.; Yang, X. Hierarchical Ag3PO4 porous microcubes with enhanced photocatalytic properties synthesized with the assistance of trisodium citrate. CrystEngComm 2012, 14, 2966–2973. [Google Scholar] [CrossRef]
- Wang, H.; He, L.; Wang, L.; Hu, P.; Guo, L.; Han, X.; Li, J. Facile Synthesis of Ag3PO4 Tetrapod Microcrystals with an Increased Percentage of Exposed {110} Facets and Highly Efficient Photocatalytic Properties. CrystEngComm 2012, 14, 8342–8344. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, X.; Liu, C.; Tan, K.; Xie, Z.; Zheng, L. High-efficiently visible light-responsive photocatalysts: Ag3PO4 tetrahedral microcrystals with exposed {111} facets of high surface energy. J. Mater. Chem. A 2013, 1, 12635–12640. [Google Scholar] [CrossRef]
- Hu, H.; Jiao, Z.; Yu, H.; Lu, G.; Ye, J.; Bi, Y. Facile synthesis of tetrahedral Ag3PO4 submicro-crystals with enhanced photocatalytic properties. J. Mater. Chem. A 2013, 1, 2387–2390. [Google Scholar] [CrossRef]
- Bi, Y.; Hu, H.; Jiao, Z.; Yu, H.; Lu, G.; Ye, J. Two-dimensional dendritic Ag3PO4 nanostructures and their photocatalytic properties. Phys. Chem. Chem. Phys. 2012, 14, 14486–14488. [Google Scholar] [CrossRef] [PubMed]
- Chong, R.; Cheng, X.; Wang, B.; Li, D.; Chang, Z.; Zhang, L. Enhanced photocatalytic activity of Ag3PO4 for oxygen evolution and Methylene blue degeneration: Effect of calcination temperature. Int. J. Hydrogen Energy 2016, 41, 2575–2582. [Google Scholar] [CrossRef]
- Yan, T.; Guan, W.; Tian, J.; Wang, P.; Li, W.; You, J.; Huang, B. Improving the photocatalytic performance of silver phosphate by thermal annealing: Influence of acetate species. J. Alloys Compd. 2016, 680, 436–445. [Google Scholar] [CrossRef]
- China Standards Publication. Test Method of Photocatalytic Materials for Purification of Water Solution; GB/T 23762-2009; China Standards Press: Beijing, China, 2009. (In Chinese) [Google Scholar]
- Yu, J.-G.; Yu, H.-G.; Cheng, B.; Zhao, X.-J.; Yu, J.C.; Ho, W.-K. The Effect of Calcination Temperature on the Surface Microstructure and Photocatalytic Activity of TiO2 Thin Films Prepared by Liquid Phase Deposition. J. Phys. Chem. B 2003, 107, 13871–13879. [Google Scholar] [CrossRef]
- Dhanabal, R.; Chithambararaj, A.; Velmathi, S.; Bose, A.C. Visible light driven degradation of methylene blue dye using Ag3PO4. J. Environ. Chem. Eng. 2015, 3, 1872–1881. [Google Scholar] [CrossRef]
- Štengl, V.; Bakardjieva, S.; Murafa, N.; Houšková, V.; Lang, K. Visible-light photocatalytic activity of TiO2/ZnS nanocomposites prepared by homogeneous hydrolysis. Microporous Mesoporous Mater. 2008, 110, 370–378. [Google Scholar] [CrossRef]
- Dong, R.; Tian, B.; Zeng, C.; Li, T.; Wang, T.; Zhang, J. Ecofriendly Synthesis and Photocatalytic Activity of Uniform Cubic Ag@AgCl Plasmonic Photocatalyst. J. Phys. Chem. C 2013, 117, 213–220. [Google Scholar] [CrossRef]
- Seo, D.; Park, J.C.; Song, H. Polyhedral Gold Nanocrystals with Oh Symmetry: From Octahedra to Cubes. J. Am. Chem. Soc. 2006, 128, 14863–14870. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Huang, B.; Zhang, Q.; Zhang, X.; Qin, X.; Dai, Y.; Zhan, J.; Yu, J.; Liu, H.; Lou, Z. Highly Efficient Visible Light Plasmonic Photocatalyst Ag@Ag(Br,I). Chem. Eur. J. 2010, 16, 10042–10047. [Google Scholar] [CrossRef] [PubMed]
- Kontoyannis, C.G.; Vagenas, N.V. Calcium carbonate phase analysis using XRD and FT-Raman spectroscopy. Analyst 2000, 125, 251–255. [Google Scholar] [CrossRef]
