Response of Soil Surface Respiration to Storm and Ips typographus (L.) Disturbance in Boreal Norway Spruce Stands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Area
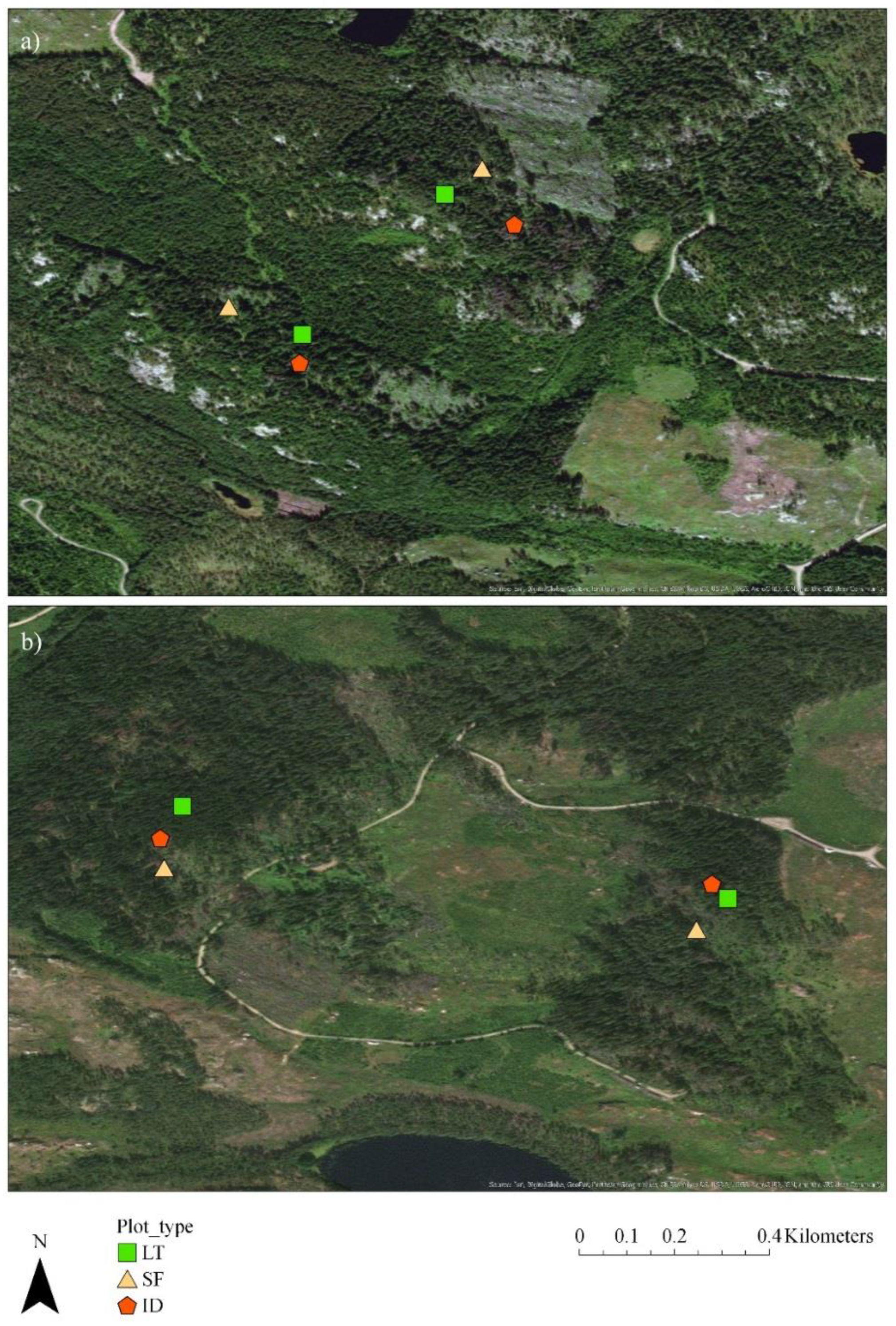
2.2. Study Layout
2.3. Tree Measurements
2.4. Soil Surface Respiration, Temperature, and Moisture Measurements
2.5. Calculation of Soil Surface Total, Heterotrophic, and Autotrophic Respiration
2.6. Statistical Analyses
3. Results
3.1. Tree Mortality
3.2. Soil Surface Total Respiration, Soil Temperature, and Soil Moisture
3.3. Heterotrophic and Autotrophic Soil Respiration
3.4. Relationships between Variables
4. Discussion
4.1. Soil Surface Total, Autotrophic, and Heterotrophic Respiration
4.2. Relationships between Basal Area, Soil Microclimate, and Respiration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mitchell, S.J. Wind as a natural disturbance agent in forests: A synthesis. Forestry 2013, 86, 147–157. [Google Scholar] [CrossRef]
- Edburg, S.L.; Hicke, J.A.; Brooks, P.D.; Pendall, E.G.; Brent, E.; Norton, U.; Gochis, D.; Gutmann, E.D.; Meddens, A.J.H.; Edburg, S.L.; et al. Cascading impacts of bark beetle-c mortality on coupled biogeophysic biogeochemical processes. Front. Ecol. Environ. 2012, 10, 416–424. [Google Scholar] [CrossRef]
- Knohl, A. Carbon exchange of a Russian boreal forest after windthrow Carbon dioxide exchange of a Russian boreal forest after disturbance by wind throw. Glob. Chang. Biol. 2002, 8, 231–246. [Google Scholar] [CrossRef]
- Kurz, W.A.; Dymond, C.C.; Stinson, G.; Rampley, G.J.; Neilson, E.T.; Carroll, A.L.; Ebata, T.; Safranyik, L. Mountain pine beetle and forest carbon feedback to climate change. Nature 2008, 452, 987–990. [Google Scholar] [CrossRef]
- Hicke, J.A.; Allen, C.D.; Desai, A.R.; Dietze, M.C.; Hall, R.J.; Hogg, E.H.T.; Kashian, D.M.; Moore, D.; Raffa, K.F.; Sturrock, R.N.; et al. Effects of biotic disturbances on forest carbon cycling in\r\nthe United States and Canada. Glob. Chang. Biol. 2012, 18, 7–34. [Google Scholar] [CrossRef]
- Mayer, M.; Sandén, H.; Rewald, B.; Godbold, D.L.; Katzensteiner, K. Increase in heterotrophic soil respiration by temperature drives decline in soil organic carbon stocks after forest windthrow in a mountainous ecosystem. Funct. Ecol. 2017, 31, 1163–1172. [Google Scholar] [CrossRef]
- Lindroth, A.; Lagergren, F.; Grelle, A.; Klemedtsson, L.; Langvall, O.; Weslien, P.; Tuulik, J. Storms can cause Europe-wide reduction in forest carbon sink. Glob. Chang. Biol. 2009, 15, 346–355. [Google Scholar] [CrossRef]
- Brown, M.; Black, T.A.; Nesic, Z.; Foord, V.N.; Spittlehouse, D.L.; Fredeen, A.L.; Grant, N.J.; Burton, P.J.; Trofymow, J.A. Impact of mountain pine beetle on the net ecosystem production of lodgepole pine stands in British Columbia. Agric. For. Meteorol. 2010, 150, 254–264. [Google Scholar] [CrossRef]
