Stereocomplexation in Copolymer Networks Incorporating Enantiomeric Glycerol-Based 3-Armed Lactide Oligomers and a 2-Armed ɛ-Caprolactone Oligomer
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Syntheses of H3LLAO and H3DLAO
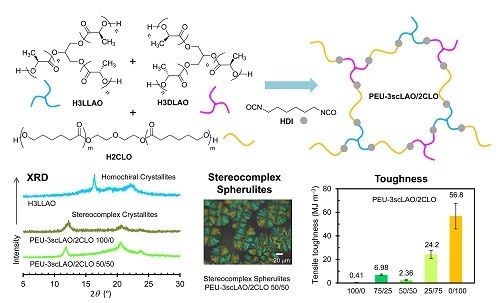
2.3. Synthesis of PEU-3scLAO/2CLO
2.4. Characterization and Measurements
3. Results and Discussion
3.1. Polymer Network Formation of PEU-3scLAO/2CLOs
3.2. Stereocomplex Formation in PEU-3scLAO/2CLOs
3.3. Thermal and Mechanical Properties of PEU-3scLAO/2CLOs
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Z.; Tan, B.H.; Lin, T.; He, C. Recent advances in stereocomplexation of enantiomeric PLA-based copolymers and applications. Prog. Polym. Sci. 2016. [Google Scholar] [CrossRef]
- Xie, L.; Xu, H.; Li, Z.M.; Hakkarainen, M. Structural hierarchy and polymorphic transformation in shear-induced shish-kebab of stereocomplex poly(lactic acid). Macromol. Rapid Commun. 2016, 37, 754–751. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.F.; Bao, R.Y.; Cao, Z.Q.; Yang, W.; Xie, B.H.; Yang, M.B. Stereocomplex crystallite network in asymmetric PLLA/PDLA blends: Formation, structure, and confining effect on the crystallization rate of homocrystallites. Macromolecules 2014, 47, 1439–1448. [Google Scholar] [CrossRef]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.H. Stereocomplex formation between enantiomeric poly(lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(lactide) stereocomplexes: Formation, structure, properties, degradation, and applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Kimura, Y. Stereocomplexed polylactides (Neo-PLA) as high-performance bio-based polymers: Their formation, properties, and application. Polym. Int. 2006, 55, 626–642. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. XI. Mechanical properties and morphology of solution-cast films. Polymer 1999, 40, 6699–6708. [Google Scholar] [CrossRef]
- Tsuji, H.; Fukui, I. Enhanced thermal stability of poly(lactide)s in the melt by enantiomeric polymer blending. Polymer 2003, 44, 2891–2896. [Google Scholar] [CrossRef]
- Tsuji, H. In vitro hydrolysis of blends from enantiomeric poly(lactide)s Part 1. Well-stereo-complexed blend and non-blended films. Polymer 2000, 41, 3621–3630. [Google Scholar] [CrossRef]
- Tsuji, H.; Yamamoto, S. Enhanced stereocomplex crystallization of biodegradable enantiomeric poly(lactic acid)s by repeated casting. Macromol. Mater. Eng. 2011, 296, 583–589. [Google Scholar] [CrossRef]
- Purnama, P.; Kim, S.H. Stereocomplex formation of high-molecular-weight polylactide using supercritical fluid. Macromolecules 2010, 43, 1137–1142. [Google Scholar] [CrossRef]
- Fujita, M.; Sawayanagi, T.; Abe, H.; Tanaka, T.; Iwata, T.; Ito, K.; Fujisawa, T.; Maeda, M. Stereocomplex formation through reorganization of poly(l-lactic acid) and poly(d-lactic acid) crystals. Macromolecules 2008, 41, 2852–2858. [Google Scholar] [CrossRef]
