The Characterization of Fixation of Ba, Pb, and Cu in Alkali-Activated Fly Ash/Blast Furnace Slag Matrix
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Methods
3. Results and Discussion
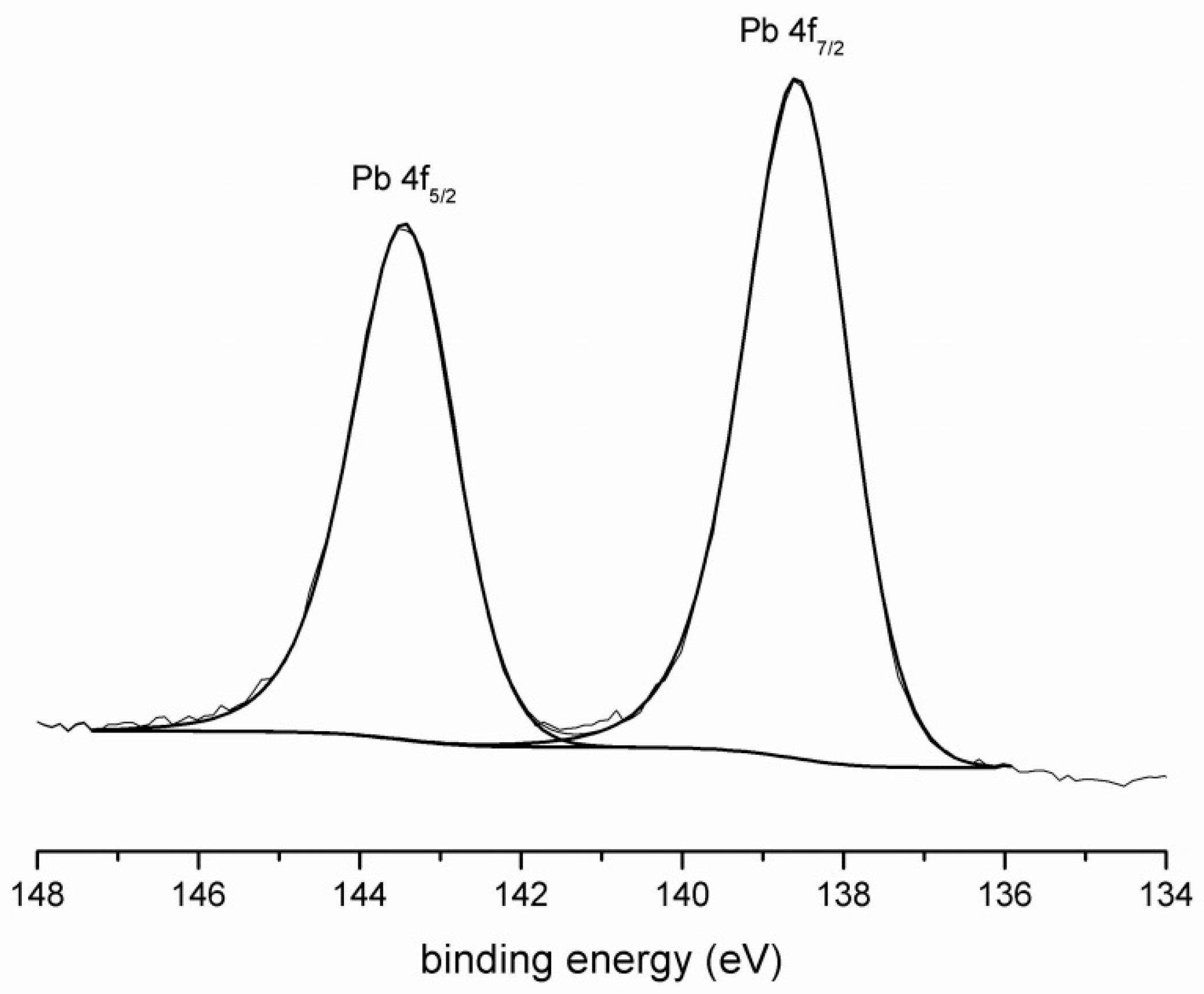
3.1. XPS
3.2. SEM
3.3. XRD
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BFS | blast furnace slag |
| XPS | X-ray photoelectron spectroscopy |
| SEM | scanning electron microscopy |
| EDS | energy dispersive X-ray spectroscopy |
| XRD | X-ray powder diffraction |
| AAM | alkali-activated material |
| HCAAMs | high-calcium alkali-activated materials |
| LCAAMs | low-calcium alkali-activated materials |
References
- Shi, C.; Fernandéz-Jimenéz, A. Stabilization/solidification of hazardous and radioactive wastes with alkali-activated cements. J. Hazard. Mater. 2006, B137, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.O. Hazardous and Radioactive Waste Treatment Technologies Handbook; CRC Press LLC: Boca Raton, FL, USA, 2001. [Google Scholar]
- Shi, C.; Krivenko, P.V.; Roy, D. Alkali-Activated Cements and Concretes; Taylor & Francis: New York, NY, USA, 2006. [Google Scholar]
- Purdon, A.O. The action of alkalis on blast-furnace slag. J. Soc. Chem. Ind. 1940, 59, 191–202. [Google Scholar]
- Provis, J.L.; van Deventer, J.S.J. Alkali Activated Materials: State-of-the Art-Report; Springer: Dorderecht, The Netherlands, 2014. [Google Scholar]
- Fernandéz-Jimenéz, A.; Palomo, A.; Revuelta, D. Akali activation of industrial by-products to develop new eath-friendly cements. In Proceeding of the 11th International Conference on Non-Conventional Materials and Technologies, Bath, UK, 6–9 September 2009; pp. 1–15.
