Structure and Transport Properties of Dense Polycrystalline Clathrate-II (K,Ba)16(Ga,Sn)136 Synthesized by a New Approach Employing SPS
Abstract
:1. Introduction
2. Sample Preparation
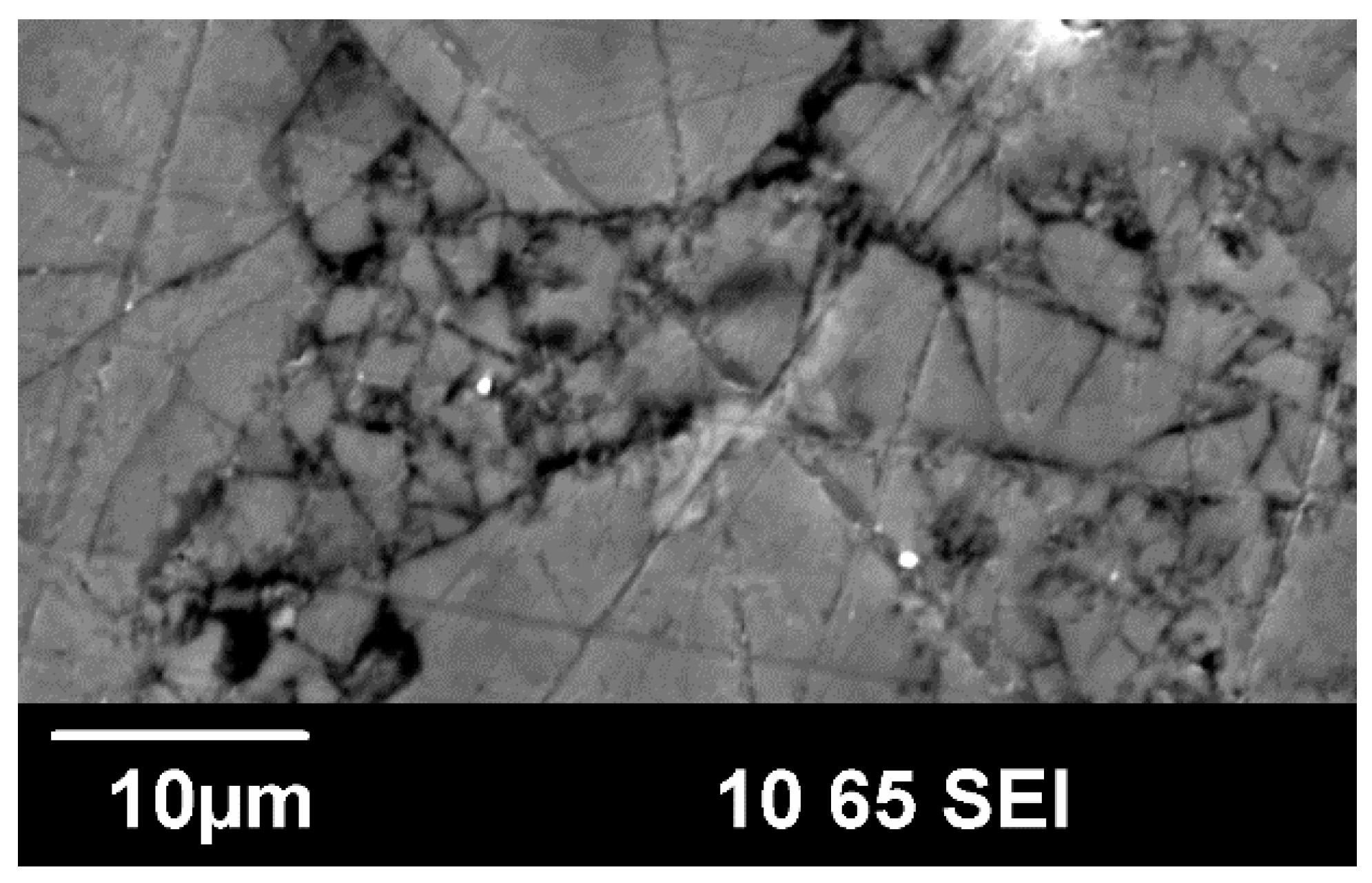
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cros, C.; Pouchard, M.; Hagenmuller, P. Sur deux nouvelles phases du système silicium-sodium. C. R. Acad. Sci. Paris 1965, 260, 4764–4767. [Google Scholar]
- Kasper, J.S.; Hagenmuller, P.; Pouchard, M.; Cros, C. Clathrate structure of silicon Na8Si46 and NaxSi136 (x < 11). Science 1965, 150, 1713–1714. [Google Scholar] [PubMed]
- Nolas, G.S. The Physics and Chemistry of Inorganic Clathrates; Springer: Berlin, Germany, 2014. [Google Scholar]
- Stefanoski, S.; Beekman, M.; Wong-Ng, W.; Zavalij, P.; Nolas, G.S. Simple approach for selective crystal growth of intermetallic clathrates. Chem. Mater. 2011, 23, 1491–1495. [Google Scholar] [CrossRef]
- Stefanoski, S.; Nolas, G.S. Synthesis and structural characterization of single-crystal K7.5Si46 and K17.8Si136 clathrates. Cryst. Growth Des. 2011, 11, 4533–4537. [Google Scholar] [CrossRef]
- Beekman, M.; Baitinger, M.; Borrmann, H.; Schnelle, W.; Meier, K.; Nolas, G.S.; Grin, Y. Preparation and crystal growth of Na24Si136. J. Am. Chem. Soc. 2009, 131, 9642–9643. [Google Scholar] [CrossRef] [PubMed]
- Stefanoski, S.; Blosser, M.C.; Nolas, G.S. Pressure effects on the size of type-I and type-II Si-clathrates synthesized by spark plasma sintering. Cryst. Growth Des. 2013, 13, 195–197. [Google Scholar] [CrossRef]
- Krishna, L.; Baranowski, L.L.; Martinez, A.D.; Koh, C.A.; Taylor, P.C.; Tamboli, A.C.; Toberer, E.S. Efficient route to phase selective synthesis of type II silicon clathrates with low sodium occupancy. CrystEngComm 2014, 16, 3940–3949. [Google Scholar] [CrossRef]
- Dong, Y.; Nolas, G.S. Rapid crystal growth of type-II clathrates A8Na16Si136 (A = K, Rb, Cs) by spark plasma sintering. CrystEngComm 2015, 17, 2242–2244. [Google Scholar] [CrossRef]
- Wei, K.; Dong, Y.; Nolas, G.S. Precursor routes to quaternary intermetallics: Synthesis, crystal structure, and physical properties of clathrate-II Cs8Na16Al24Si112. J. Solid State Chem. 2016, 237, 81–85. [Google Scholar] [CrossRef]
