Effects of Seed Size and Sand Burial on Germination and Early Growth of Seedlings for Coastal Pinus thunbergii Parl. in the Northern Shandong Peninsula, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Seed Collection
2.3. Seed Germination Test
2.4. Seedling Growth Test
2.5. Data Analysis
3. Results
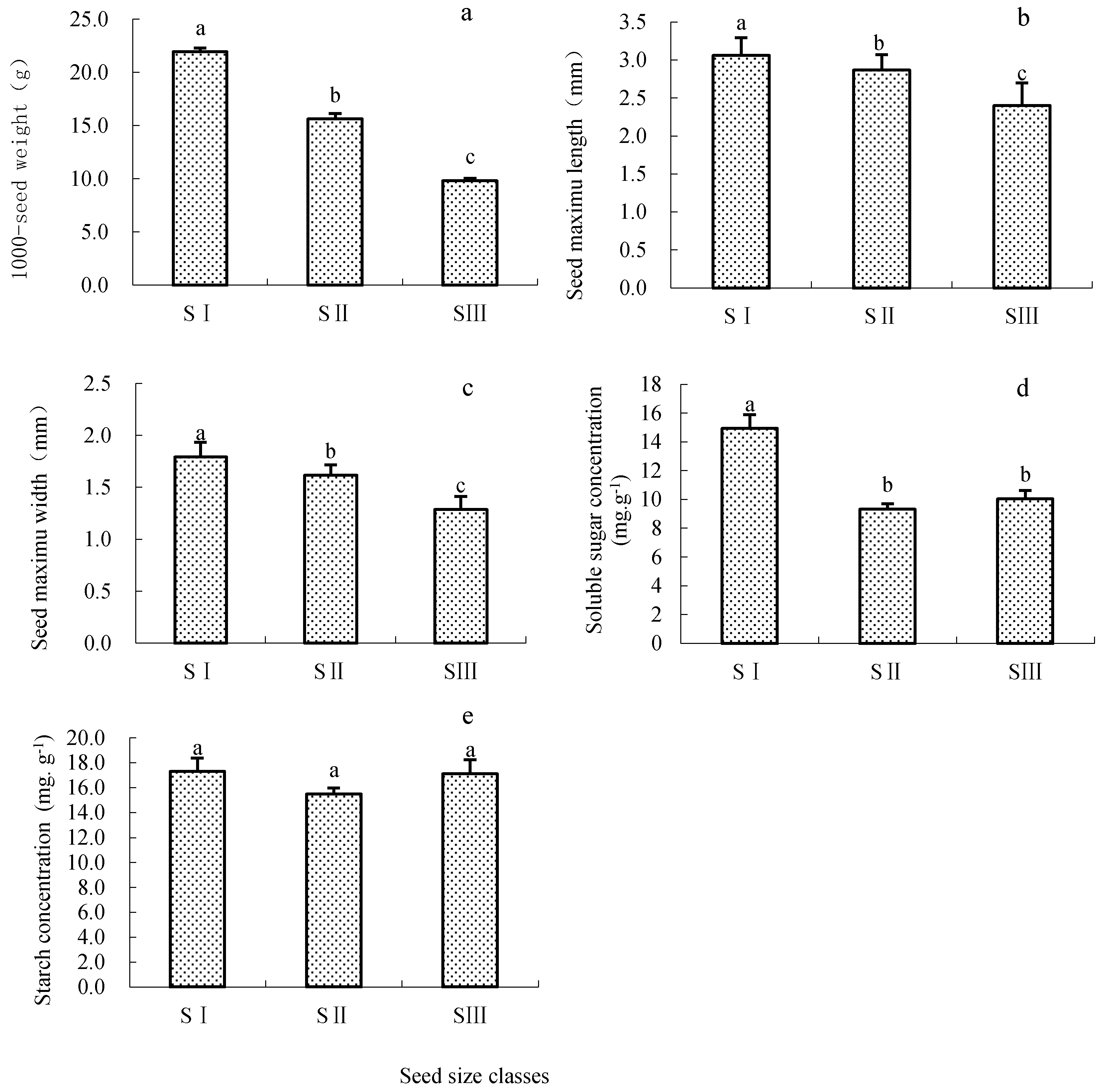
3.1. Characteristics of Seeds in Different Size Groups
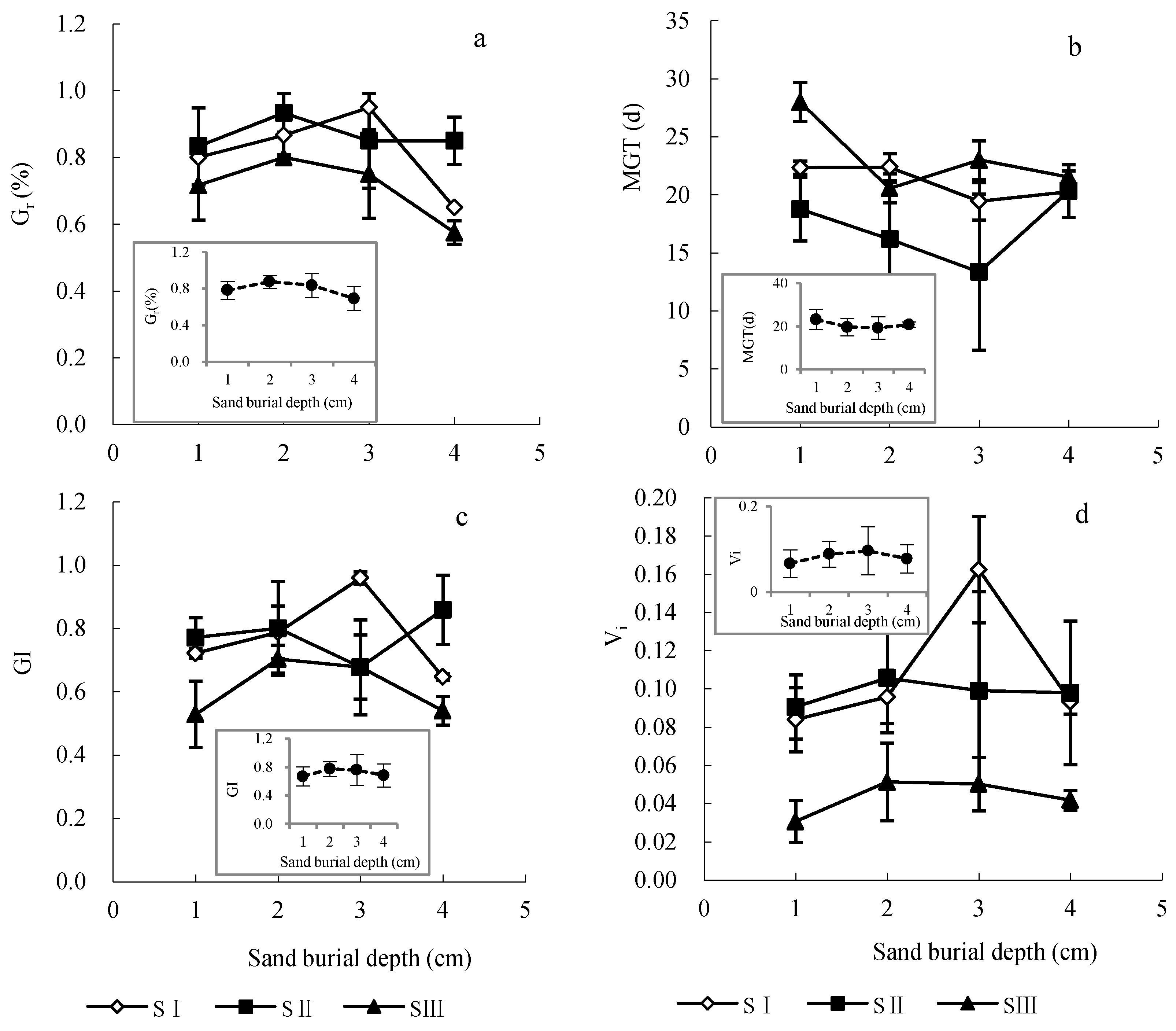
3.2. Effects of Seed Size and Sand Burial Depth on Seed Germination
3.3. Effects of Seed Size and Sand Burial Depth on Seedling Growth
3.4. Effects of Seed Size and Sand Burial Depth on Seedling Biomass Allocation
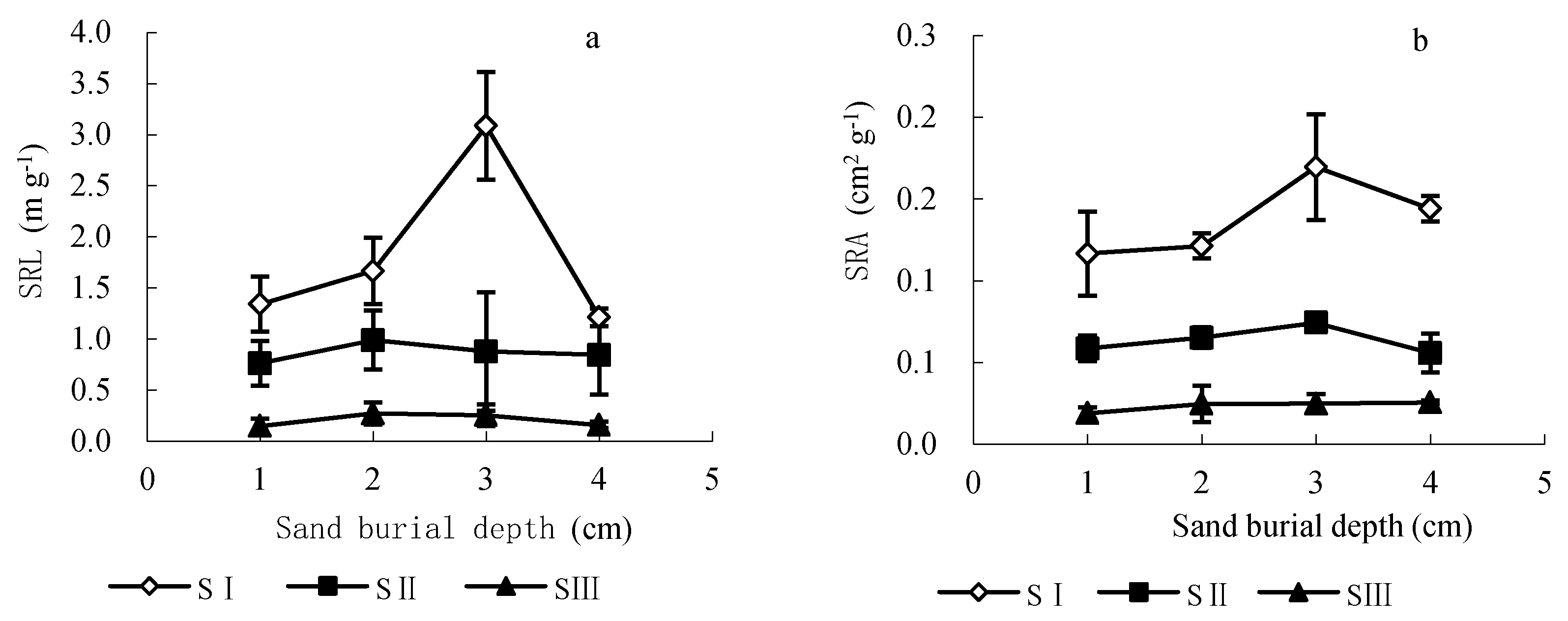
3.5. Effects of Seed Size and Sand Burial Depth on Seedling Root Characteristics
3.6. Correlation Analysis of Seed Germination and Seedling Growth Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Payn, T.; Carnus, J.M.; Freer-Smith, P.; Kimberley, M.; Kollert, W.; Liu, S.; Orazio, C.; Rodriguez, L.; Silva, L.N.; Wingfield, M.J. Changes in planted forests and future global implications. For. Ecol. Manag. 2015, 352, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Yang, Y.; Wang, H. Development strategy and management countermeasures of planted forests in China: Transforming from timber-centered single objective management towards multi-purpose management for enhancing quality and benefits of ecosystem services. Acta Ecol. Sin. 2018, 38, 1–10. [Google Scholar]
- Taniguchi, T.; Tamai, S.; Yamanaka, N.; Futai, K. Inhibition of the regeneration of Japanese black pine (Pinus thunbergii) by black locust (Robinia pseudoacacia) in coastal sand dunes. J. For. Res. 2017, 12, 350–357. [Google Scholar] [CrossRef]
- Hayasaka, D.; Fujiwara, K.; Box, E.O. Recovery of sandy beach and maritime forest vegetation on Phuket Island (Thailand) after the major Indian Ocean tsunami of 2004. Appl. Veg. Sci. 2009, 12, 211–224. [Google Scholar] [CrossRef]