- Myers, R.J.; Bernal, S.A.; San Nicolas, R.; Provis, J.L. Generalized structural description of calcium-sodium aluminosilicate hydrate gels: The cross linked substituted tobermorite model. Langmuir 2013, 29, 5294–5306. [Google Scholar] [CrossRef] [PubMed]
- Bernal, S.A.; Provis, J.L.; de Mejía Gutierez, R.; Rose, V. Evolution of binder structure in sodium silicate-activated slag-metakaolin blends. Cem. Concr. Compos. 2011, 33, 46–54. [Google Scholar] [CrossRef]
- Escalante-Garcia, J.; Fuentes, A.F.; Gorokhovsky, A.; Fraire-Luna, P.E.; Mendoza-Suarez, G. Hydration products and reactivity of blast-furnace slag activated by various alkalis. J. Am. Ceram. Soc. 2003, 86, 2148–2153. [Google Scholar] [CrossRef]
- Ben Haha, M.; Lothenbach, B.; Le Saout, G.; Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part I: Effect of MgO. Cem. Concr. Res. 2011, 41, 955–963. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Geopolymerisation: A review and prospects for the minerals industry. Min. Eng. 2007, 20, 1261–2277. [Google Scholar] [CrossRef]
- Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. Do geopolymers actually contain zeolites? A reexamination of existing results. Chem. Mater. 2005, 17, 3075–3085. [Google Scholar] [CrossRef]
- Puertas, F.; Martínez-ramírez, S.; Alonso, S.; Vázquez, T. Alkali-activated fly ash/slag cement. Strength behavior and hydration products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; van Deventer, J.S.J.; Lorenzen, L. Factor affecting the immobilization of metals in geopolymerized fly ash. Metall. Mater. Trans. 1998, 29B, 283–291. [Google Scholar] [CrossRef]
- Phair, J.W.; van Deventer, J.S.J.; Smith, J.D. Effect of Al source and alkali activation on Pb and Cu immobilization in fly-ash based “geopolymers”. App. Geochem. 2004, 19, 423–434. [Google Scholar] [CrossRef]
- Xu, J.Z.; Zhou, Y.L.; Chang, Q.; Qu, H.Q. Study on the factors affecting the immobilization of heavy metals in fly ash-based geopolymers. Mater. Lett. 2006, 60, 820–822. [Google Scholar] [CrossRef]
- Palomo, A.; de la Fuente, J.I.L. Alkali-activated cementious materials: Alternative matrices for the immobilization of hazardous wastes Part I. Stabilization of boron. Cem. Conc. Res. 2003, 33, 281–288. [Google Scholar] [CrossRef]
- Zhang, J.; Provis, J.L.; Feng, S.; van Deventer, J.S.J. Geopolymers for immobilization of Cr6+, Cd2+, and Pb2+. J. Hazard. Mater. 2008, 157, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Jiménez, A.; Palomo, A.; Macphee, D.E.; Lachowski, E.E. Fixing arsenic in alkali-activated cementious matrices. J. Am. Ceram. Soc. 2005, 88, 1122–1126. [Google Scholar] [CrossRef]
- Phair, J.W.; van Deventer, J.S.J. Effect of silicate activator pH on the leaching and material characteristic of waste-based inorganic polymers. Min. Eng. 2001, 14, 289–304. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, W.; Chen, Q.; Chen, L. Synthesis and heavy metal immobilization behaviors of slag based geopolymer. J. Hazard. Mater. 2007, 143, 206–213. [Google Scholar]
- Deja, J. Immobilization of Cr6+, Cd2+, Zn2+ and Pb2+ in alkali-activated slag binders. Cem. Concr. Res. 2002, 32, 1971–1979. [Google Scholar] [CrossRef]
