Biochar Is Comparable to Dicyandiamide in the Mitigation of Nitrous Oxide Emissions from Camellia oleifera Abel. Fields
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Soil Collection
2.2. Experimental Design and Field Procedures
2.3. Analysis of Soil and Biochar Characteristics
2.4. Measurement of Soil N2O Emission Rates and Cumulative Soil N2O Emissions
2.5. Statistical Analysis
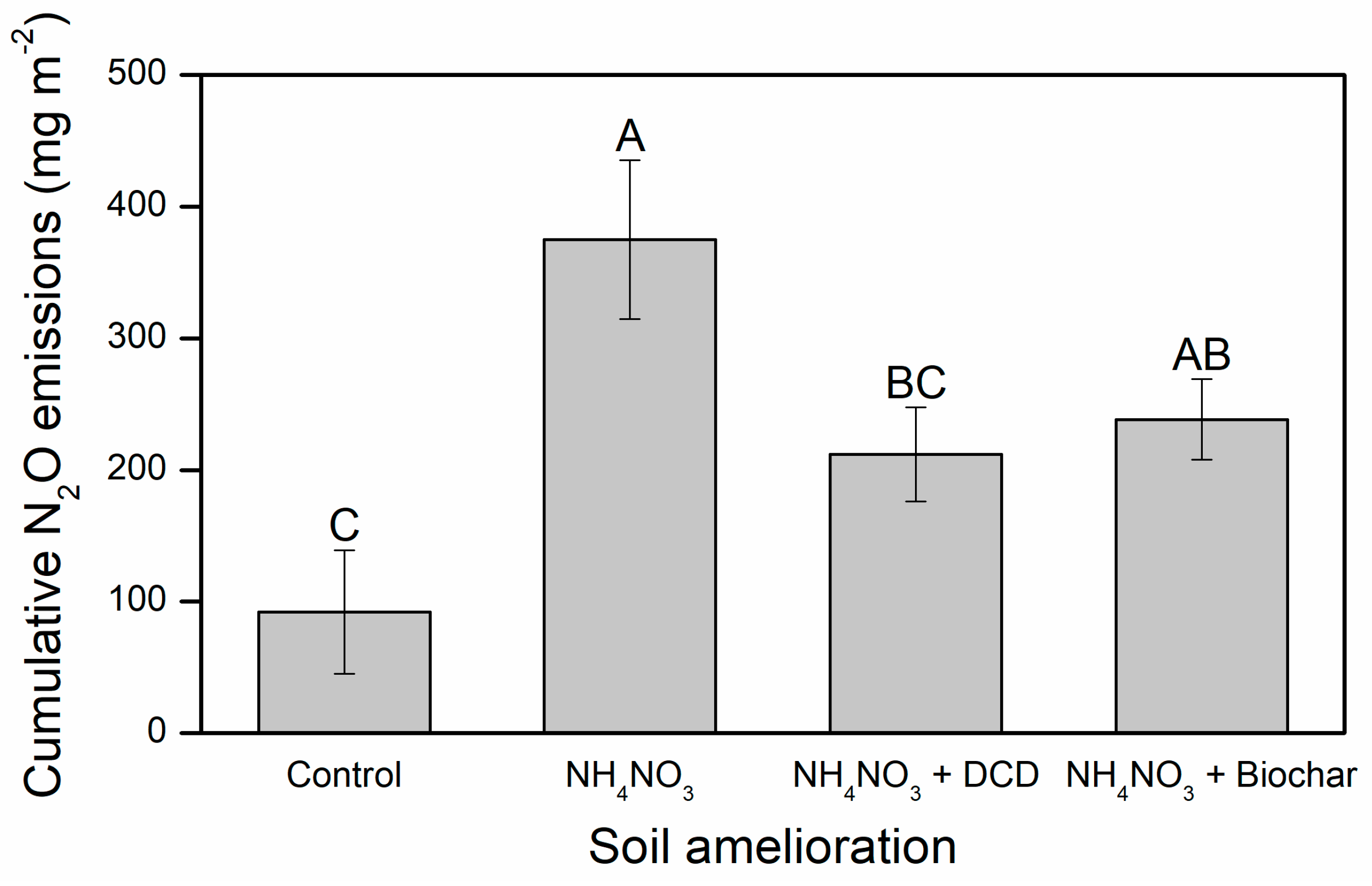
3. Results
4. Discussion
4.1. Nitrogen Fertilization Stimulated Soil N2O Emissions
4.2. Biochar Reduced Soil N2O Emission Rates as Affected by N Fertilization
4.3. DCD Reduced Soil N2O Emissions as Affected by N Fertilization
4.4. Biochar and DCD Effects on Soil N2O Emissions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Synthesis Report, Climate Change 2014; IPCC: Geneva, Switzerland, 2014; pp. 1–164. [Google Scholar]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed]
- WMO. WMO Greenhouse Gas Bulletin: The State of Greenhouse Gases in the Atmosphere Based on Global Observations Through 2017; Atmospheric Environment Research Division: Geneva, Switzerland, 2018; pp. 1–8. [Google Scholar]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 1–13. [Google Scholar] [CrossRef]
- Gerber, J.S.; Carlson, K.M.; Makowski, D.; Mueller, N.D.; Garcia De Cortazar-Atauri, I.; Havlík, P.; Herrero, M.; Launay, M.; O’Connell, C.S.; Smith, P.; et al. Spatially explicit estimates of N2O emissions from croplands suggest climate mitigation opportunities from improved fertilizer management. Glob. Chang. Biol. 2016, 22, 3383–3394. [Google Scholar] [CrossRef] [PubMed]
- Carlson, K.M.; Gerber, J.S.; Mueller, N.D.; Herrero, M.; MacDonald, G.K.; Brauman, K.A.; Havlik, P.; Connell, C.S.; Johnson, J.A.; Saatchi, S.; et al. Greenhouse gas emissions intensity of global croplands. Nat. Clim. Chang. 2017, 7, 63–68. [Google Scholar] [CrossRef]
- He, Y.; Zhou, X.; Jiang, L.; Li, M.; Du, Z.; Zhou, G.; Shao, J.; Wang, X.; Xu, Z.; Hosseini Bai, S.; et al. Effects of biochar application on soil greenhouse gas fluxes: A meta-analysis. GCB Bioenergy 2017, 9, 743–755. [Google Scholar] [CrossRef]
- Gu, J.; Nie, H.; Guo, H.; Xu, H.; Gunnathorn, T. Nitrous oxide emissions from fruit orchards: A review. Atmos. Environ. 2019, 201, 166–172. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, B.; Zhang, Y.; Hu, T.; Lin, Z.; Liu, G.; Wang, X.; Ma, J.; Wang, H.; Jin, H.; et al. Biochar application as a tool to decrease soil nitrogen losses (NH3 volatilization, N2O emissions, and N leaching) from croplands: Options and mitigation strength in a global perspective. Glob. Chang. Biol. 2019, 25, 2077–2093. [Google Scholar] [CrossRef]
- Shrestha, B.; Chang, S.; Bork, E.; Carlyle, C. Enrichment planting and soil amendments enhance carbon sequestration and reduce greenhouse gas emissions in agroforestry systems: A review. Forests 2018, 9, 369. [Google Scholar] [CrossRef]
- Johannes, L.; Stephen, J. Biochar for Environmental Management; Routledge: New York, NY, USA, 2015; pp. 1–944. [Google Scholar]