- Na, B.; Zhu, J.; Lv, R.; Ju, Y.; Tian, R.; Chen, B. Stereocomplex formation in enantiomeric polylactides by melting recrystallization of homocrystals: Crystallization kinetics and crystal morphology. Macromolecules 2014, 47, 347–352. [Google Scholar] [CrossRef]
- Bao, R.Y.; Yang, W.; Jiang, W.R.; Liu, Z.Y.; Xie, B.H.; Yang, M.B.; Fu, Q. Stereocomplex formation of high-molecular-weight polylactide: A low temperature approach. Polymer 2012, 53, 5449–5454. [Google Scholar] [CrossRef]
- Bao, R.Y.; Yang, W.; Jiang, W.R.; Liu, Z.Y.; Xie, B.H.; Yang, M.B. Polymorphism of racemic poly(l-lactide)/Poly(d-lactide) blend: Effect of melt and cold crystallization. J. Phys. Chem. B 2013, 117, 3667–3674. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acids). 9. Stereocomplexation from the melt. Macromolecules 1993, 26, 6918–6926. [Google Scholar] [CrossRef]
- Yui, N.; Dijkstra, P.J.; Feijen, J. Stereo block copolymers of L- and d-lactides. Makromol. Chem. 1990, 191, 481–488. [Google Scholar] [CrossRef]
- Li, L.; Zhong, Z.; Jeu, W.H.; Dijkstra, P.J.; Feijen, J. Crystal structure and morphology of poly(l-lactide-b-d-lactide) diblock copolymers. Macromolecules 2004, 37, 8641–8646. [Google Scholar] [CrossRef]
- Hirata, M.; Kobayashi, K.; Kimura, Y. Synthesis and properties of high-molecular-weight stereo di-block polylactides with nonequivalent d/l ratios. J. Polym. Sci. Part. A Polym. Chem. 2010, 48, 794–801. [Google Scholar] [CrossRef]
- Hirata, M.; Kobayashi, K.; Kimura, Y. Enhanced stereocomplexation by enantiomer adjustment for stereo diblock polylactides with non-equivalent d/l ratios. Macromol. Chem. Phys. 2010, 211, 1426–1432. [Google Scholar] [CrossRef]
- Sugai, N.; Yamamoto, T.; Tezuka, Y. Synthesis of orientationally isomeric cyclic stereoblock polylactides with head-to-head and head-to-tail linkages of the enantiomeric segments. ACS Macro Lett. 2012, 1, 902–906. [Google Scholar] [CrossRef]
- Isono, T.; Kondo, Y.; Ozawa, S.; Chen, Y.; Sakai, R.; Sato, S.; Tajima, K.; Kakuchi, T.; Satoh, T. Stereoblock-like brush copolymers consisting of poly(l-lactide) and poly(d‑lactide) side chains along poly(norbornene) backbone: Synthesis, stereocomplex formation, and structure−property relationship. Macromolecule 2014, 47, 7118–7128. [Google Scholar] [CrossRef]
- Isono, T.; Kondo, Y.; Otsuka, I.; Nishiyama, Y.; Borsali, R.; Kakuchi, T.; Satoh, T. Synthesis and stereocomplex formation of star-shaped stereoblock polylactides consisting of poly(l‑lactide) and poly(d‑lactide) arms. Macromolecules 2013, 46, 8509–8518. [Google Scholar] [CrossRef]
- Shao, J.; Tang, Z.; Sun, J.; Li, G.; Chen, X. Linear and four-armed poly(l-lactide)-block-poly(d-lactide) copolymers and their stereocomplexation with poly(lactide). J. Polym. Sci. Part B Polym. Phys. 2014, 52, 1560–1567. [Google Scholar] [CrossRef]
- Han, L.; Shan, G.; Bao, Y.; Pan, P. Exclusive stereocomplex crystallization of linear and multiarm star-shaped high-molecular-weight stereo diblock poly(lactic acid)s. J. Phys. Chem. B 2015, 119, 14270–14279. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Jones, A.E.; Waymouth, R.M. Stereocomplexation in cyclic and linear polylactide blends. Macromolecules 2012, 45, 595–598. [Google Scholar] [CrossRef]