- Zhu, J.; Gonda, Y.; Yu, L.; Li, F.; Yan, Q.; Sun, Y. Regeneration of a coastal pine (Pinus thunbergii Parl.) forest 11 years after thinning, Niigata, Japan. PLoS ONE 2012, 7, e47593. [Google Scholar] [CrossRef] [PubMed]
- Badalamenti, E.; da Silveira Bueno, R.; Campo, O.; Gallo, M.; La Mela Veca, D.; Pasta, S.; Sala, G.; La Mantia, T. Pine stand density influences the regeneration of Acacia saligna Labill. H.L. Wendl. and native woody species in a Mediterranean coastal pine plantation. Forests 2018, 9, 359. [Google Scholar] [CrossRef]
- Han, G.; Mao, P.; Liu, S.; Wang, G.; Zhang, Z.; Xue, Q. Effects of sea water salinity and mother tree size on the seed germination and seedling early growth of Pinus thunbergii coastal protection forest. Chin. J. Ecol. 2009, 28, 2171–2176. [Google Scholar]
- Mao, P.; Yu, X.; Cao, B.; Ma, X.; Zhai, Q.; Dong, J.; Zhao, S.; Li, X. Effects of water stress and mother tree age on seed germination of the coastal Pinus thunbergii protection forest in Northern Shandong peninsula. Seed 2016, 35, 10–13, 17. [Google Scholar]
- Mao, P.; Mu, H.; Cao, B.; Liu, Y.; Fan, Z.; Wang, S. Effects of sand burial and overstory tree age on seedling establishment in coastal Pinus thunbergii forests in the northern Shandong Peninsula, China. For. Chron. 2016, 92, 357–365. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, G.; Mao, P.; Wang, G.; Xue, Q. Effects of adult tree density, distance to coast and understory on natural regeneration of Pinus thunbergii coastal protection forests in Yantai region. J. Nat. Resour. 2009, 24, 782–790. [Google Scholar]
- Han, G.; Wang, G.; Zhang, Z.; Li, Q.; Xue, Q. Population structure of the Pinus thunbergii coastal protection forest and its spatial variation at different distance to coastline in Yantai. Sci. Silvae Sin. 2008, 44, 8–13. [Google Scholar]