- Obia, A.; Cornelissen, G.; Mulder, J.; Dörsch, P. Effect of soil pH increase by biochar on NO, N2O and N2 production during denitrification in acid soils. PLoS ONE 2015, 10, 1–19. [Google Scholar] [CrossRef]
- Zakir, H.A.; Subbarao, G.V.; Pearse, S.J.; Gopalakrishnan, S.; Ito, O.; Ishikawa, T.; Kawano, N.; Nakahara, K.; Yoshihashi, T.; Ono, H.; et al. Detection, isolation and characterization of a root-exuded compound, methyl 3-(4-hydroxyphenyl) propionate, responsible for biological nitrification inhibition by sorghum (Sorghum bicolor). New Phytol. 2008, 180, 442–451. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Nakahara, K.; Hurtado, M.P.; Ono, H.; Moreta, D.E.; Salcedo, A.F.; Yoshihashi, A.T.; Ishikawa, T.; Ishitani, M.; Ohnishi-Kameyama, M.; et al. Evidence for biological nitrification inhibition in Brachiaria pastures. Proc. Natl. Acad. Sci. USA 2009, 106, 17302–17307. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Liu, L.; Hu, S.; Compton, J.E.; Greaver, T.L.; Li, Q. How inhibiting nitrification affects nitrogen cycle and reduces environmental impacts of anthropogenic nitrogen input. Glob. Chang. Biol. 2015, 21, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Ruser, R.; Schulz, R. The effect of nitrification inhibitors on the nitrous oxide (N2O) release from agricultural soils-a review. J. Plant Nutr. Soil Sci. 2015, 178, 171–188. [Google Scholar] [CrossRef]
- Liu, J.; Wu, L.; Chen, D.; Li, M.; Wei, C. Soil quality assessment of different Camellia oleifera stands in mid-subtropical China. Appl. Soil Ecol. 2017, 113, 29–35. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, C.; Chen, H.; Zhou, H.; Ye, J. Prediction of fatty acid composition in Camellia oleifera oil by near infrared transmittance spectroscopy (NITS). Food Chem. 2013, 138, 1657–1662. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Tang, W.; Peng, S.; Li, M.; Deng, N.; Chen, Y. Predicting potential distribution and evaluating suitable soil condition of oil tea Camellia in China. Forests 2018, 9, 487. [Google Scholar] [CrossRef]
- Martins, M.R.; Sant Anna, S.A.C.; Zaman, M.; Santos, R.C.; Monteiro, R.C.; Alves, B.J.R.; Jantalia, C.P.; Boddey, R.M.; Urquiaga, S. Strategies for the use of urease and nitrification inhibitors with urea: Impact on N2O and NH3 emissions, fertilizer-15N recovery and maize yield in a tropical soil. Agric. Ecosyst. Environ. 2017, 247, 54–62. [Google Scholar] [CrossRef]
- Liu, S.; Lin, F.; Wu, S.; Ji, C.; Sun, Y.; Jin, Y.; Li, S.; Li, Z.; Zou, J. A meta-analysis of fertilizer-induced soil NO and combined NO+N2O emissions. Glob. Chang. Biol. 2017, 23, 2520–2532. [Google Scholar] [CrossRef]