- Nolas, G.S.; Slack, G.A. Thermoelectric clathrates. Am. Sci. 2001, 89, 136–141. [Google Scholar] [CrossRef]
- Nolas, G.S.; Slack, G.A.; Schujman, S.B. Semiconductor clathrates: A phonon-glass electron-crystal material with potential for thermoelectric applications. In Semiconductors and Semimetals; Tritt, T.M., Ed.; Academic Press: New York, NY, USA, 2001. [Google Scholar]
- Nolas, G.S. Structure, Thermal Conductivity, and Thermoelectric Properties of Clathrate Compounds. In Thermoelectrics Handbook: Macro to Nano; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 2006; p. 33. [Google Scholar]
- Rogl, P. Formation and Crystal Chemistry of Clathrates. In Thermoelectrics Handbook: Macro to Nano; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 2006; p. 32. [Google Scholar]
- Beekman, M.; Nolas, G.S. Inorganic clathrate-II materials of group 14: Synthetic routes and physical properties. J. Mater. Chem. 2008, 18, 842–851. [Google Scholar] [CrossRef]
- Kovnir, K.A.; Shevelkov, A.V. Semiconducting clathrates: Synthesis, structure and properties. Russ. Chem. Rev. 2004, 73, 923–938. [Google Scholar] [CrossRef]
- Beekman, M.; Morelli, D.T.; Nolas, G.S. Better thermoelectrics through glass-like crystals. Nat. Mater. 2015, 14, 1182–1185. [Google Scholar] [CrossRef] [PubMed]
- Mano, S.; Onimaru, T.; Yamanaka, S.; Takabatake, T. Off-center rattling and thermoelectric properties of type-II clathrate (K,Ba)24(Ga,Sn)136 single crystals. Phys. Rev. B 2011, 84, 214101/1–214101/6. [Google Scholar] [CrossRef]
- Koda, S.; Kishimoto, K.; Akai, K.; Asada, H.; Koyanagi, T. Thermoelectric and transport properties of sintered n-type K8Ba16Ga40Sn96 with type-II clathrate structure. J. Appl. Phys. 2014, 116, 023710/1–023710/9. [Google Scholar] [CrossRef]
- Kishimoto, K.; Koda, S.; Akai, K.; Koyanagi, T. Thermoelectric properties of sintered type-II clathrates (K,Ba)24(Ga,Sn)136 with various carrier concentrations. J. Appl. Phys. 2015, 118, 125103/1–125103/7. [Google Scholar] [CrossRef]
- Schäfer, M.C.; Bobev, S. Tin Clathrates with the Type II Structure. J. Am. Chem. Soc. 2013, 135, 1696–1699. [Google Scholar] [CrossRef] [PubMed]