- Mao, P.; Han, G.; Wang, G.; Yu, J.; Shao, H. Effects of age and stand density of mother trees on early Pinus thunbergii seedling establishment in the coastal zone, China. Sci. World J. 2014, 2014, 468036. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, Y. Signaling pathways of seed size control in plants. Curr. Opin. Plant Biol. 2016, 33, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Larios, E.; Búrquez, A.; Becerra, J.X.; Venable, D.L. Natural selection on seed size through the life cycle of a desert annual plant. Ecology 2014, 95, 3213–3220. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Chu, G.; Jiang, P.; Niu, P.; Wang, M. Effects of sand burial and seed size on seed germination, seedling emergence and seedling biomass of Anabasis aphylla. Pak. J. Bot. 2017, 49, 391–396. [Google Scholar]
- Baraloto, C.; Forget, P.M.; Goldberg, D.E. Seed mass, seedling size and neotropical tree seedling establishment. J. Ecol. 2005, 9393, 1156–1166. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, Z.; Chu, Y.; Zhang, S.; Liu, H.; Dong, M. Effects of burial in sand and seed size on seed germination and seedling emergence in two leguminous shrubs in the Otindag Sandland, China. Isr. J. Plant Sci. 2004, 52, 133–142. [Google Scholar] [CrossRef]
- Chen, H.; Maun, M.A. Effects of sand burial depth on seed germination and seedling emergence of Cirsium pitcher. Plant Ecol. 1999, 140, 53–60. [Google Scholar] [CrossRef]
- Venable, D.L.; Brown, J.S. The selective interactions of dispersal, dormancy, and seed size as adaptations for reducing risk in variable environments. Am. Nat. 1988, 131, 360–384. [Google Scholar] [CrossRef]
- Chacón, P.; Bustamante, R.O. The effects of seed size and pericarp on seedling recruitment and biomass in Cryptocarya alba (Lauraceae) under two contrasting moisture regimes. Plant Ecol. 2001, 152, 137–144. [Google Scholar] [CrossRef]
- Guo, C.; Wang, Z.; Lu, J. Seed germination and seedling development of Prunus armeniaca under different burial depths in soil. J. For. Res. 2010, 21, 492–496. [Google Scholar] [CrossRef]