- Deng, B.; Wang, S.; Xu, X.; Wang, H.; Hu, D.; Guo, X.; Shi, Q.; Siemann, E.; Zhang, L. Effects of biochar and dicyandiamide combination on nitrous oxide emissions from Camellia oleifera field soil. Environ. Sci. Pollut. Res. 2019, 26, 4070–4077. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.; Liu, S.; Liu, X.; Zou, J.; Siemann, E. Perennial forb invasions alter greenhouse gas balance between ecosystem and atmosphere in an annual grassland in China. Sci. Total Environ. 2018, 642, 781–788. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, X.; Wang, H.; Liu, S.; Siemann, E.; Zou, J. Soil respiration and litter decomposition increased following perennial forb invasion into an annual grassland. Pedosphere 2016, 26, 567–576. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis; CRC Press: Boca Raton, IL, USA, 2007; pp. 1–1224. [Google Scholar]
- Pärn, J.; Verhoeven, J.T.A.; Butterbach-Bahl, K.; Dise, N.B.; Ullah, S.; Aasa, A.; Egorov, S.; Espenberg, M.; Järveoja, J.; Jauhiainen, J.; et al. Nitrogen-rich organic soils under warm well-drained conditions are global nitrous oxide emission hotspots. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Domeignoz-Horta, L.A.; Philippot, L.; Peyrard, C.; Bru, D.; Breuil, M.; Bizouard, F.; Justes, E.; Mary, B.; Léonard, J.; Spor, A. Peaks of in situ N2O emissions are influenced by N2O-producing and reducing microbial communities across arable soils. Glob. Chang. Biol. 2018, 24, 360–370. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Yuan, J.; Luo, J.; Wang, W.; Fan, J.; Liu, D.; Ding, W. Organic fertilizers have divergent effects on soil N2O emissions. Biol. Fertil. Soils 2019, 55, 685–699. [Google Scholar] [CrossRef]
- Lourenço, K.S.; Dimitrov, M.R.; Pijl, A.; Soares, J.R.; Do Carmo, J.B.; van Veen, J.A.; Cantarella, H.; Kuramae, E.E. Dominance of bacterial ammonium oxidizers and fungal denitrifiers in the complex nitrogen cycle pathways related to nitrous oxide emission. GCB Bioenergy 2018, 10, 645–660. [Google Scholar] [CrossRef]
- Pauleta, S.R.; Dell Acqua, S.; Moura, I. Nitrous oxide reductase. Coord. Chem. Rev. 2013, 257, 332–349. [Google Scholar] [CrossRef]
- Hu, H.; Chen, D.; He, J. Microbial regulation of terrestrial nitrous oxide formation: Understanding the biological pathways for prediction of emission rates. FEMS Microbiol. Rev. 2015, 39, 729–749. [Google Scholar] [CrossRef]
- Shcherbak, I.; Millar, N.; Robertson, G.P. Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proc. Natl. Acad. Sci. USA 2014, 111, 9199–9204. [Google Scholar] [CrossRef]
- Tian, D.; Niu, S. A global analysis of soil acidification caused by nitrogen addition. Environ. Res. Lett. 2015, 10, 24019. [Google Scholar] [CrossRef]
- Matson, P.A.; McDowell, W.H.; Townsend, A.R.; Vitousek, P.M. The globalization of N deposition: Ecosystem consequences in tropical environments. Biogeochemistry 1999, 46, 67–83. [Google Scholar] [CrossRef]
- Carey, C.J.; Dove, N.C.; Beman, J.M.; Hart, S.C.; Aronson, E.L. Meta-analysis reveals ammonia-oxidizing bacteria respond more strongly to nitrogen addition than ammonia-oxidizing archaea. Soil Biol. Biochem. 2016, 99, 158–166. [Google Scholar] [CrossRef] [Green Version]