- Fukushima, K.; Pratt, R.C.; Nederberg, F.; Tan, J.P.K.; Yang, Y.Y.; Waymouth, R.M.; Hedrick, J.L. Organocatalytic approach to amphiphilic comb-block copolymers capable of stereocomplexation and self-assembly. Biomacromolecules 2008, 9, 3051–3058. [Google Scholar] [CrossRef] [PubMed]
- Biela, T.; Duda, A.; Penczek, S. Enhanced melt stability of star-shaped stereocomplexes as compared with linear stereocomplexes. Macromolecules 2006, 39, 3710–3713. [Google Scholar] [CrossRef]
- Hiemstra, C.; Zhong, Z.; Li, L.; Dijkstra, P.J.; Feijen, J. In-situ formation of biodegradable hydrogels by stereocomplexation of PEG-(PLLA)8 and PEG-(PDLA)8 star block copolymers. Biomacromolecules 2006, 7, 2790–2795. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, K.; Fujiura, K.; Enami, S.; Ouchi, T.; Ohya, Y. Irreversible Temperature-responsive formation of high-strength hydrogel from an enantiomeric mixture of starburst triblock copolymers consisting of 8-arm PEG and PLLA or PDLA. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6317–6332. [Google Scholar] [CrossRef]
- Shaver, M.P.; Cameron, D.J.A. Tacticity control in the synthesis of poly(lactic acid) polymer stars with dipentaerythritol cores. Biomacromolecules 2010, 11, 3673–3679. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.H.; Hussain, H.; Lin, T.T.; Chua, Y.C.; Leong, Y.W.; Tjiu, W.W.; Wong, P.K.; He, C.B. Stable dispersions of hybrid nanoparticles induced by stereocomplexation between enantiomeric poly(lactide) star polymers. Langmuir 2011, 27, 10538–10547. [Google Scholar] [CrossRef] [PubMed]
- Calucci, L.; Forte, C.; Buwalda, S.J.; Dijkstra, P.J. Solid-state NMR study of stereocomplexes formed by enantiomeric star-shaped PEGPLA copolymers in water. Macromolecules 2011, 44, 7288–7295. [Google Scholar] [CrossRef]
- Shao, J.; Sun, J.; Bian, X.; Cui, Y.; Li, G.; Chen, X. Investigation of poly(lactide) stereocomplexes: 3-Armed poly(l-lactide) blended with linear and 3-armed enantiomers. J. Phys. Chem. B 2012, 116, 9983–9991. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.J.; Calucci, L.; Forte, C.; Dijkstra, P.J.; Feijen, J. Stereocomplexed 8-armed poly(ethylene glycol)−poly(lactide) star block copolymer hydrogels: Gelation mechanism, mechanical properties and degradation behavior. Polymer 2007, 53, 2809–2817. [Google Scholar] [CrossRef]
- Shao, J.; Sun, J.; Bian, X.; Cui, Y.; Zhou, Y.; Li, G.; Chen, X. Modified PLA homochiral crystallites facilitated by the confinement of PLA stereocomplexes. Macromolecules 2013, 46, 6963–6971. [Google Scholar] [CrossRef]
- Purnama, P.; Jung, Y.; Kim, S.H. Melt stability of 8-arms star-shaped stereocomplex polylactide with three-dimensional core structures. Polym. Degrad. Stab. 2013, 98, 1097–1101. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Tsuji, H. Stereocomplex crystallization behavior and physical properties of linear 1-arm, 2-arm, and branched 4-arm poly(l-lactide)/poly(d-lactide) blends: Effects of chain directional change and branching. Macromol. Chem. Phys. 2013, 214, 776–786. [Google Scholar] [CrossRef]