- Škvára, F.; Kopecký, L.; Šmilauer, V.; Bittnar, Z. Material and structural characterization of alkali activated low-calcium brown coal fly ash. J. Hazard. Mater. 2009, 168, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Provis, J.L.; Feng, D.; van Deventer, J.S.J. The role of sulfide in the immobilization of Cr(VI) in fly ash geopolymers. Cem. Concr. Res. 2008, 38, 681–688. [Google Scholar] [CrossRef]
- Bankowski, P.; Zhou, L.; Hodges, R. Using inorganic polymer to reduce leach rates of metals from brown coal fly ash. Min. Eng. 2004, 17, 159–166. [Google Scholar] [CrossRef]
- Álvarez-Ayuso, E.; Querol, X.; Plana, F.; Alastuey, A.; Moreno, N.; Izquierdo, M.; Font, O.; Moreno, T.; Diez, S.; Vázquez, E.; et al. Environmental, physical and structural characterization of geopolymer matrixes synthesized from coal (co-)combustion fly ash. J. Hazard. Mater. 2008, 154, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Fernández Pereira, C.; Luna, Y.; Querol, X.; Antenucci, D.; Vale, J. Waste stabilization/solidification of an electric arc furnace dust using fly ash-based geopolymers. Fuel 2009, 88, 1185–1193. [Google Scholar] [CrossRef]
- Li, F.; Li, Q.; Zhai, J.; Sheng, G. Effect of zeolitization of CFBC fly ash on immobilization of Cu, Pb and Cr. Ind. Eng. Chem. Res. 2007, 46, 7087–7095. [Google Scholar] [CrossRef]
- Palomo, A.; Palacios, M. Alkali-activated matrices for the immobilization of hazardous waste Part II. Cem. Concr. Res. 2003, 33, 289–295. [Google Scholar] [CrossRef]
- Watts, J.F.; Wolstenholme, J. An Introduction to Surface Analysis by XPS and AES; John Wiley & Sons: Chichester, UK, 2005. [Google Scholar]







| Fly Ash | |||||||||
| SiO2 | Al2O3 | CaO | Na2O | K2O | MgO | SO3 | Fe2O3 | TiO2 | P2O5 |
| 50.30 | 27.70 | 3.84 | 0.76 | 2.67 | 1.15 | 0.87 | 10.40 | 1.45 | 0.28 |
| Blast Furnace Slag | |||||||||
| SiO2 | Al2O3 | CaO | Na2O | K2O | MgO | SO3 | Fe2O3 | TiO2 | MnO |
| 34.70 | 9.05 | 41.1 | 0.41 | 0.90 | 10.5 | 1.46 | 0.25 | 0.96 | 0.55 |
| Slag | Fly ash | NaOH | Na2O.SiO2 | Water | Ba, Pb, Cu |
|---|---|---|---|---|---|
| 15.6 | 57.4 | 6.1 | 6.2 | 12.2 | 2.5 |
| O | Na | Si | S | Cl | K | Ca | Fe | Ba |
|---|---|---|---|---|---|---|---|---|
| 62.86 | 0.59 | 1.96 | 14.44 | 1.76 | 1.12 | 0.32 | 0.59 | 16.36 |
| O | Al | Si | Cl | Ca | Fe | Cu | Pb |
|---|---|---|---|---|---|---|---|
| 55.8 | 0.87 | 6.91 | 1.31 | 0.34 | 0.28 | 33.79 | 0.38 |
| O | Mg | Al | Si | S | K | Ca | Ti | Fe | Cu | Pb |
|---|---|---|---|---|---|---|---|---|---|---|
| 58.36 | 5.48 | 5.64 | 20.08 | 0.58 | 3.29 | 4.25 | 0.28 | 0.57 | 0.42 | 1.04 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koplík, J.; Kalina, L.; Másilko, J.; Šoukal, F. The Characterization of Fixation of Ba, Pb, and Cu in Alkali-Activated Fly Ash/Blast Furnace Slag Matrix. Materials 2016, 9, 533. https://doi.org/10.3390/ma9070533
Koplík J, Kalina L, Másilko J, Šoukal F. The Characterization of Fixation of Ba, Pb, and Cu in Alkali-Activated Fly Ash/Blast Furnace Slag Matrix. Materials. 2016; 9(7):533. https://doi.org/10.3390/ma9070533
Chicago/Turabian StyleKoplík, Jan, Lukáš Kalina, Jiří Másilko, and František Šoukal. 2016. "The Characterization of Fixation of Ba, Pb, and Cu in Alkali-Activated Fly Ash/Blast Furnace Slag Matrix" Materials 9, no. 7: 533. https://doi.org/10.3390/ma9070533
APA StyleKoplík, J., Kalina, L., Másilko, J., & Šoukal, F. (2016). The Characterization of Fixation of Ba, Pb, and Cu in Alkali-Activated Fly Ash/Blast Furnace Slag Matrix. Materials, 9(7), 533. https://doi.org/10.3390/ma9070533