- Liu, H.; Shi, X.; Wang, J.; Yin, L.; Huang, Z.; Zhang, D. Effects of sand burial, soil water content and distribution pattern of seeds in sand on seed germination and seedling survival of Eremosparton songoricum (Fabaceae), a rare species inhabiting the moving sand dunes of the Gurbantunggut Desert of China. Plant Soil 2011, 345, 69–87. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, X.; Baskin, C.C.; Baskin, J.M.; Dong, M.; Huang, Z. Effects of amount and frequency of precipitation and sand burial on seed germination, seedling emergence and survival of the dune grass Leymus secalinus in semiarid China. Plant Soil 2013, 374, 399–409. [Google Scholar] [CrossRef]
- Harper, J.L.; Benton, R.A. The behaviour of seeds in soil. The germination of seeds on the surface of a water supplying substrate. J. Ecol. 1966, 54, 151–156. [Google Scholar] [CrossRef]
- Vleeshouwers, L.M. Modeling the effect of temperature, soil penetration resistance, burial depth and seed weight on pre-emergence growth of weeds. Ann. Bot. Lond. 1997, 79, 553–563. [Google Scholar] [CrossRef]
- Cendán, C.; Sampedro, L.; Zas, R. The maternal environment determines the timing of germination in Pinus pinaster. Environ. Exp. Bot. 2013, 94, 66–72. [Google Scholar] [CrossRef]
- Zhao, S.; Shi, G.; Dong, X. Techniques of Plant Physiological Experiment; China Agricultural Science Press: Beijing, China, 2002; pp. 83–88. [Google Scholar]
- Huang, Z.; Footitt, S.; Tang, A.; Finch-Savage, W.E. Predicted global warming scenarios impact on the mother plant to alter seed dormancy and germination behaviour in Arabidopsis. Plant Cell Environ. 2018, 41, 187–197. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Wang, N.; Jiang, X.; Mao, H.; Zhu, C.; Wen, F.; Wang, X.; Lu, Z.; Yue, G.; et al. Manipulation of Auxin Response Factor 19 affects seed size in the woody perennial Jatropha curcas. Sci. Rep. 2017, 7, 40844. [Google Scholar] [CrossRef]