- Shäfer, M.C.; Bobev, S. K and Ba distribution in the structures of the clathrate compounds KxBa16−x(Ga,Sn)136 (x = 0.8, 4.4, and 12.9) and KxBa8−x(Ga,Sn)46 (x = 0.3). Acta Cryst. C 2013, 69, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, A.P.; Lind, C.; Young, R.A.; Shastri, S.D.; Lee, P.L.; Nolas, G.S. Preparation, transport properties and structure analysis by resonant X-ray scattering of the type-I clathrate Cs8Cd4Sn42. Chem. Mater. 2002, 14, 1300–1305. [Google Scholar]
- Stefanoski, S.; Dong, Y.; Nolas, G.S. Structural characterization and low-temperature physical properties of p-type single-crystal K8Ga8.5Sn37.5 grown by self-flux method. J. Solid State Chem. 2013, 204, 166–169. [Google Scholar] [CrossRef]
- Suekuni, K.; Avila, M.A.; Umeo, K.; Fukuoka, H.; Yamanaka, S.; Nakagawa, T.; Takabatake, T. Simultaneous structure and carrier tuning of dimorphic clathrate Ba8Ga16Sn30. Phys. Rev. B 2008, 77, 235119/1–235119/8. [Google Scholar] [CrossRef]
- Tanaka, T.; Onimaru, T.; Suekuni, K.; Mano, S.; Fukuoka, H.; Yamanaka, S.; Takabatake, T. Interplay between thermoelectric and structural properties of type-I clathrate K8Ga8Sn38 single crystals. Phys. Rev. B 2010, 81, 165110/1–165110/6. [Google Scholar] [CrossRef]
- Nolas, G.S.; Weakley, T.J.R.; Cohn, J.L. Structural, chemical, and transport properties of a new clathrate compound: Cs8Zn4Sn42. Chem. Mater. 1999, 11, 2470–2473. [Google Scholar] [CrossRef]
- Avila, M.A.; Suekuni, K.; Umeo, K.; Fukuoka, K.; Yamanaka, S.; Takabatake, T. Ba8Ga16Sn30Ba8Ga16Sn30 with type-I clathrate structure: Drastic suppression of heat conduction. Appl. Phys. Lett. 2008, 92, 041901/1–041901/3. [Google Scholar] [CrossRef]
- Hayashi, M.; Kishimoto, K.; Asada, H.; Kishio, K.; Koyanagi, T. Preparation and thermoelectric properties of sintered n-type K8M8Sn38 (M = Al, Ga and In) with the type-I clathrate structure. J. Phys. D Appl. Phys. 2012, 45, 455308–455318. [Google Scholar] [CrossRef]
- Nolas, G.S.; Chakoumakos, B.C.; Mahieu, B.; Long, G.J.; Weakley, T.J.R. Structural characterization and thermal conductivity of type-I tin clathrates. Chem. Mater. 2000, 12, 1947–1953. [Google Scholar] [CrossRef]
- Schujman, S.B.; Nolas, G.S.; Young, R.A.; Lindt, C.; Slack, G.A.; Patschke, R.; Kanatzidis, M.G.; Ulutagay, M.; Hwu, S.-J. Structural analysis of the thermoelectric clathrate compound Sr8Ga16Ge30. J. Appl. Phys. 2000, 87, 1529–1533. [Google Scholar] [CrossRef]