- Moore, D.J.P.; Trahan, N.A.; Wilkes, P.; Quaife, T.; Stephens, B.B.; Elder, K.; Desai, A.R.; Negron, J.; Monson, R.K. Persistent reduced ecosystem respiration after insect disturbance in high elevation forests. Ecol. Lett. 2013, 16, 731–737. [Google Scholar] [CrossRef]
- Hicke, J.A.; Meddens, A.J.H.; Allen, C.D.; Kolden, C.A. Carbon stocks of trees killed by bark beetles and wildfire in the western United States. Environ. Res. Lett. 2013, 8, 035032. [Google Scholar] [CrossRef]
- Hansen, E.M.; Amacher, M.C.; Van Miegroet, H.; Long, J.N.; Ryan, M.G. Carbon dynamics in central US Rockies lodgepole pine type after mountain pine beetle outbreaks. For. Sci. 2015, 61, 665–679. [Google Scholar] [CrossRef]
- Reed, D.E.; Ewers, B.E.; Pendall, E. Impact of mountain pine beetle induced mortality on forest carbon and water fluxes. Environ. Res. Lett. 2014, 9, 105004. [Google Scholar] [CrossRef]
- Jonášová, M.; Prach, K. The influence of bark beetles outbreak vs. salvage logging on ground layer vegetation in Central European mountain spruce forests. Biol. Conserv. 2008, 141, 1525–1535. [Google Scholar] [CrossRef]
- Seidl, R.; Rammer, W.; Jäger, D.; Lexer, M.J. Impact of bark beetle (Ips typographus L.) disturbance on timber production and carbon sequestration in different management strategies under climate change. For. Ecol. Manag. 2008, 256, 209–220. [Google Scholar] [CrossRef]
- Jonášová, M.; Vávrová, E.; Cudlín, P. Western Carpathian mountain spruce forest after a windthrow: Natural regeneration in cleared and uncleared areas. For. Ecol. Manag. 2010, 259, 1127–1134. [Google Scholar] [CrossRef]
- Schlesinger, W.; Andrews, J. Soil Respiration and Global Carbon Cycle. Biogeochemistry 2000, 48, 7–20. [Google Scholar] [CrossRef]
- Janssens, I.A.; Lankreijer, H.; Matteucci, G.; Kowalski, A.S.; Buchmann, N.; Epron, D.; Pilegaard, K.; Kutsch, W.; Longdoz, B.; Grünwald, T.; et al. Productivity overshadows temperature in determining soil and ecosystem respiration across European forests. Glob. Chang. Biol. 2002, 7, 269–278. [Google Scholar] [CrossRef]
- Bhupinderpal-Singh; Nordgren, A.; Löfvenius Ottosson, M.; Högberg, M.N.; Mellander, P.E.; Högberg, P. Tree root and soil heterotrophic respiration as revealed by girdling of boreal Scots pine forest: Extending observations beyond the first year. Plant Cell Environ. 2003, 26, 1287–1296. [Google Scholar] [CrossRef]
- Högberg, P.; Bhupinderpal-Singh; Löfvenius, M.O.; Nordgren, A. Partitioning of soil respiration into its autotrophic and heterotrophic components by means of tree-girdling in old boreal spruce forest. For. Ecol. Manag. 2009, 257, 1764–1767. [Google Scholar]