- Mani, P.D. Reactive Sputter Deposition of Lithium Phosphorus Oxynitride Thin Films, A Li Battery Solid State Electrolyte. Ph.D. Thesis, University of Central Florida, Orlando, FL, USA, 2015. [Google Scholar]
- Li, X.B.; Ma, S.Y.; Li, F.M.; Chen, Y.; Zhang, Q.Q.; Yang, X.H.; Wang, C.Y.; Zhu, J. Porous spheres-like ZnO nanostructure as sensitive gas sensors for acetone detection. Mater. Lett. 2013, 100, 119–123. [Google Scholar] [CrossRef]
- Kumar, V.; Swart, H.C.; Ntwaeaborwa, O.M.; Kroon, R.E.; Terblans, J.J.; Shaat, S.K.K.; Yousif, A.; Duvenhage, M.M. Origin of the red emission in zinc oxide nanophosphors. Mater. Lett. 2013, 101, 57–60. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, J.; Xue, Y.; Yu, P.; Zhang, B.; Wang, L.; Liu, R. Effect of aspect ratio and surface defects on the photocatalytic activity of ZnO nanorods. Sci. Rep. 2014, 4, 4596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, J.; Lu, H.; Gong, L.; Li, L.; Zheng, J.; Li, H.; Zhu, Z. High sensitive and selective formaldehyde sensors based on nanoparticle-assembled ZnO micro-octahedrons synthesized by homogeneous precipitation method. Sens. Actuator B Chem. 2011, 160, 364–370. [Google Scholar] [CrossRef]
- Perron, H.; Vandenborre, J.; Domain, C.; Drot, R.; Roques, J.; Simoni, E.; Ehrhardt, J.J.; Catalette, H. Combined investigation of water sorption on TiO2 rutile (1 1 0) single crystal face: XPS vs. periodic DFT. Surf. Sci. 2007, 601, 518–527. [Google Scholar] [CrossRef] [Green Version]
- Weaver, J.F.; Hoflund, G.B. Surface Characterization Study of the Thermal Decomposition of Ag2O. Chem. Mater. 1994, 6, 1693–1699. [Google Scholar] [CrossRef]
- Mani, P.D.; Saraf, S.; Singh, V.; Real-Robert, M.; Vijayakumar, A.; Duranceau, S.J.; Seal, S.; Coffey, K.R. Ionic conductivity of bias sputtered lithium phosphorus oxy-nitride thin films. Solid State Ion. 2016, 287, 48–59. [Google Scholar] [CrossRef]
- Amano, F.; Nakata, M. High-temperature calcination and hydrogen reduction of rutile TiO2: A method to improve the photocatalytic activity for water oxidation. Appl. Catal. B 2014, 158–159, 202–208. [Google Scholar] [CrossRef]
- Ma, X.; Li, H.; Wang, Y.; Li, H.; Liu, B.; Yin, S.; Sato, T. Substantial change in phenomenon of “self-corrosion” on Ag3PO4/TiO2 compound photocatalyst. Appl. Catal. B 2014, 158, 314–320. [Google Scholar] [CrossRef]
- Lv, Y.H.; Liu, Y.F.; Zhu, Y.Y.; Zhu, Y.F. Surface oxygen vacancy induced photocatalytic performance enhancement of a BiPO4 nanorod. J. Mater. Chem. A 2014, 2, 1174–1182. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef] [PubMed]
- Liqiang, J.; Yichun, Q.; Baiqi, W.; Shudan, L.; Baojiang, J.; Libin, Y.; Wei, F.; Honggang, F.; Jiazhong, S. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energy Mater. Sol. C 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Jing, L.; Yuan, F.; Hou, H.; Xin, B.; Cai, W.; Fu, H. Relationships of surface oxygen vacancies with photoluminescence and photocatalytic performance of ZnO nanoparticles. Sci. China Ser. B Chem. 2005, 48, 25–30. [Google Scholar] [CrossRef]
- Cui, E.; Lu, G. New evidence for the regulation of photogenerated electron transfer on surface potential energy controlled co-catalyst on TiO2—The investigation of hydrogen production over selectively exposed Au facet on Au/TiO2. Int. J. Hydrogen Energy 2014, 39, 7672–7685. [Google Scholar] [CrossRef]