- Kröner, R.; Peters, K.; von Schnering, H.G.; Nesper, R.Z. Crystal structure of the clathrate-II, Ba16Ga32Sn104. New Cryst. Struct. 1998, 213, 664. [Google Scholar]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System; Report LAUR 86-748; Los Alamos National Laboratory: Los Alamos, NM, USA, 2004. [Google Scholar]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–221. [Google Scholar] [CrossRef]
- Wang, H.W.; Porter, D.; Bottner, H.; Kronig, J.; Chen, L.; Bai, S.; Tritt, T.M.; Mayolet, A.; Senawiratne, J.; Smith, C.; et al. Transport properties of bulk thermoelectrics: An international round-robin study, part II: Thermal diffusivity, specific heat, and thermal conductivity. J. Electron. Mater. 2013, 42, 1073–1084. [Google Scholar] [CrossRef]
- Shannonm, R.D.; Prewitt, C.T. Effective ionic radii in oxides and fluorides. Acta Cryst. 1969, B25, 925–946. [Google Scholar] [CrossRef]
- Khabibullin, A.R.; Nolas, G.S.; Woods, L.M. Cage disorder and gas encapsulation as routes to tailor properties of clathrates. 2016, in press. [Google Scholar]
- Dong, J.; Sankey, O.; Ramachandran, G.K.; McMillan, P.F. Chemical trends of the rattling phonon modes in alloyed germanium clathrates. J. Appl. Phys. 2000, 87, 7726–7734. [Google Scholar] [CrossRef]
- Nolas, G.S. Semiconducting clathrates: A PGEC system with potential for thermoelectric applications. Mater. Res. Soc. Symp. Proc. 1999, 545, 435. [Google Scholar] [CrossRef]
- Nolas, G.S.; Cohn, J.L.; Slack, G.A.; Schujman, S.B. Semiconductor Ge-clathrates: Promising candidates for thermoelectric applications. Appl. Phys. Lett. 1998, 73, 178–180. [Google Scholar] [CrossRef]
- Slack, G.A.; Hussain, M.A. The maximum possible conversion efficiency of silicon-germanium thermoelectric generators. J. Appl. Phys. 1991, 70, 2694–2718. [Google Scholar] [CrossRef]
- Goldsmid, H.J.; Sharp, J.W. Estimation of the thermal band gap of a semiconductor from seebeck measurements. J. Electron. Mater. 1999, 28, 869–872. [Google Scholar] [CrossRef]
- Nolas, G.S.; Sharp, J.W.; Goldsmid, H.J. Thermoelectrics: Basics Principles and New Materials Developments; Springer-Verlag: Berlin, Germany, 2001. [Google Scholar]




| K7.1(2)Ba8.8(3)Ga25.1(4)Sn110.8(3) | K2.9(4)Ba13.1(2)Ga23.2(3)Sn112.7(5) | |
|---|---|---|
| Space group, Z | Fdm, 1 | |