- Tsuji, H.; Suzuki, M. Hetero-stereocomplex crystallization between star-shaped 4-arm poly(l-2-hydroxybutanoic acid) and poly(d-lactic acid) from the melt. Macromol. Chem. Phys. 2014, 215, 1879–1888. [Google Scholar] [CrossRef]
- Tsuji, H.; Yamashita, Y. Highly accelerated stereocomplex crystallization by blending star-shaped 4-armed stereo diblock poly(lactide)s with poly(d-lactide) and poly(l-lactide) cores. Polymer 2014, 55, 6444–6450. [Google Scholar] [CrossRef]
- Tsuji, H.; Tamai, K.; Kimura, T.; Kubota, A.; Tahahashi, A.; Kuzuya, A.; Ohya, Y. Stereocomplex- and homo-crystallization of blends from 2-armed poly(l-lactide) and poly(d-lactide) with identical and opposite chain directional architectures and of 2-armed stereo diblock poly(lactide). Polymer 2016, 96, 167–181. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, D.; Bai, G.; Guo, Y.; Hu, Z. Stable stereocomplex micelles from Y-shaped amphiphilic copolymers MPEG–(scPLA)2: Preparation and characteristics. RSC Adv. 2016, 6, 20761–20771. [Google Scholar] [CrossRef]
- Fan, X.; Wang, Z.; Yuan, D.; Sun, Y.; Li, Z.; He, C. Novel linear-dendritic-like amphiphilic copolymers: Synthesis and self-assembly characteristics. Polym. Chem. 2014, 5, 4069–4075. [Google Scholar] [CrossRef]
- Shibata, M.; Katoh, M.; Takase, H.; Shibita, A. Stereocomplex formation in stereoblock copolymer networks composed of 4-armed star-shaped lacide oligomers and a 2-armed ɛ-caprolactone oligomer. Polym. Chem. 2015, 6, 4123–4132. [Google Scholar] [CrossRef]
- Crescenzi, V.; Manzini, G.; Calzolari, G.; Borri, C. Thermodynamics of fusion of poly-β-propiolactone and poly-ɛ-caprolactone. Comparative analysis of the melting of aliphatic polylactone and polyester chains. Eur. Polym. J. 1972, 8, 449–463. [Google Scholar] [CrossRef]
- Kucharczyk, P.; Pavelková, A.; Stloukal, P.; Sedlarík, V. Degradation behaviour of PLA-based polyesterurethanes under abiotic and biotic environments. Polym. Degrad. Stab. 2016, 129, 222–230. [Google Scholar] [CrossRef]
- Liu, Q.; Cheng, S.; Li, Z.; Xu, K.; Chen, G.Q. Characterization, biodegradability and blood compatibility of poly[(R)-3-hydroxybutyrate] based poly(ester-urethane)s. J. Biomed. Mater. Res. 2009, 90, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Hoogsteen, W.; Postema, A.R.; Pennings, A.J.; Brinke, G.T.; Zugenmaier, P. Crystal structure, conformation and morphology of solution-spun poly(l-lactide) fibers. Macromolecules 1990, 23, 634–642. [Google Scholar] [CrossRef]
- Cartier, L.; Okihara, T.; Lotz, B. Triangular polymer single crystals: Stereocomplexes, twins, and frustrated structures. Macromolecules 1997, 30, 6313–6322. [Google Scholar] [CrossRef]
- Pant, H.R.; Neupane, M.P.; Pant, B.; Panthia, G.; Oh, H.J.; Lee, M.H.; Kim, H.Y. Fabrication of highly porous poly (ɛ-caprolactone) fibers for novel tissue scaffold via water-bath electrospinning. Colloids Surf. B 2011, 88, 587–592. [Google Scholar] [CrossRef] [PubMed]