- Weller, S.G. Establishment of Lithospermum caroliniense on sand dunes: The role of nutlet mass. Ecology 1985, 66, 1893–1901. [Google Scholar] [CrossRef]
- Zheng, M.; Lai, L.; Jiang, L.; An, P.; Yu, Y.; Zheng, Y.; Shimizu, H.; Baskin, J.M.; Baskin, C.C. Moderate water supply and partial sand burial increase relative growth rate of two Artemisia species in an inland sandy land. J. Arid Environ. 2012, 85, 105–113. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Gross, K.L. Effects of seed size and growth from on seedling establishment of six monocarpic perennial plants. J. Ecol. 1984, 72, 369–387. [Google Scholar] [CrossRef]
- Saeed, S.; Shaukat, S.S. Effect of seed size on germination, emergence, growth and seedling survival of Senna occidentalis Link. Pak. J. Bot. Sci. 2000, 33, 292–295. [Google Scholar]
- Brown, J.F. Effects of experimental burial on survival, growth, and resource allocation of three species of dune plants. J. Ecol. 1997, 85, 151–158. [Google Scholar] [CrossRef]
- Xu, L.; Huber, H.; During, H.J.; Dong, M.; Anten, N.P. Intraspecific variation of a desert shrub species in phenotypic plasticity in response to sand burial. New Phytol. 2013, 199, 991–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, A.; Rewald, B.; Rachmilevitch, S. Belowground dynamics in two olive varieties as affected by saline irrigation. Sci. Hortic. Amst. 2013, 162, 313–319. [Google Scholar] [CrossRef]
- Frosini, S.; Lardicci, C.; Balestri, E. Global change and response of coastal dune plants to the combined effects of increased sand accretion (burial) and nutrient availability. PLoS ONE 2012, 7, e47561. [Google Scholar] [CrossRef] [PubMed]
- Sykes, M.T.; Wilson, J.B. An experimental investigation into the response of New Zealand sand dune species to different depths of burial by sand. Acta Bot. Neerl. 1990, 39, 17–181. [Google Scholar] [CrossRef]
- Paz, H.; Mazer, S.J.; Martinez-Ramos, M. Comparative ecology of seed mass in Psychotria (Rubiaceae): Within- and between-species effects of seed mass on early performance. Funct. Ecol. 2005, 19, 707–718. [Google Scholar] [CrossRef]


