Effects of Long-Term Successive Rotations, Clear-Cutting and Stand Age of Prince Rupprecht’s larch (Larix principis-rupprechtii Mayr) on Soil Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design and Sampling
2.3. Physical Analysis
2.4. Chemical Analysis
2.5. Microbial Properties
2.6. Soil Enzyme Activity
2.7. Statistical Analysis
3. Results
3.1. Soil Physical Properties
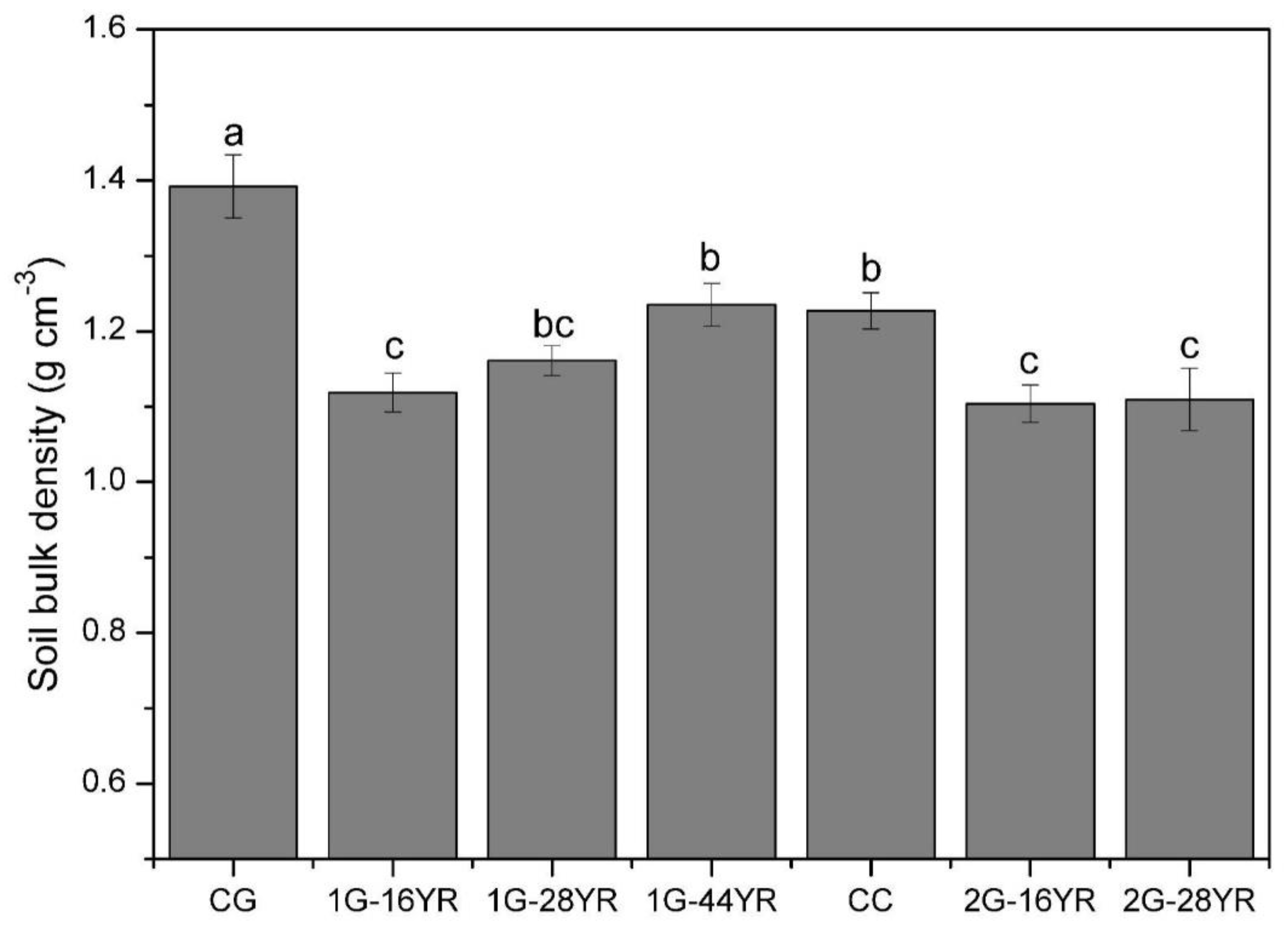
3.1.1. Soil Bulk Density
3.1.2. Soil Porosity
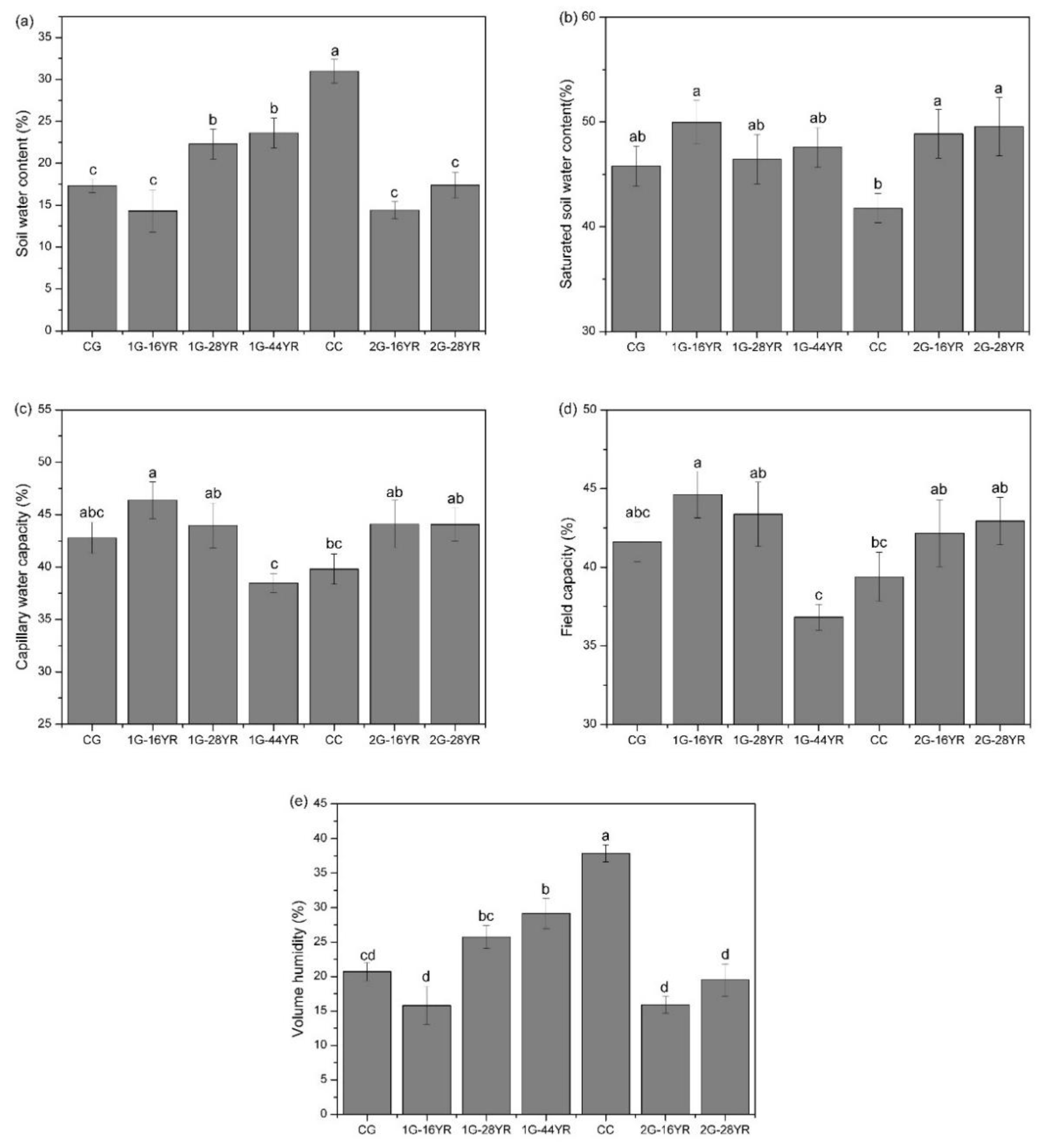
3.1.3. Soil Water Content
3.2. Soil Chemical Properties