- Sariyildiz, T.; Akkuzu, E.; Küçük, M.; Duman, A.; Aksu, Y. Effects of Ips typographus (L.) damage on litter quality and decomposition rates of oriental spruce [Picea orientalis (L.) Link.] in Hatila Valley National Park, Turkey. Eur. J. For. Res. 2008, 127, 429–440. [Google Scholar] [CrossRef]
- Kopáček, J.; Cudlín, P.; Fluksová, H.; Kaňa, J.; Picek, T.; Šantrůčková, H.; Svoboda, M.; Vaněk, D. Dynamics and composition of litterfall in an unmanaged Norway spruce (Picea abies) forest after bark-beetle outbreak. Boreal Environ. Res. 2015, 20, 305–323. [Google Scholar]
- Morehouse, K.; Johns, T.; Kaye, J.; Kaye, M. Carbon and nitrogen cycling immediately following bark beetle outbreaks in southwestern ponderosa pine forests. For. Ecol. Manag. 2008, 255, 2698–2708. [Google Scholar] [CrossRef]
- Mayer, M.; Matthews, B.; Schindlbacher, A.; Katzensteiner, K. Soil CO2 efflux from mountainous windthrow areas: Dynamics over 12 years post-disturbance. Biogeosciences 2014, 11, 6081–6093. [Google Scholar] [CrossRef]
- Štursová, M.; Šnajdr, J.; Cajthaml, T.; Bárta, J.; Šantrůčková, H.; Baldrian, P. When the forest dies: The response of forest soil fungi to a bark beetle-induced tree dieback. ISME J. 2014, 8, 1920–1931. [Google Scholar] [CrossRef]
- Mikkelson, K.M.; Brouillard, B.M.; Bokman, C.M.; Sharpa, J.O. Ecosystem resilience and limitations revealed by soil bacterial community dynamics in a bark beetle-impacted forest. MBio 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Pec, G.J.; Karst, J.; Taylor, D.L.; Cigan, P.W.; Erbilgin, N.; Cooke, J.E.K.; Simard, S.W.; Cahill, J.F. Change in soil fungal community structure driven by a decline in ectomycorrhizal fungi following a mountain pine beetle (Dendroctonus ponderosae) outbreak. New Phytol. 2017, 213, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Lindner, M.; Abs, C.; Lasch, P. Vegetation dynamics in central european forest ecosystems (near-natural as well as managed) after storm events. Folia Geobot. 2002, 37, 17–32. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, X.; Zhou, L.; Ju, R. A global synthesis of below-ground carbon responses to biotic disturbance: A meta-analysis. Glob. Ecol. Biogeogr. 2015, 24, 126–138. [Google Scholar] [CrossRef]
- Kobler, J.; Jandl, R.; Dirnböck, T.; Mirtl, M.; Schindlbacher, A. Effects of stand patchiness due to windthrow and bark beetle abatement measures on soil CO2 efflux and net ecosystem productivity of a managed temperate mountain forest. Eur. J. For. Res. 2015, 134, 683–692. [Google Scholar] [CrossRef]
- Köster, K.; Püttsepp, Ü.; Pumpanen, J. Comparison of soil CO2 flux between uncleared and cleared windthrow areas in Estonia and Latvia. For. Ecol. Manag. 2011, 262, 65–70. [Google Scholar] [CrossRef]