- Shaaban, M.; Wu, Y.; Khalid, M.S.; Peng, Q.; Xu, X.; Wu, L.; Younas, A.; Bashir, S.; Mo, Y.; Lin, S.; et al. Reduction in soil N2O emissions by pH manipulation and enhanced nosZ gene transcription under different water regimes. Environ. Pollut. 2018, 235, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.; Su, Q.; Ma, Y.; Valverde-Pérez, B.; Domingo-Félez, C.; Jensen, M.M.; Smets, B.F. The pH dependency of N-converting enzymatic processes, pathways and microbes: Effect on net N2O production. Environ. Microbiol. 2018, 20, 1623–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Z.; Wang, J.; Almøy, T.; Bakken, L.R. Excessive use of nitrogen in Chinese agriculture results in high N2O/(N2O+N2) product ratio of denitrification, primarily due to acidification of the soils. Glob. Chang. Biol. 2014, 20, 1685–1698. [Google Scholar] [CrossRef] [Green Version]
- Schindlbacher, A. Effects of soil moisture and temperature on NO, NO2, and N2O emissions from European forest soils. J. Geophys. Res. 2004, 109, 1–12. [Google Scholar] [CrossRef]
- Pinheiro, P.L.; Recous, S.; Dietrich, G.; Weiler, D.A.; Schu, A.L.; Bazzo, H.L.S.; Giacomini, S.J. N2O emission increases with mulch mass in a fertilized sugarcane cropping system. Biol. Fertil. Soils 2019, 55, 511–523. [Google Scholar] [CrossRef] [Green Version]
- Yin, C.; Fan, F.; Song, A.; Fan, X.; Ding, H.; Ran, W.; Qiu, H.; Liang, Y. The response patterns of community traits of N2O emission-related functional guilds to temperature across different arable soils under inorganic fertilization. Soil Biol. Biochem. 2017, 108, 65–77. [Google Scholar] [CrossRef]
- Chen, T.; Oenema, O.; Li, J.; Misselbrook, T.; Dong, W.; Qin, S.; Yuan, H.; Li, X.; Hu, C. Seasonal variations in N2 and N2O emissions from a wheat–maize cropping system. Biol. Fertil. Soils 2019, 55, 539–551. [Google Scholar] [CrossRef]
- Quick, A.M.; Reeder, W.J.; Farrell, T.B.; Tonina, D.; Feris, K.P.; Benner, S.G. Nitrous oxide from streams and rivers: A review of primary biogeochemical pathways and environmental variables. Earth-Sci. Rev. 2019, 191, 224–262. [Google Scholar] [CrossRef]
- Chen, H.; Mothapo, N.V.; Shi, W. Soil moisture and pH control relative contributions of fungi and bacteria to N2O production. Microb. Ecol. 2015, 69, 180–191. [Google Scholar] [CrossRef]
- Burgin, A.J.; Groffman, P.M. Soil O2 controls denitrification rates and N2O yield in a riparian wetland. J. Geophys. Res. Biogeosci. 2012, 117, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Ding, W.; Xu, Y.; Müller, C.; Yu, H.; Fan, J. Increased N2O emissions during soil drying after waterlogging and spring thaw in a record wet year. Soil Biol. Biochem. 2016, 101, 152–164. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Singh, B.P.; Kimber, S.W.L.; Murphy, D.V.; Macdonald, L.M.; Rust, J.; Morris, S. An incubation study investigating the mechanisms that impact N2O flux from soil following biochar application. Agric. Ecosyst. Environ. 2014, 191, 53–62. [Google Scholar] [CrossRef]