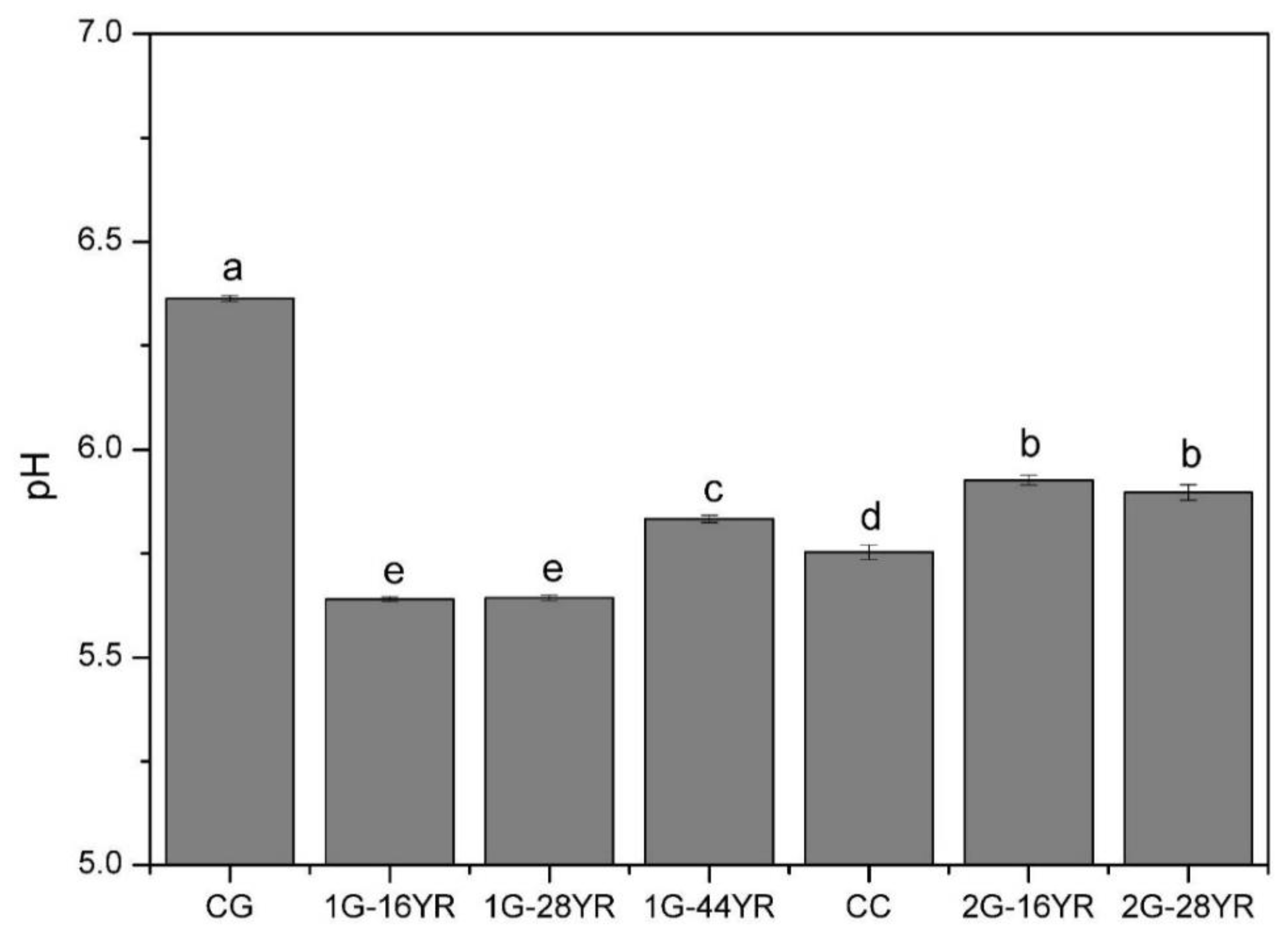
3.2.1. Soil pH Value
3.2.2. Soil Nutrients
3.3. Soil Biological Properties
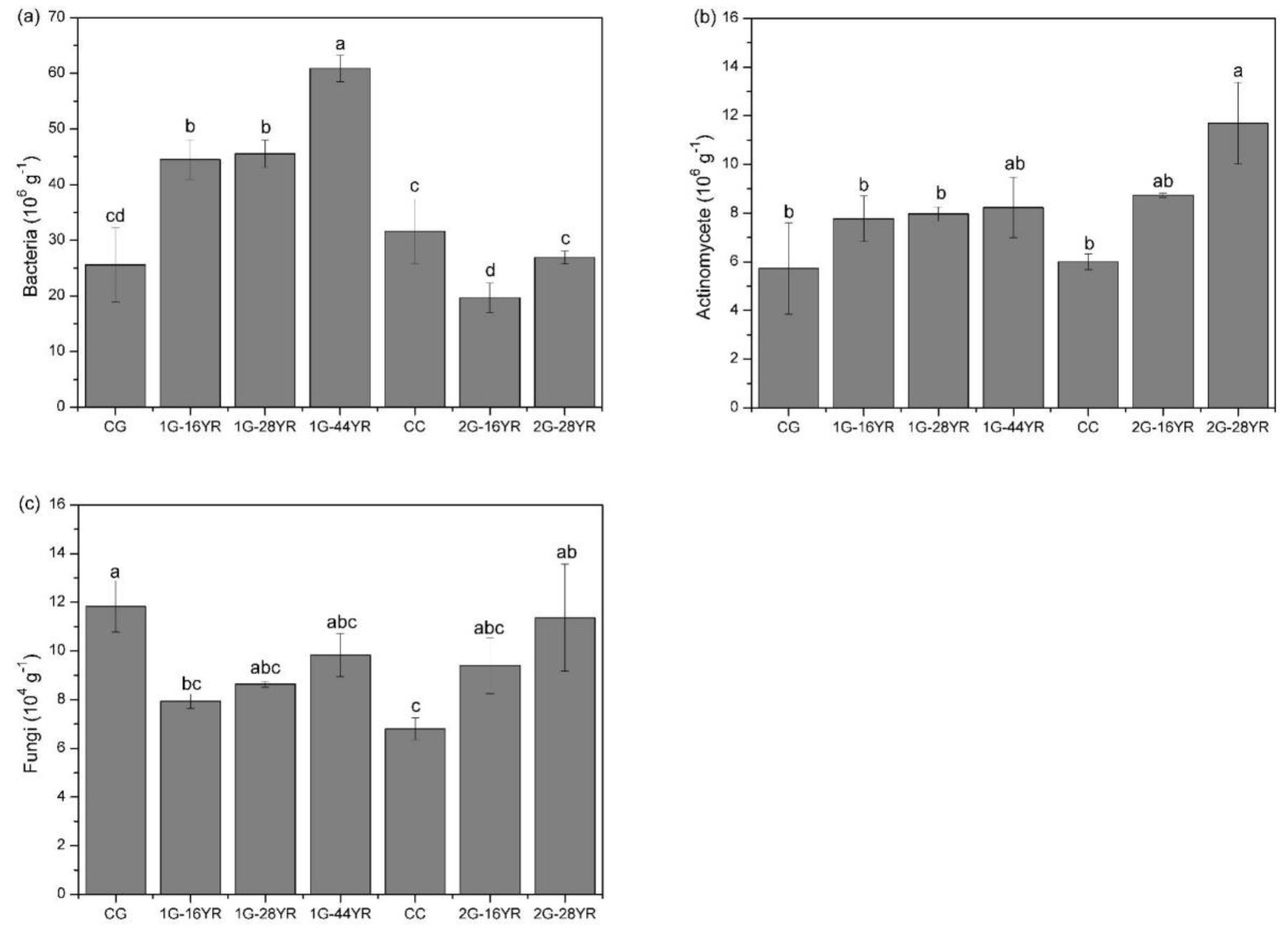
3.3.1. Soil Microorganisms
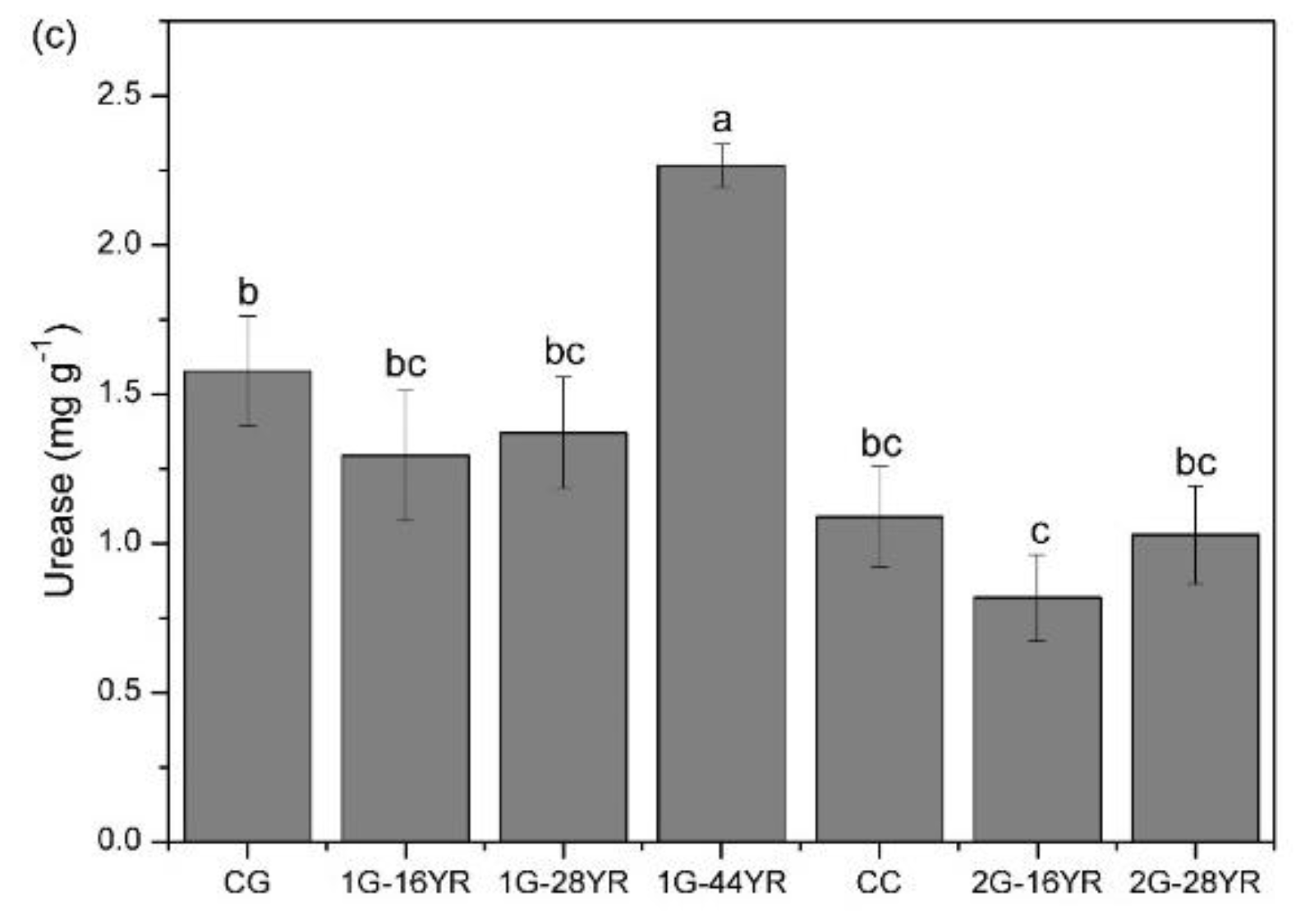
3.3.2. Soil Enzyme Activity
3.4. Soil Quality Index
4. Discussion
4.1. Stand Age
4.2. Forest Generation
4.3. Clear-Cutting
4.4. Forest Cultivation Cycle and Soil Quality Recovery Time
5. Conclusions
- (1)
- Extending the cultivation cycle of Larix principis-rupprechtii forest was beneficial to improving soil quality.