| Indexes | Seed Size | Sand Burial Depth | Seed Size × Sand Burial Depth | |||
|---|---|---|---|---|---|---|
| F Value | p Value | F Value | p Value | F Value | p Value | |
| Germination rate | 8.43 | <0.01 | 5.70 | <0.01 | 1.21 | 0.35 |
| Mean germination time | 11.92 | <0.01 | 3.41 | <0.05 | 1.75 | 0.17 |
| Germination index | 5.08 | <0.05 | 1.14 | 0.36 | 1.44 | 0.26 |
| Vigor index | 22.16 | <0.01 | 3.06 | 0.07 | 1.57 | 0.22 |
| Seedling height | 58.74 | <0.01 | 19.56 | <0.01 | 5.62 | <0.01 |
| Ground diameter | 27.70 | <0.01 | 3.25 | <0.05 | 3.05 | <0.05 |
| Seedling biomass | 38.85 | <0.01 | 4.52 | <0.05 | 1.32 | 0.30 |
| Root mass ratio | 20.50 | <0.01 | 0.34 | 0.80 | 2.25 | 0.09 |
| Stem mass ratio | 4.64 | <0.05 | 0.83 | 0.50 | 0.46 | 0.83 |
| Leaf mass ratio | 18.47 | <0.01 | 0.28 | 0.84 | 2.39 | 0.07 |
| Root/shoot ratio | 17.28 | <0.01 | 0.26 | 0.85 | 1.92 | 0.14 |
| Specific root length | 75.12 | <0.01 | 8.21 | <0.01 | 6.16 | <0.01 |
| Specific root area | 210.40 | <0.01 | 5.53 | <0.01 | 2.65 | 0.05 |
| Index | SB | SH | GD | Gr | MGT | GI | Vi | RMR | SMR | LMR | RSR | SRL | SRA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SB | 1.00 | 0.83 ** | −0.23 | 0.48 ** | −0.56 ** | 0.51 ** | 0.82 ** | −0.56 ** | −0.12 | 0.68 ** | −0.58 ** | 0.79 ** | 0.83 ** |
| SH | 1.00 | −0.04 | 0.41 * | −0.52 ** | 0.47 * | 0.69 ** | −0.49 ** | 0.01 | 0.54 ** | −0.49 ** | 0.67 ** | 0.73 ** | |
| GD | 1.00 | 0.05 | 0.05 | 0.07 | −0.11 | −0.11 | 0.44 * | −0.10 | −0.12 | −0.11 | −0.15 | ||
| Gr | 1.00 | −0.32 | 0.81 ** | 0.80 ** | −0.15 | −0.22 | 0.27 | −0.17 | 0.57 ** | 0.32 | |||
| MGT | 1.00 | −0.15 | −0.35 | 0.24 | 0.27 | −0.39 * | −0.27 | −0.18 | −0.25 | ||||
| GI | 1.00 | 0.86 ** | −0.31 | 0.06 | 0.31 | −0.33 | 0.69 ** | 0.44 * | |||||
| Vi | 1.00 | −0.40 * | −0.12 | 0.50 ** | −0.42 * | 0.86 ** | 0.68 ** | ||||||
