1. Introduction
Soil is the largest terrestrial carbon reservoir [
1]; the distribution of soil organic carbon in terrestrial ecosystems has, therefore, attracted considerable interest from the perspective of global carbon budget study [
2]. However, soil carbon stocks are highly variable, particularly in sloping landscapes such as hills and mountains.
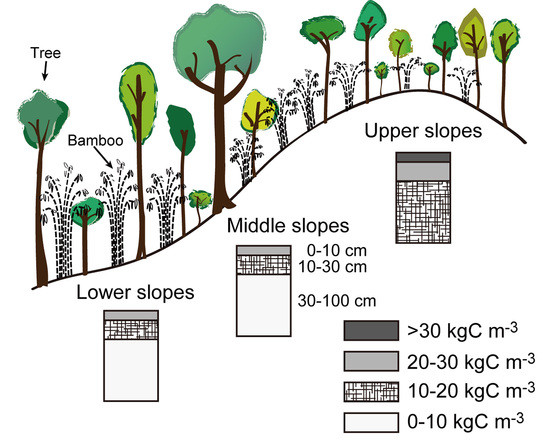
In forest ecosystems, soil organic carbon tends to accumulate in the lower slopes because of deposition of eroded materials from upper slopes, large biomass production, and chemical stabilization by soil minerals [
3,
4]. For example, in the brown forest soils of Japan, the upper-slope stock is 17.2 kg C m
−2 up to depths of 100 cm, whereas the lower-slope stock is 22.0 kg C m
−2 [
5]. Such general tendency has been reported in many countries, including a hill evergreen forest in North Thailand [
6] and a mature mesophytic forest in Kentucky, USA [
7]. However, an atypical tendency, i.e., high carbon stock in the upper slopes and vice versa, has sometimes been reported, for instance, in a lowland evergreen broad-leaved forest in Taiwan [
8], seasonal dry tropical forest in India [
9], and tropical forest in a steepland of Puerto Rico [
10].
In our previous studies on soil respiration at different positions on the slope (lower, upper, and ridge) in a seasonally dry tropical forest in Thailand [
11,
12], we found that carbon release rates by heterotrophic respiration are lower, whereas those by root respiration are higher, in a ridge and an upper slope position than they are in a lower slope position. Further, the distribution of soil carbon stock was found to be atypical, in that the stock was larger in the ridge than in the lower slope, which could be attributed to the differences in carbon cycling among slope positions.
Our aim in this study was to examine the reasons for this atypical distribution of carbon stock. We speculated that the atypical pattern of soil carbon stock is distributed in the whole watershed because soil carbon cycling is restricted by the soil moisture conditions, which are influenced by the slope position of a small watershed unit [
13,
14]. We also assumed that root development, being responsive to soil moisture conditions, influences the variation in soil carbon accumulation. To verify these conjectures, we aimed to compare the densities and stocks of soil carbon and root biomass among different slope positions in a seasonally dry tropical forest.
2. Materials and Methods
2.1. Study Site
The soil survey was conducted at the Mae Klong Watershed Research Station (14°35′ N, 98°52′ E), Kanchanaburi Province, Thailand [
12]. The annual mean air temperature and precipitation of the watershed are 25 °C and 1660 mm, respectively, and the rainy season is from April to October [
15]. The average soil moisture contents (0–30 cm layer) in the upper and lower slopes were, respectively, 0.225 and 0.211 m
3 m
−3 in the rainy season and 0.127 and 0.157 m
3 m
−3 in the dry season [
12]. The seasonally dry tropical forest is classified as mixed deciduous forest (MDF) type, in which the predominant tree species are
Shorea siamensis,
Vitex peduncularis, and
Dillenia parviflora var.
kerrii [
16]. The understory vegetation is characterized by dense bamboo species [
11]. The soil is deeply weathered and well drained, and the predominant soil types are Haplustalfs and Paleustalfs [
17]. The contents (mean ± standard deviation) of clay, silt, and sand in the B horizons, regardless of the soil type, are 385 ± 140, 215 ± 109, and 398 ± 161 g kg
−1, respectively [
18]. The clay mineralogy primarily consists of kaolinite and small quantities of illite.
2.2. Soil Survey and Slope Positions
We used 13 soil profiles reported previously to calculate carbon stock in the soil [
11,
18,
19,
20]. The sites of soil pits were classified according to the relative height of the slope in a small watershed unit into upper, middle, and lower. These positions differ in the relative dryness of soil in the watershed. The locations of the soil sampling sites are shown in
Figure 1.
2.3. Determination of the Density and Stock of Soil Carbon
Soil samples were collected from the soil horizons identified by the soil profile description. The dry bulk density of the soil horizon was measured in a cylindrical core (4 cm high × 100 cm2). Organic carbon concentration in the fine soil (<2 mm) was analyzed using a dry combustion method (Sumigraph NC-22F; Sumika Chemical Analysis Service, Ltd. Tokyo, Japan). All data are expressed on an oven-dry basis (105 °C) (Hot Air Circulating Oven, GT-150PS, ALP CO., Ltd, Tokyo, Japan).
Soil carbon density was calculated from the concentration of carbon and dry bulk density of the soil horizons as follows:
where CC is the carbon concentration of the fine soil in the soil horizon, and DBD is the dry bulk density of the soil horizon.