- (2)
- Increasing forest generation did not significantly reduce soil quality.
- (3)
- Clear-cutting could greatly decrease soil quality, and returning the harvesting residues to the forest land may reduce the negative impact of clear-cutting on soil quality.
- (4)
- In order to maintain soil health and achieve sustainable planting, the planting period of Larix principis-rupprechtii forests should be more than 39 years.
- (5)
- SQI provided a more intelligible and comprehensive measurement of soil quality with the identification of a minimum data set. Future studies should compare SQI with other soil quality indicators to further optimize SQI calculation method.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lal, R. Restoring Soil Quality to Mitigate Soil Degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef] [Green Version]
- Lal, R. Soil degradation as a reason for inadequate human nutrition. Food Sec. 2009, 1, 45–57. [Google Scholar] [CrossRef]
- León, J.D.; Osorio, N.W. Role of Litter Turnover in Soil Quality in Tropical Degraded Lands of Colombia. Sci. World J. 2014, 2014, 693981. [Google Scholar] [CrossRef] [PubMed]
- Bini, C. Soil: A precious natural resource. In Conservation of Natural Resources; Kudrow, N.J., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2009; pp. 1–48. [Google Scholar]
- Yu, X.B.; Yang, G.Q.; Li, S.K. Study on the changes of soil properties in Eucalyptus plantation under continuous-planting practices. In Studies on Longterm Productivity Management of Eucalyptus Plantation; Yu, X.B., Ed.; China Forestry Publishing House: Beijing, China, 2000; pp. 94–103. [Google Scholar]
- Zhang, K.; Zheng, H.; Chen, F.L.; Ouyang, Z.Y.; Wang, Y.; Wu, Y.F.; Lan, J.; Fu, M.; Xiang, X.W. Changes in Soil Quality after Converting Pinus to Eucalyptus Plantations in Southern China. Solid Earth 2015, 6, 115–123. [Google Scholar] [CrossRef]
- LeBauer, D.S.; Treseder, K.K. Nitrogen Limitation of Net Primary Productivity in Terrestrial Ecosystems Is Globally Distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Laclau, J.P.; Ranger, J.; Goncalves, J.L.D.; Maquere, V.; Krusche, A.V.; M’Bou, A.T.; Nouvellon, Y.; Saint-Andre, L.; Bouillet, J.P.; Piccolo, M.D.; et al. Biogeochemical Cycles of Nutrients in Tropical Eucalyptus Plantations Main Features Shown by Intensive Monitoring in Congo and Brazil. For. Ecol. Manag. 2010, 259, 1771–1785. [Google Scholar] [CrossRef]
- Yu, F.K.; Huang, X.H.; Wang, K.Q.; Duang, C.Q. An overview of ecological degradation and restoration of Eucalyptus plantations. Chin. J. Eco-Agric. 2009, 17, 393–398. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Pulleman, M. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Garay, I.; Pellens, R.; Kindel, A.; Barros, E.; Franco, A.A. Evaluation of soil conditions in fast-growing plantations of Eucalyptus grandis and Acacia mangium in Brazil: A contribution to the study of sustainable land use. Appl. Soil Ecol. 2004, 27, 177–187. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M.; Jordán, A.; Zavala, L.M.; De la Rosa, D.; AbdElmabod, S.K.; Anaya-Romero, M. Organic carbon stocks in Mediterranean soil types under different land uses (Southern Spain). Solid Earth 2012, 3, 375–386. [Google Scholar] [CrossRef] [Green Version]
- Parras-Alcántara, L.; Martín-Carrillo, M.; Lozano-García, B. Impacts of land use change in soil carbon and nitrogen in a Mediterranean agricultural area (Southern Spain). Solid Earth 2013, 4, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wei, Z.C.; Li, H.T.; Guo, F.T.; Wu, P.F.; Zhou, L.L.; Ma, X.Q. Biochemical quality and accumulation of soil organic matter in an age sequence of Cunninghamia lanceolata plantations in southern China. J. Soils Sediments 2017, 17, 2218–2229. [Google Scholar] [CrossRef]
- Filip, Z. International approach to assessing soil quality by ecologically-related biological parameters. Agric. Ecosyst. Environ. 2002, 88, 169–174. [Google Scholar] [CrossRef]
- Parisi, V.; Menta, C.; Gardi, C.; Jacomini, C.; Mozzanica, E. Microarthropod communities as a tool to assess soil quality and biodiversity: A new approach in Italy. Agric. Ecosyst. Environ. 2005, 105, 323–333. [Google Scholar] [CrossRef]