| RMR | 1.00 | −0.42 * | −0.89 ** | 1.00 ** | −0.57 ** | −0.72 ** | |||||||
| SMR | 1.00 | −0.04 | −039 * | 0.11 | 0.19 | ||||||||
| LMR | 1.00 | −0.90 ** | 0.57 ** | 0.70 ** | |||||||||
| RSR | 1.00 | −0.58 ** | −0.71 ** | ||||||||||
| SRL | 1.00 | 0.90 ** | |||||||||||
| SRA | 1.00 |
| Index | The First Principal Component | The Second Principal Component | The Third Principal Component | The Fourth Principal Component |
|---|---|---|---|---|
| SB | 0.74 | 0.46 | −0.27 | −0.27 |
| SH | 0.65 | 0.43 | −0.08 | −0.30 |
| GD | −0.08 | 0.05 | 0.93 | −0.06 |
| Gr | 0.12 | 0.89 | 0.04 | −0.20 |
| MGT | −0.33 | −0.14 | 0.01 | 0.87 |
| GI | 0.27 | 0.89 | 0.13 | 0.08 |
| Vi | 0.49 | 0.85 | −0.13 | −0.08 |
| RMR | −0.94 | 0.03 | −0.24 | −0.06 |
| SMR | 0.28 | −0.15 | 0.65 | 0.53 |
| LMR | 0.90 | 0.05 | −0.06 | −0.20 |
| RSR | −0.94 | 0.01 | −0.25 | −0.02 |
| SRL | 0.68 | 0.63 | −0.14 | 0.19 |
| SRA | 0.86 | 0.32 | −0.16 | 0.15 |
| Eigenvalue | 5.27 | 3.25 | 1.56 | 1.36 |
| Variance contribution rate (%) | 40.57 | 25.01 | 11.99 | 10.44 |
| Accumulated contribution rate (%) | 40.57 | 65.58 | 77.56 | 88.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, P.; Guo, L.; Gao, Y.; Qi, L.; Cao, B. Effects of Seed Size and Sand Burial on Germination and Early Growth of Seedlings for Coastal Pinus thunbergii Parl. in the Northern Shandong Peninsula, China. Forests 2019, 10, 281. https://doi.org/10.3390/f10030281
Mao P, Guo L, Gao Y, Qi L, Cao B. Effects of Seed Size and Sand Burial on Germination and Early Growth of Seedlings for Coastal Pinus thunbergii Parl. in the Northern Shandong Peninsula, China. Forests. 2019; 10(3):281. https://doi.org/10.3390/f10030281
Chicago/Turabian StyleMao, Peili, Longmei Guo, Yunxiao Gao, Lin Qi, and Banghua Cao. 2019. "Effects of Seed Size and Sand Burial on Germination and Early Growth of Seedlings for Coastal Pinus thunbergii Parl. in the Northern Shandong Peninsula, China" Forests 10, no. 3: 281. https://doi.org/10.3390/f10030281