To compare uniform soil depths, carbon stock in the 0–10 cm (surface), 10–30 cm (subsurface), and 30–100 cm (deep) soil layers was calculated by apportioning the soil horizons. The volume of stones and gravel (>2 mm) was ignored, because their average volume was 0.013 m3 m−3, ranging from 0.001 to 0.030 m3 m−3, in the soil layers.
2.4. Root Density and Biomass Measurement
During the dry season, root density (dry root weight in a unit volume, kg m
−3) and biomass (dry root weight in a sampling layer, kg m
−2) within a 15 cm × 15 cm area in the 0–15, 15–30, 30–60, 60–90, and 90–120 cm soil layers was measured in triplicate in a soil profile using the method described by Takahashi et al. [
12]. Dead roots were removed, and bamboo roots were separated by visual inspection. Measurements were performed at three soil profiles in the lower slopes, three profiles in the middle slopes, and two profiles in the upper slopes. The roots were divided according to their diameter into <1, 1–3, 3–5, 5–10, and >10 mm, and their dry weights at 70 °C were determined.
2.5. Statistical Analysis
The effects of soil layers and slope positions on mean carbon density, dry bulk density, and root density were assessed using two-way analysis of variance (ANOVA). The difference between the slope positions in total soil carbon stocks and root biomass in the soil profiles were analyzed using one-way ANOVA. Because of the differences in the number of pits at each slope position, type III sum of squares was used for the ANOVA. When the result of ANOVA detected a significant difference, a post-hot multiple comparison, the Shaffer’s modified sequentially rejective Bonferroni procedure, was performed. All statistical analyses were performed using R statistical software v. 3.4.0 [
21].
4. Discussion
We speculated that the upper slopes (relatively drier sites) of a small watershed unit have higher soil carbon stocks than the lower slopes. We verified this hypothesis in the watershed where we carried out our study. This atypical pattern of soil carbon accumulation was probably because of the differences in soil carbon cycling along the hillslope, as observed in our previous soil respiration study [
12].
Roots can be an important source, both directly and indirectly, of soil organic matter. In the present study, the average root density of the medium size class in the upper slopes was significantly higher than that in the middle and lower slopes. The development of root systems in the upper slopes might be induced by the responses of tree physiology to dry soil conditions. Under dry soil conditions, photosynthates are preferentially translocated to the belowground parts of plants, thus enhancing the development of long, large root systems that can obtain water from an extensive soil area [
22,
23]. Not only the fine roots with short turnover rates but also the coarse roots can be a source of carbon after their death. Indeed, biomass of the medium and coarse roots was abundant in the 15–60 cm layers, especially in the upper and middle slopes (
Table 3). This high root biomass might contribute to relatively large carbon accumulation in the subsurface soil layers. Further, when coarse roots die and decompose, they create macropores in the soil, which have been recognized to constitute a pathway for carbon migration in deep soil layers [
24]. In addition, root exudates and symbiotic fungi can also be sources of carbon in the soil [
25,
26].
Regarding root decomposition, Rasse et al. [
27] reported that the mean residence time of soil carbon derived from roots is 2.4-fold longer than that derived from aboveground biomass. Furthermore, with respect to soil moisture conditions, Fujimaki et al. [
28] showed that decomposition of the dead fine roots of
Hopea ferrea was faster in a mesic site than in a xeric site in Northern Thailand. Thus, decomposition of roots is expected to be slow in the upper slopes, resulting in the accumulation of soil carbon.
From the perspective of evaluating soil carbon distribution, it is important to take into consideration soil erosion on a slope [
3,
29]. According to the erosion risk evaluated using the Universal Soil Loss Equation (USLE) [
30], the C factor (a factor for the relative effectiveness of cover management in terms of preventing soil loss) is low in Thai forests, with values of 0.02 for MDFs and 0.015 for bamboo forests [
31,
32]. Zhou et al. [
33] indicated that fibrous bamboo root systems that develop in the surface soil protect the soil from the risk of erosion. In Thailand, MDFs are often accompanied by bamboo undergrowth, which may also stabilize the surface soil via root development. Indeed, in the middle slopes, which are steeper than the upper and lower slopes, the abundant bamboo roots might play an important role in preventing sheet erosion. In addition, bamboo species enhance soil carbon sequestration via the occlusion of carbon in silica phytoliths [
34]. Further study, however, is needed to examine the effects of bamboos in enhancing soil carbon accumulation via physical and chemical processes.
Low erosion risk will result in stable stand conditions, which might enable trees to develop large root systems in the soil. In Puerto Rican tabonuco forests, there is a high accumulation of soil carbon along ridges [
10]; these are aged forests in which the stable conditions have allowed large root systems to form. In contrast, the forests on the slopes are young and accumulate low soil carbon because of erosion [
10]. In Thailand, MDFs generally establish on deeply weathered and well-drained soils in limestone areas [
35,
36]. Thus, stable and deeply weathered soils might be a basic requirement for high accumulation of soil carbon by developing large root systems. Indeed, in the watershed, we observed that, if the soil is shallow on steep slopes or narrow ridges, the soil carbon stock is low, leading to the establishment of a different forest type, i.e., dry dipterocarp forest.