- Case, S.D.C.; McNamara, N.P.; Reay, D.S.; Whitaker, J. The effect of biochar addition on N2O and CO2 emissions from a sandy loam soil - The role of soil aeration. Soil Biol. Biochem. 2012, 51, 125–134. [Google Scholar] [CrossRef]
- Case, S.D.C.; McNamara, N.P.; Reay, D.S.; Stott, A.W.; Grant, H.K.; Whitaker, J. Biochar suppresses N2O emissions while maintaining N availability in a sandy loam soil. Soil Biol. Biochem. 2015, 81, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Dai, Q.; Jin, X.; Dong, X.; Peng, J.; Wu, M.; Liang, N.; Pan, B.; Xing, B. Negative impacts of biochars on urease activity: High pH, heavy metals, polycyclic aromatic hydrocarbons, or free radicals? Environ. Sci. Technol. 2018, 52, 12740–12747. [Google Scholar] [CrossRef]
- Shaaban, M.; Hu, R.; Wu, Y.; Younas, A.; Xu, X.; Sun, Z.; Jiang, Y.; Lin, S. Mitigation of N2O emissions from urine treated acidic soils by liming. Environ. Pollut. 2019, 225, 113237. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Sánchez-Monedero, M.A.; Roig, A.; Hanley, K.; Enders, A.; Lehmann, J. Biochar and denitrification in soils: When, how much and why does biochar reduce N2O emissions? Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Hu, H.W.; Shen, J.P.; Du, S.; Zhang, L.M.; He, J.Z.; Han, L.L. Effects of the nitrification inhibitor dicyandiamide (DCD) on N2O emissions and the abundance of nitrifiers and denitrifiers in two contrasting agricultural soils. J. Soils Sediments 2017, 17, 1635–1643. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Yi, Q.; Tang, S.; Fan, X.; Zhang, M.; Pang, Y.; Huang, X.; Huang, Q. Effects of nitrogen application rate, nitrogen synergist and biochar on nitrous oxide emissions from vegetable field in south China. PLoS ONE 2017, 12, 1–15. [Google Scholar] [CrossRef]



| Parameters | Soil Temperature | Soil Moisture | NH4+-N | NO3−-N |
|---|---|---|---|---|
| Soil moisture | 0.275 *** | |||
| NH4+-N | 0.051 | −0.050 | ||
| NO3−-N | 0.188 * | −0.003 | 0.414 *** | |
| N2O | 0.216 *** | 0.201 *** | 0.285 *** | 0.221 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, B.; Fang, H.; Jiang, N.; Feng, W.; Luo, L.; Wang, J.; Wang, H.; Hu, D.; Guo, X.; Zhang, L. Biochar Is Comparable to Dicyandiamide in the Mitigation of Nitrous Oxide Emissions from Camellia oleifera Abel. Fields. Forests 2019, 10, 1076. https://doi.org/10.3390/f10121076
Deng B, Fang H, Jiang N, Feng W, Luo L, Wang J, Wang H, Hu D, Guo X, Zhang L. Biochar Is Comparable to Dicyandiamide in the Mitigation of Nitrous Oxide Emissions from Camellia oleifera Abel. Fields. Forests. 2019; 10(12):1076. https://doi.org/10.3390/f10121076
Chicago/Turabian StyleDeng, Bangliang, Haifu Fang, Ningfei Jiang, Weixun Feng, Laicong Luo, Jiawei Wang, Hua Wang, Dongnan Hu, Xiaomin Guo, and Ling Zhang. 2019. "Biochar Is Comparable to Dicyandiamide in the Mitigation of Nitrous Oxide Emissions from Camellia oleifera Abel. Fields" Forests 10, no. 12: 1076. https://doi.org/10.3390/f10121076