| Sample | Tg,LAO (°C) | Tm,CLO (°C) | ∆Hm,CLO (J·g−1) | χc,C/LO (%) | Tc,LAO (°C) | ∆Hc,LAO (J·g−1) | Tm,LAO (°C) | ∆Hm,LAO (J·g−1) | χc,LAO (%) |
|---|---|---|---|---|---|---|---|---|---|
| H3DLAO | 59.9 | - | - | - | - | 0 | 118.0 | 22.2 | 23.9 |
| PEU-3DLAO | 64.7 | - | - | - | - | 0 | - | 0 | 0 |
| H3scLAO | 45.3 | - | - | - | 88.9 | –31.2 | 186.5 | 40.1 | 28.2 |
| PEU-3scLAO/2CLO | |||||||||
| 100/0 | 65.9 | - | - | - | - | 0 | 178.1 | 31.5 | 23.8 |
| 75/25 | - | - | 0 | 0 | - | 0 | 173.1 | 27.4 | 27.7 |
| 50/50 | (–45.2) 1 | −31.7 | 1.67 | 2.6 | - | 0 | 176.5 | 19.4 | 29.6 |
| 25/75 | (–45.5) 1 | 36.0 | 7.72 | 8.0 | - | 0 | 178.5 | 8.46 | 25.6 |
| 0/100 | - | 53.1 | 30.5 | 23.8 | - | - | - | - | - |
| Sample | Tg,LAO (°C) | Tm,CLO (°C) | ∆Hm,CLO (J·g−1) | χc,CLO (%) | Tc,LAO (°C) | ∆Hc,LAO (J·g−1) | Tm,LAO (°C) | ∆Hm,LAO (J·g−1) | χc,LAO (%) |
|---|---|---|---|---|---|---|---|---|---|
| H3DLAO | 35.0 | - | - | - | - | 0 | - | 0 | 0 |
| PEU-3DLAO | 58.9 | - | - | - | - | 0 | - | 0 | 0 |
| H3scLAO | 47.8 | - | - | - | 88.9 | −31.6 | 186.5 | 37.8 | 26.6 |
| PEU-3scLAO/2CLO | |||||||||
| 100/0 | 42.3 | - | - | - | 90.4 | −25.9 | 176.6 | 21.7 | 16.4 |
| 75/25 | - | - | 0 | 0 | 68.5 | −21.0 | 176.9 | 21.1 | 21.3 |
| 50/50 | 56.9 | - | 0 | 0 | 91.8 | −12.9 | 174.6 | 12.9 | 19.7 |
| 25/75 | (−32.7) 1 | 36.6 | 18.6 | 19.4 | 81.3 | −3.85 | 183.3 | 2.10 | 6.3 |
| 0/100 | (−42.8) 1 | 35.7 | 22.2 | 17.3 | - | - | - | - | - |
| Sample | Tensile Strength (MPa) | Tensile Modulus (MPa) | Elongation at Break (%) | Tensile Toughness (MJ·m−3) |
|---|---|---|---|---|
| PEU-3DLAO | 42.9 ± 3.0 | 2214 ± 187 | 2.26 ± 0.32 | 0.62 ± 0.25 |
| PEU-3scLAO/2CLO | ||||
| 100/0 | 54.7 ± 3.6 | 2374 ± 262 | 2.98 ± 0.79 | 0.41 ± 0.24 |
| 75/25 | 13.3 ± 0.5 | 163 ± 16 | 66.8 ± 4.7 | 6.98 ± 0.69 |
| 50/50 | 3.37 ± 0.42 | 26.3 ± 2.0 | 92.3 ± 8.4 | 2.36 ± 0.46 |
| 25/75 | 11.3 ± 0.9 | 17.3 ± 2.5 | 378 ± 27 | 24.2 ± 3.3 |
| 0/100 | 10.4 ± 0.9 | 136 ± 13 | 726 ± 113 | 56.8 ± 10.9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibita, A.; Kawasaki, S.; Shimasaki, T.; Teramoto, N.; Shibata, M. Stereocomplexation in Copolymer Networks Incorporating Enantiomeric Glycerol-Based 3-Armed Lactide Oligomers and a 2-Armed ɛ-Caprolactone Oligomer. Materials 2016, 9, 591. https://doi.org/10.3390/ma9070591
Shibita A, Kawasaki S, Shimasaki T, Teramoto N, Shibata M. Stereocomplexation in Copolymer Networks Incorporating Enantiomeric Glycerol-Based 3-Armed Lactide Oligomers and a 2-Armed ɛ-Caprolactone Oligomer. Materials. 2016; 9(7):591. https://doi.org/10.3390/ma9070591
Chicago/Turabian StyleShibita, Ayaka, Seina Kawasaki, Toshiaki Shimasaki, Naozumi Teramoto, and Mitsuhiro Shibata. 2016. "Stereocomplexation in Copolymer Networks Incorporating Enantiomeric Glycerol-Based 3-Armed Lactide Oligomers and a 2-Armed ɛ-Caprolactone Oligomer" Materials 9, no. 7: 591. https://doi.org/10.3390/ma9070591