- Huang, G.; Shi, R.; Zhu, Y. Photocatalytic activity and photoelectric performance enhancement for ZnWO4 by fluorine substitution. J. Mol. Catal. A Chem. 2011, 348, 100–105. [Google Scholar] [CrossRef]
- Bai, X.; Wang, L.; Wang, Y.; Yao, W.; Zhu, Y. Enhanced oxidation ability of g-C3N4 photocatalyst via C60 modification. Appl. Catal. B 2014, 152, 262–270. [Google Scholar] [CrossRef]
- Lv, Y.H.; Pan, C.S.; Ma, X.G.; Zong, R.L.; Bai, X.J.; Zhu, Y.F. Production of visible activity and UV performance enhancement of ZnO photocatalyst via vacuum deoxidation. Appl. Catal. B 2013, 138, 26–32. [Google Scholar] [CrossRef]
- Lee, H.; Choi, W. Photocatalytic Oxidation of Arsenite in TiO2 Suspension: Kinetics and Mechanisms. Environ. Sci. Technol. 2002, 36, 3872–3878. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, Q.; Lei, P.; Song, W.; Ma, W.; Zhao, J. Photodegradation of Dye Pollutants Catalyzed by Porous K3PW12O40 under Visible Irradiation. Environ. Sci. Technol. 2006, 40, 3965–3970. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Zhu, N.; Zhao, Y.; Li, J.; Liu, L. Sunlight-Assisted Degradation of Dye Pollutants in Ag3PO4 Suspension. Ind. Eng. Chem. Res. 2012, 51, 5167–5173. [Google Scholar] [CrossRef]
- Hou, Y.; Zuo, F.; Ma, Q.; Wang, C.; Bartels, L.; Feng, P. Ag3PO4 Oxygen Evolution Photocatalyst Employing Synergistic Action of Ag/AgBr Nanoparticles and Graphene Sheets. J. Phys. Chem. C 2012, 116, 20132–20139. [Google Scholar] [CrossRef]
- Bamwenda, G.R.; Arakawa, H. The visible light induced photocatalytic activity of tungsten trioxide powders. Appl. Catal. A 2001, 210, 181–191. [Google Scholar] [CrossRef]
- Lee, H.S.; Woo, C.S.; Youn, B.K.; Kim, S.Y.; Oh, S.T.; Sung, Y.E.; Lee, H.I. Bandgap Modulation of TiO2 and its Effect on the Activity in Photocatalytic Oxidation of 2-isopropyl-6-methyl-4-pyrimidinol. Top. Catal. 2005, 35, 255–260. [Google Scholar] [CrossRef]
- Abe, R.; Takami, H.; Murakami, N.; Ohtani, B. Pristine Simple Oxides as Visible Light Driven Photocatalysts: Highly Efficient Decomposition of Organic Compounds over Platinum-Loaded Tungsten Oxide. J. Am. Chem. Soc. 2008, 130, 7780–7781. [Google Scholar] [CrossRef] [PubMed]
- Liqiang, J.; Xiaojun, S.; Baifu, X.; Baiqi, W.; Weimin, C.; Honggang, F. The preparation and characterization of La doped TiO2 nanoparticles and their photocatalytic activity. J. Solid State Chem. 2004, 177, 3375–3382. [Google Scholar] [CrossRef]
- Arai, T.; Yanagida, M.; Konishi, Y.; Iwasaki, Y.; Sugihara, H.; Sayama, K. Efficient Complete Oxidation of Acetaldehyde into CO2 over CuBi2O4/WO3 Composite Photocatalyst under Visible and UV Light Irradiation. J. Phys. Chem. C 2007, 111, 7574–7577. [Google Scholar] [CrossRef]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, P.; Hou, G.; Liu, C.; Zhang, X.; Tian, H.; Xu, F.; Xi, X.; Shao, R. Origin of Activity and Stability Enhancement for Ag3PO4 Photocatalyst after Calcination. Materials 2016, 9, 968. https://doi.org/10.3390/ma9120968
Dong P, Hou G, Liu C, Zhang X, Tian H, Xu F, Xi X, Shao R. Origin of Activity and Stability Enhancement for Ag3PO4 Photocatalyst after Calcination. Materials. 2016; 9(12):968. https://doi.org/10.3390/ma9120968
Chicago/Turabian StyleDong, Pengyu, Guihua Hou, Chao Liu, Xinjiang Zhang, Hao Tian, Fenghua Xu, Xinguo Xi, and Rong Shao. 2016. "Origin of Activity and Stability Enhancement for Ag3PO4 Photocatalyst after Calcination" Materials 9, no. 12: 968. https://doi.org/10.3390/ma9120968