| a, Å | 17.0001 (7) | 17.0182 (5) |
| V, Å3 | 4913.10 (6) | 4928.80 (4) |
| Radiation | Graphite monochromated Cu Kα (1.5405 Å) | |
| T (K) | 295 | 295 |
| θ limits, deg. | 5 to 30 | 5 to 30 |
| R indices | 0.1637, 0.0995 (wRp, Rp) a | 0.1261, 0.0992 (wRp, Rp) a |
| Goodness-of-fit on F2 | 2.08 | 2.23 |
| Ga1/Sn1 x and y | 0.06791 | 0.06794 |
| Ga1/Sn1 z | 0.37267 | 0.37276 |
| Sn2 x, y, and z | 0.21816 | 0.21815 |
| Ga3/Sn3 x, y, and z | 0.125 | 0.125 |
| Uiso for K/Ba | 0.0146 | 0.0209 |
| Uiso for Ga1/Sn1 | 0.0141 | 0.0145 |
| Uiso for Sn2 | 0.0149 | 0.0142 |
| Uiso for Ga3/Sn3 | 0.0121 | 0.0286 |
| K/Ba—Ga1/Sn1, Å | 3.90693 (12) | 3.91165 (8) |
| K/Ba—Sn2, Å | 3.78691 (15) | 3.79083 (11) |
| K/Ba—Ga3/Sn3, Å | 3.68062 (11) | 3.68454 (8) |
| Ga1/Sn1—Sn2, Å | 2.76613 (10) | 2.77084 (7) |
| Sn2—Ga3/Sn3, Å | 2.74310 (8) | 2.74572 (6) |
| Stoichiometry | K6.9(4)Ba8.9(5)Ga23.5(2)Sn112.3(3) |
|---|---|
| Space group, Z | Fdm, 1 |
| a, Å | 17.0006 (3) |
| V, Å3 | 4917.08 (2) |
| Radiation | Cu Kα, INCOATEC Imus micro-focus source (λ = 1.54178 Å) |
| T (K) | 100 |
| Absorption coefficient (mm−1) | 10.615 |
| θ limits, degree | 6.07 to 72 |
| No. Unique data with | 6310/210 [R(int) = 0.0386] |
| No. of unique data with > 2σ () | 212 |
| R indices | 0.0508, 0.0370 (wR2, R1) a |
| Goodness-of-fit on F2 | 2.994 |
| Max. and Min. residual e- density(e/Å3) | 0.260/−0.259 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, K.; Zeng, X.; Tritt, T.M.; Khabibullin, A.R.; Woods, L.M.; Nolas, G.S. Structure and Transport Properties of Dense Polycrystalline Clathrate-II (K,Ba)16(Ga,Sn)136 Synthesized by a New Approach Employing SPS. Materials 2016, 9, 732. https://doi.org/10.3390/ma9090732
Wei K, Zeng X, Tritt TM, Khabibullin AR, Woods LM, Nolas GS. Structure and Transport Properties of Dense Polycrystalline Clathrate-II (K,Ba)16(Ga,Sn)136 Synthesized by a New Approach Employing SPS. Materials. 2016; 9(9):732. https://doi.org/10.3390/ma9090732
Chicago/Turabian StyleWei, Kaya, Xiaoyu Zeng, Terry M. Tritt, Artem R. Khabibullin, Lilia M. Woods, and George S. Nolas. 2016. "Structure and Transport Properties of Dense Polycrystalline Clathrate-II (K,Ba)16(Ga,Sn)136 Synthesized by a New Approach Employing SPS" Materials 9, no. 9: 732. https://doi.org/10.3390/ma9090732