- Borkhuu, B.; Peckham, S.D.; Ewers, B.E.; Norton, U.; Pendall, E. Does soil respiration decline following bark beetle induced forest mortality? Evidence from a lodgepole pine forest. Agric. For. Meteorol. 2015, 214–215, 201–207. [Google Scholar] [CrossRef]
- Schelhaas, M.-J.; Nabuurs, G.-J.; Schuck, A. Natural disturbances in the European forests in the 19th and 20th centuries. Glob. Chang. Biol. 2003, 9, 1620–1633. [Google Scholar] [CrossRef]
- Jeger, M.; Bragard, C.; Caffier, D.; Candresse, T.; Chatzivassiliou, E.; Dehnen-Schmutz, K.; Gilioli, G.; Jaques Miret, J.A.; MacLeod, A.; Navajas Navarro, M.; et al. Pest categorisation of Ips amitinus. EFSA J. 2017, 15, 5038. [Google Scholar]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Seidl, R.; Schelhaas, M.J.; Lexer, M.J. Unraveling the drivers of intensifying forest disturbance regimes in Europe. Glob. Chang. Biol. 2011, 17, 2842–2852. [Google Scholar] [CrossRef]
- Ulanova, N.G. The effects of windthrow on forest at different spatial scales: A review. For. Ecol. Manag. 2000, 135, 155–167. [Google Scholar] [CrossRef]
- Pumpanen, J.; Westman, C.J.; Ilvesniemi, H. Soil CO2 efflux from a podzolic forest soil before and after forest clear-cutting and site preparation. Boreal Environ. Res. 2004, 9, 199–212. [Google Scholar]
- Müller, K.H.; Wagner, S. Fine root dynamics in gaps of Norway spruce stands in the German Ore Mountains. Forestry 2003, 76, 149–158. [Google Scholar] [CrossRef]
- Gray, A.N.; Spies, T.A.; Easter, M.J. Microclimatic and soil moisture responses to gap formation in coastal Douglas-fir forests. Can. J. For. Res. 2002, 32, 332–343. [Google Scholar] [CrossRef]
- Wermelinger, B. Ecology and management of the spruce bark beetle Ips typographus—A review of recent research. For. Ecol. Manag. 2004, 202, 67–82. [Google Scholar] [CrossRef]
- Eriksson, M.; Pouttu, A.; Roininen, H. The influence of windthrow area and timber characteristics on colonization of wind-felled spruces by Ips typographus (L.). For. Ecol. Manag. 2005, 216, 105–116. [Google Scholar] [CrossRef]
- Mikola, P. Application of vegetation science to forestry in Finland. In Handbook of Vegetation Science, Part 12; Jahn, G., Ed.; Dr W. Junk Publishers: The Hague, The Netherlands; Boston, MA, USA; London, UK, 1982; pp. 199–224. [Google Scholar]
- Pirinen, P.; Simola, H.; Aalto, J.; Kaukoranta, J.-P.; Karlsson, P.; Ruuhela, R. CLIMATOLOGICAL statistics of Finland 1981–2010; Reports 2012-1; Finnish Meteorological Institute: Helsinki, Finland, 2012. [Google Scholar]
- Finnish Meteorological Institute. Open Source Weather Observations. Available online: https://en.ilmatieteenlaitos.fi/download-observations#!/ (accessed on 19 October 2018).