- Ritz, K.; Black, H.I.J.; Campbell, C.D.; Harris, J.A.; Wood, C. Selecting biological indicators for monitoring soils: A framework for balancing scientific and technical opinion to assist policy development. Ecol. Indic. 2009, 9, 1212–1221. [Google Scholar] [CrossRef] [Green Version]
- Andrews, S.S.; Carroll, C.R. Designing a soil quality assessment tool for sustainable agroecosystem management. Ecol. Appl. 2001, 11, 1573–1585. [Google Scholar] [CrossRef]
- Zornoza, R.; Acosta, J.A.; Bastida, F.; Domínguez, S.G.; Toledo, D.M.; Faz, A. Identification of sensitive indicators to assess the interrelationship between soil quality, management practices and human health. Soil 2015, 1, 173–185. [Google Scholar] [CrossRef] [Green Version]
- Gruba, P.; Socha, J.; Pietrzykowski, M.; Pasichnyk, D. Tree species affects the concentration of total mercury (Hg) in forest soils: Evidence from a forest soil inventory in Poland. Sci. Total Environ. 2019, 647, 141–148. [Google Scholar] [CrossRef]
- Bouma, J.; Van Ittersum, M.K.; Stoorvogel, J.J.; Batjes, N.H.; Droogers, P.; Pulleman, M.M. Soil capability: Exploring the functional potentials of soils. Glob. Soil Secur. 2017, 27–44. [Google Scholar] [CrossRef]
- Weis, W.; Huber, C.; Gattlein, A. Regeneration of mature Norway spruce stands: Early effects of selective cutting and clear cutting on seepage water quality and soil fertility. Sci. World J. 2001, 1, 493–499. [Google Scholar] [CrossRef]
- Zhou, X.N.; Zhou, Y.; Zhou, C.J.; Wu, Z.L.; Zheng, L.F.; Hu, X.S.; Chen, H.X.; Gan, J.B. Effects of Cutting Intensity on Soil Physical and Chemical Properties in a Mixed Natural Forest in Southeastern China. Forests 2015, 6, 4495–4509. [Google Scholar] [CrossRef]
- Aleksandrowicz-Trzcinska, M.; Drozdowski, S.; Zybura, H. Effect of Mechanical Site Preparation on Features of the Soil in a Clear-Cut Area. Sylwan 2018, 162, 648–657. [Google Scholar]
- Hume, A.M.; Chen, H.Y.H.; Taylor, A.R. Intensive Forest Harvesting Increases Susceptibility of Northern Forest Soils to Carbon, Nitrogen and Phosphorus Loss. J. Appl. Ecol. 2018, 55, 246–255. [Google Scholar] [CrossRef]
- Zhao, K.J.; Zheng, M.X.; Fahey, T.J.; Jia, Z.K.; Ma, Y.L. Vertical gradients and seasonal variations in the stem CO2 efflux of Larix principis-rupprechtii Mayr. Agric. For. Meteorol. 2018, 262, 71–80. [Google Scholar] [CrossRef]
- Yao, Y.T.; Zhang, J.Z.; Hu, J.F. Larix Principis-Rupprechtii; Agricultural Science and Technology Press of China: Beijing, China, 2013. [Google Scholar]
- McKenzie, N.; Coughlan, K.; Cresswell, H. Soil Physical Measurement and Interpretation for Land Evaluation; Csiro Publishing: Collingwood, Australia, 2002; Volume 5. [Google Scholar]
- Wu, G.L.; Yang, Z.; Cui, Z.; Liu, Y.; Fang, N.F.; Shi, Z.H. Mixed artificial grasslands with more roots improved mine soil infiltration capacity. J. Hydrol. 2016, 535, 54–60. [Google Scholar] [CrossRef]
- Jiao, F.; Wen, Z.M.; An, S.S. Changes in soil properties across a chronosequence of vegetation restoration on the Loess Plateau of China. Catena 2011, 86, 110–116. [Google Scholar] [CrossRef]
- Huang, C.Y. Soil Science; China Agriculture Press: Beijing, China, 2003; pp. 89–92. [Google Scholar]
- Hardie, M.; Clothier, B.; Bound, S.; Oliver, G.; Close, D. Does Biochar Influence Soil Physical Properties and Soil Water Availability? Plant Soil 2014, 376, 347–361. [Google Scholar] [CrossRef]
- Clark, M.D.; Gilmour, J.T. The effect of temperature on decomposition at optimum and saturated soil water contents. Soil Sci. Soc. Am. J. 1983, 47, 927–929. [Google Scholar] [CrossRef]
- Rowell, D.L. Soil Science: Methods and Applications; Routledge: London, UK, 2014. [Google Scholar]
- Cassel, D.K.; Nielsen, D.R. Field capacity and available water capacity. In Methods of Soil Analysis. Part 1, 2nd ed.; Klute, A., Ed.; Agron. Monogr. 9. ASA and SSSA: Madison, WI, USA, 1986; pp. 901–926. [Google Scholar]