- Hurlbert, S.H. Pseudoreplication and the Design of Ecological Field Experiments. Ecol. Monogr. 1984, 54, 187–211. [Google Scholar] [CrossRef]
- Blomqvist, M.; Kosunen, M.; Starr, M.; Kantola, T.; Holopainen, M.; Lyytikäinen-Saarenmaa, P. Modelling the predisposition of Norway spruce to Ips typographus L. infestation by means of environmental factors in southern Finland. Eur. J. For. Res. 2018, 137, 675–691. [Google Scholar] [CrossRef]
- Lyytikäinen-Saarenmaa, P.; (Department of Forest Sciences, University of Helsinki). Personal communication, 2014.
- Finnish Meteorological Institute. Annual Weather Statistics. Available online: https://ilmatieteenlaitos.fi/vuositilastot (accessed on 3 January 2019).
- Helmisaari, H.-S.; Derome, J.; Nojd, P.; Kukkola, M. Fine root biomass in relation to site and stand characteristics in Norway spruce and Scots pine stands. Tree Physiol. 2007, 27, 1493–1504. [Google Scholar] [CrossRef]
- Zehetgruber, B.; Kobler, J.; Dirnböck, T.; Jandl, R.; Seidl, R.; Schindlbacher, A. Intensive ground vegetation growth mitigates the carbon loss after forest disturbance. Plant Soil 2017, 420, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.; Taylor, J. On the Temperature Dependence of Soil Respiration. Funct. Ecol. 1994, 8, 315–323. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 2nd ed.; Sage: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Lenth, R. Emmeans: Estimated Marginal Means, aka Least-Squares Means; R Core Team: Vienna, Austria, 2018. [Google Scholar]
- Seidl, R.; Blennow, K. Pervasive Growth Reduction in Norway Spruce Forests following Wind Disturbance. PLoS ONE 2012, 3, e33301. [Google Scholar] [CrossRef]
- Högberg, M.N.; Högberg, P.; Högberg, M.N. Extramatrical ectomycorrhizal mycelium contributes one-third of microbial biomass and produces, together with associated roots, half the dissolved organic carbon in a forest soil. New Phytol. 2002, 791–795. [Google Scholar] [CrossRef]
- Lõhmus, K.; Ivask, M. Decomposition and nitrogen dynamics of fine roots of Norway spruce (Picea abies (L.) Karst.) at different sites. Plant Soil 1995, 168, 89–94. [Google Scholar] [CrossRef]
- Hyvonen, R.; Olsson, B.A.; Lundkvist, H.; Staaf, H. Decomposition and nutrient release from Picea abies (L.) Karst. and Pinus sylvestris L. logging residues. For. Ecol. Manag. 2000, 126, 97–112. [Google Scholar] [CrossRef]



| Basal Area (m2/ha) | Species (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Year | Plot Type | Site Type | dbh (cm) | Stems/ha | Living | Dead | Total | Sp | Pi | De |
| PS | 2015 | LT | MT | 21 ± 8 | 1350 | 42.2 ± 0.6 | 9.3 ± 1.4 | 51.5 ± 0.8 | 65 | 22 | 13 |
| PS | 2015 | SF | MT | 22 ± 7 | 925 | 13.6 ± 0.7 | 24.6 ± 0.6 | 38.2 ± 0.7 | 81 | 14 | 5 |
| PS | 2015 | ID | OMT | 25 ± 8 | 625 | 5.5 ± 1.2 | 27.4 ± 0.7 | 33.0 ± 0.8 | 96 | 0 | 4 |
| PS | 2016 | LT | MT | 20 ± 10 | 925 | 34.3 ± 0.8 | 0.9 ± 0.2 | 35.2 ± 0.8 | 89 | 0 | 11 |
| PS | 2016 | SF | MT | 17 ± 7 | 1725 | 10.6 ± 0.3 | 36.8 ± 0.7 | 47.4 ± 0.6 | 88 | 0 | 12 |
| PS | 2016 | ID | MT | 15 ± 7 | 1525 | 2.3 ± 0.3 | 29.3 ± 0.5 | 31.7 ± 0.5 | 100 | 0 | 0 |
| VL | 2015 | LT | MT | 21 ± 7 | 975 | 35.9 ± 0.4 | 0.4 ± 0.0 | 36.3 ± 0.5 | 97 | 0 | 3 |
| VL | 2015 | SF | MT | 25 ± 3 | 600 | 0 ± 0.0 | 28.6 ± 0.3 | 28.6 ± 0.3 | 100 | 0 | 0 |
| VL | 2015 | ID | MT | 24 ± 4 | 650 | 9.8 ± 0.4 | 20.1 ± 0.3 | 30.0 ± 0.3 | 100 | 0 | 0 |
| VL | 2016 | LT | MT | 20 ± 10 | 1275 | 39.8 ± 0.8 | 9.5 ± 1.0 | 49.2 ± 0.9 | 45 | 4 | 51 |
| VL | 2016 | SF | MT | 19 ± 9 | 800 | 0.7 ± 0.1 | 26 ± 0.8 | 26.7 ± 0.8 | 63 | 0 | 38 |
| VL | 2016 | ID | OMT | 19 ± 9 | 1800 | 22.8 ± 0.7 | 39.7 ± 0.7 | 62.5 ± 0.7 | 96 | 0 | 4 |
| LT | SFd | SFo | ID | |||||
|---|---|---|---|---|---|---|---|---|
| Paajasensalo | ||||||||
| SRtot (mg CO2 m−2 s−1) | 0.28 | a | 0.23 | ab | 0.18 | b | 0.22 | ab |
| SRtot_STadj. (mg CO2 m−2 s−1) | 0.24 | a | 0.22 | ab | 0.17 | b | 0.20 | ab |
| ST (°C) | 10.7 | a | 10.2 | b | 10.2 | b | 10.5 | ab |
| SM (% vol/vol) | 13.3 | a | 18.3 | b | 22.8 | bc | 26.0 | c |
| SRh (mg CO2 m−2 s−1) | 0.14 | a | 0.13 | ab | 0.10 | b | 0.12 | ab |
| SRh_STadj. (mg CO2 m−2 s−1) | 0.15 | a | 0.15 | a | 0.12 | a | 0.13 | a |
| Viitalampi | ||||||||
| SRtot (mg CO2 m−2 s−1) | 0.22 | a | 0.18 | a | 0.21 | a | 0.20 | a |
| SRtot_STadj. (mg CO2 m−2 s−1) | 0.20 | a | 0.17 | a | 0.19 | a | 0.17 | a |
| ST (°C) | 10.5 | a | 10.3 | a | 10.6 | a | 11.0 | b |
| SM (% vol/vol) | 20.1 | a | 26.1 | a | 26.3 | a | 26.3 | a |
| SRh (mg CO2 m−2 s−1) | 0.11 | a | 0.14 | a | 0.12 | a | 0.11 | a |
| SRh_STadj. (mg CO2 m−2 s−1) | 0.12 | a | 0.15 | a | 0.12 | a | 0.11 | a |
| BAL | BAD | BAtot | ST | SM | |
|---|---|---|---|---|---|
| SRtot | 0.55 * | −0.41 | 0.40 | 0.54 * | −0.67 ** |
| SRtot_STadj. | 0.43 | −0.36 | 0.31 | - | −0.69 ** |
| SRa | 0.14 | −0.12 | 0.17 | - | - |
| SRh | 0.02 | 0.17 | 0.12 | −0.29 | −0.83 * |
| SRh_STadj. | 0.16 | 0.13 | 0.33 | - | −0.88 ** |
| ST | 0.17 | −0.18 | −0.01 | - | −0.13 |
| SM | −0.56 * | 0.25 | −0.59 ** | −0.13 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosunen, M.; Lyytikäinen-Saarenmaa, P.; Ojanen, P.; Blomqvist, M.; Starr, M. Response of Soil Surface Respiration to Storm and Ips typographus (L.) Disturbance in Boreal Norway Spruce Stands. Forests 2019, 10, 307. https://doi.org/10.3390/f10040307
Kosunen M, Lyytikäinen-Saarenmaa P, Ojanen P, Blomqvist M, Starr M. Response of Soil Surface Respiration to Storm and Ips typographus (L.) Disturbance in Boreal Norway Spruce Stands. Forests. 2019; 10(4):307. https://doi.org/10.3390/f10040307
Chicago/Turabian StyleKosunen, Maiju, Päivi Lyytikäinen-Saarenmaa, Paavo Ojanen, Minna Blomqvist, and Mike Starr. 2019. "Response of Soil Surface Respiration to Storm and Ips typographus (L.) Disturbance in Boreal Norway Spruce Stands" Forests 10, no. 4: 307. https://doi.org/10.3390/f10040307