- Givi, J.; Prasher, S.O.; Patel, R.M. Evaluation of pedotransfer functions in predicting the soil water contents at field capacity and wilting point. Agric. Water Manag. 2004, 70, 83–96. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agro-Chemical Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Chen, H.K. Soil Microbiology; Shanghai Science and Technology Press: Shanghai, China, 1981; pp. 44–45. [Google Scholar]
- Waldrop, M.P.; Balser, T.C.; Firestone, M.K. Linking microbial community composition to function in a tropical soil. Soil Biol. Biochem. 2000, 32, 1837–1846. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Zheng, H.; Ouyang, Z.Y.; Wang, X.K.; Miao, H.; Zhao, T.Q.; Peng, T.B. How different reforestation approaches affect red soil properties in Southern China. Land Degrad. Dev. 2005, 16, 387–396. [Google Scholar] [CrossRef]
- Andrews, S.S.; Karlen, D.L.; Mitchell, J.P. A comparison of soil quality indexing methods for vegetable production systems in Northern California. Agric. Ecosyst. Environ. 2002, 90, 25–45. [Google Scholar] [CrossRef]
- Lima, A.M.; Silva, I.R.; Neves, J.C.; Novais, R.F.; Barros, N.F.; Mendonça, E.S.; Leite, F.P. Soil organic carbon dynamics following afforestation of degraded pastures with eucalyptus in southeastern Brazil. For. Ecol. Manag. 2006, 235, 219–231. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Hedo, J.; Cerdá, A.; Candel-Pérez, D.; Viñegla, B. Unravelling the importance of forest age stand and forest structure driving microbiological soil properties, enzymatic activities and soil nutrients content in Mediterranean Spanish black pine (Pinus nigra Ar. ssp. salzmannii) Forest. Sci. Total Environ. 2016, 562, 145–154. [Google Scholar] [CrossRef]
- Mao, R.; Zeng, D.H.; Hu, Y.L.; Li, L.J.; Yang, D. Soil organic carbon and nitrogen stocks in an age-sequence of poplar stands planted on marginal agricultural land in Northeast China. Plant Soil 2010, 332, 277–287. [Google Scholar] [CrossRef]
- Cheng, J.; Lee, X.; Theng, B.K.; Zhang, L.; Fang, B.; Li, F. Biomass accumulation and carbon sequestration in an age-sequence of Zanthoxylum bungeanum plantations under the Grain for Green Program in karst regions, Guizhou province. Agric. For. Meteorol. 2015, 203, 88–95. [Google Scholar] [CrossRef]
- Zhou, G.Y.; Liu, S.G.; Li, Z.; Zhang, D.Q.; Tang, X.L.; Zhou, C.Y.; Yan, J.H.; Mo, J.M. Old-Growth Forests Can Accumulate Carbon in Soils. Science 2006, 314, 1417. [Google Scholar] [CrossRef]
- Luyssaert, S.; Schulze, E.D.; Borner, A.; Knohl, A.; Hessenmoller, D.; Law, B.E.; Ciais, P.; Grace, J. Old-growth forests as global carbon sinks. Nature 2008, 455, 213–215. [Google Scholar] [CrossRef]
- Chen, G.S.; Yang, Z.J.; Gao, R.; Xie, J.S.; Guo, J.F.; Huang, Z.Q.; Yang, Y.S. Carbon Storage in a Chronosequence of Chinese Fir Plantations in Southern China. For. Ecol. Manag. 2013, 300, 68–76. [Google Scholar] [CrossRef]
- Xu, D.P. An approach to nutrient balance in tropical and south subtropical short rotation plantations. In Studies on Long-Term Productivity Management of Eucalyptus Plantation; Yu, X.B., Ed.; China Forestry Publishing House: Beijing, China, 2000; pp. 27–35. [Google Scholar]
- Diochon, A.C.; Kellman, L. Physical fractionation of soil organic matter: Destabilization of deep soil carbon following harvesting of a temperate coniferous forest. J. Geophys. Res. Biogeosci. 2009, 114, G01016. [Google Scholar] [CrossRef]
- Selvaraj, S.; Duraisamy, V.; Huang, Z.J.; Guo, F.T.; Ma, X.Q. Influence of Long-Term Successive Rotations and Stand Age of Chinese Fir (Cunninghamia lanceolata) Plantations on Soil Properties. Geoderma 2017, 306, 127–134. [Google Scholar] [CrossRef]
- Jennings, T.N.; Smith, J.E.; Cromack, K.; Sulzman, E.W.; McKay, D.; Caldwell, B.A.; Beldin, S.I. Impact of postfire logging on soil bacterial and fungal communities and soil biogeochemistry in a mixed-conifer forest in central Oregon. Plant Soil 2012, 350, 393–411. [Google Scholar] [CrossRef]
- Yu, X.B.; Chen, Q.B.; Wang, S.M.; Mo, X.Y. Research on land degradation in forest plantation and preventive strategies. In Studies on Long-Term Productivity Management of Eucalyptus Plantation; Yu, X.B., Ed.; China Forestry Publishing House: Beijing, China, 2000; pp. 1–7. [Google Scholar]
- Zhang, X.; Kirschbaum, M.U.F.; Hou, Z.; Guo, Z. Carbon stock changes in successive rotations of Chinese fir (Cunninghamia lanceolata (Lamb.) Hook.) plantations. For. Ecol. Manag. 2004, 202, 131–147. [Google Scholar] [CrossRef]
- Kibblewhite, M.G.; Ritz, K.; Swift, M.J. Soil health in agricultural systems. Philos. Trans. R. Soc. Lond. Ser. B 2008, 363, 685–701. [Google Scholar] [CrossRef]
- Brussaard, L. Ecosystem services provided by the soil biota. In Soil Ecology and Ecosystem Services; Wall, D.H., Bardgett, R.D., Behan-Pelletier, V., Herrick, J.E., Jones, H., Ritz, K., Six, J., Strong, D.R., van der Putten, W.H., Eds.; Oxford University Press: Oxford, UK, 2012; pp. 45–58. [Google Scholar]
- Zinn, Y.L.; Resck, D.V.; da Silva, J.E. Soil organic carbon as affected by afforestation with Eucalyptus and Pinus in the Cerrado region of Brazil. For. Ecol. Manag. 2002, 166, 285–294. [Google Scholar] [CrossRef]
- Nave, L.E.; Vance, E.D.; Swanston, C.W.; Curtis, P.S. Harvest impacts on soil carbon storage in temperate forests. For. Ecol. Manag. 2010, 259, 857–866. [Google Scholar] [CrossRef]
- Achat, D.L.; Deleuze, C.; Landmann, G.; Pousse, N.; Ranger, J.; Augusto, L. Quantifying consequences of removing harvesting residues on forest soils and tree growth—A meta-analysis. For. Ecol. Manag. 2015, 348, 124–141. [Google Scholar] [CrossRef]
- Thiffault, E.; Hannam, K.D.; Pare, D.; Titus, B.D.; Hazlett, P.W.; Maynard, D.G.; Brais, S. Effects of forest biomass harvesting on soil productivity in boreal and temperate forests—A review. Environ. Rev. 2011, 19, 278–309. [Google Scholar] [CrossRef]
- Cambi, M.; Grigolato, S.; Neri, F.; Picchio, R.; Marchi, E. Effects of forwarder operation on soil physical characteristics: A case study in the Italian alps. Croat. J. For. Eng. 2016, 37, 233–239. [Google Scholar]





| Samples | Aspect | Angle of the Slope (°) | Slope Position | Canopy Density | Age (YR) | Altitude (m) | Mean DBH (cm) | Mean Tree Height (m) |
|---|---|---|---|---|---|---|---|---|
| Control grassland | North | 1° | Above | - | - | 1657.50 | - | - |
| 1G-16YR | South | 3° | Below | 0.80 | 16 | 1666.20 | 8.80 | 9.40 |
| 1G-28YR | - | - | 0.50 | 28 | 1702.40 | 23.90 | 15.68 | |
| 1G-44YR | North | 5° | Middle | 0.70 | 44 | 1712.00 | 35.50 | 20.50 |
| Clear-cutting forest land | - | - | - | - | 1672.00 | - | - | |
| 2G-16YR | - | - | 0.90 | 16 | 1696.00 | 7.70 | 6.90 | |
| 2G-28YR | South | 2° | Middle | 0.90 | 28 | 1692.20 | 11.00 | 9.60 |
| CG | 1G-16YR | 1G-28YR | 1G-44YR | CC | 2G-16YR | 2G-28YR | |
|---|---|---|---|---|---|---|---|
| SBD | 0.76 ± 0.04 a | 0.29 ± 0.04 c | 0.36 ± 0.04 bc | 0.49 ± 0.01 b | 0.48 ± 0.04 b | 0.27 ± 0.06 c | 0.28 ± 0.04 c |
| NCP | 0.48 ± 0.02 b | 0.25 ± 0.11 b | 0.33 ± 0.07 b | 0.75 ± 0.11 a | 0.42 ± 0.03 b | 0.37 ± 0.06 b | 0.44 ± 0.13 b |
| VH | 0.46 ± 0.05 cd | 0.33 ± 0.09 d | 0.59 ± 0.03 bc | 0.67 ± 0.03 b | 0.90 ± 0.04 a | 0.33 ± 0.03 d | 0.43 ± 0.05 d |
| SOC | 0.24 ± 0.04 c | 0.39 ± 0.06 bc | 0.43 ± 0.04 b | 0.71 ± 0.09 a | 0.36 ± 0.01 bc | 0.43 ± 0.02 b | 0.47 ± 0.02 b |
| PPO | 0.47 ± 0.02 bc | 0.43 ± 0.06 c | 0.58 ± 0.09 ab | 0.63 ± 0.02 a | 0.43 ± 0.01 c | 0.25 ± 0.02 d | 0.45 ± 0.01 bc |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Fahey, T.J.; Liang, D.; Jia, Z.; Ma, L. Effects of Long-Term Successive Rotations, Clear-Cutting and Stand Age of Prince Rupprecht’s larch (Larix principis-rupprechtii Mayr) on Soil Quality. Forests 2019, 10, 932. https://doi.org/10.3390/f10100932
Zhao K, Fahey TJ, Liang D, Jia Z, Ma L. Effects of Long-Term Successive Rotations, Clear-Cutting and Stand Age of Prince Rupprecht’s larch (Larix principis-rupprechtii Mayr) on Soil Quality. Forests. 2019; 10(10):932. https://doi.org/10.3390/f10100932
Chicago/Turabian StyleZhao, Kuangji, Timothy J. Fahey, Dong Liang, Zhongkui Jia, and Lvyi Ma. 2019. "Effects of Long-Term Successive Rotations, Clear-Cutting and Stand Age of Prince Rupprecht’s larch (Larix principis-rupprechtii Mayr) on Soil Quality" Forests 10, no. 10: 932. https://doi.org/10.3390/f10100932





