Eucalyptus Short-Rotation Management Effects on Nutrient and Sediments in Subtropical Streams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Areas
2.2. Hydrological Data and Water Quality
2.3. Data Analysis
3. Results
3.1. Catchment Water Availability
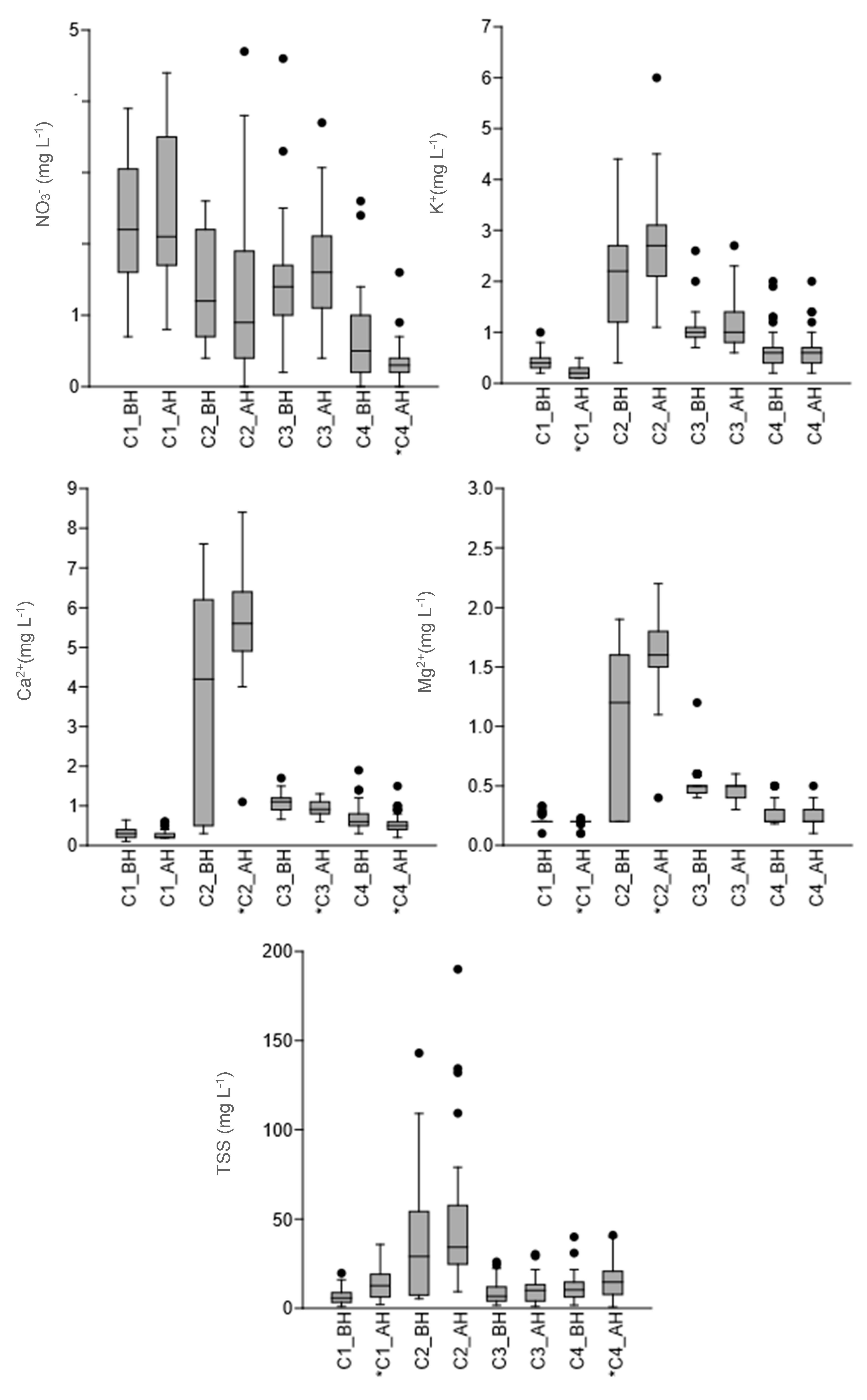
3.2. Nutrient and Suspended Solids Concentrations
3.3. Nutrient and Suspended Solids Exportations
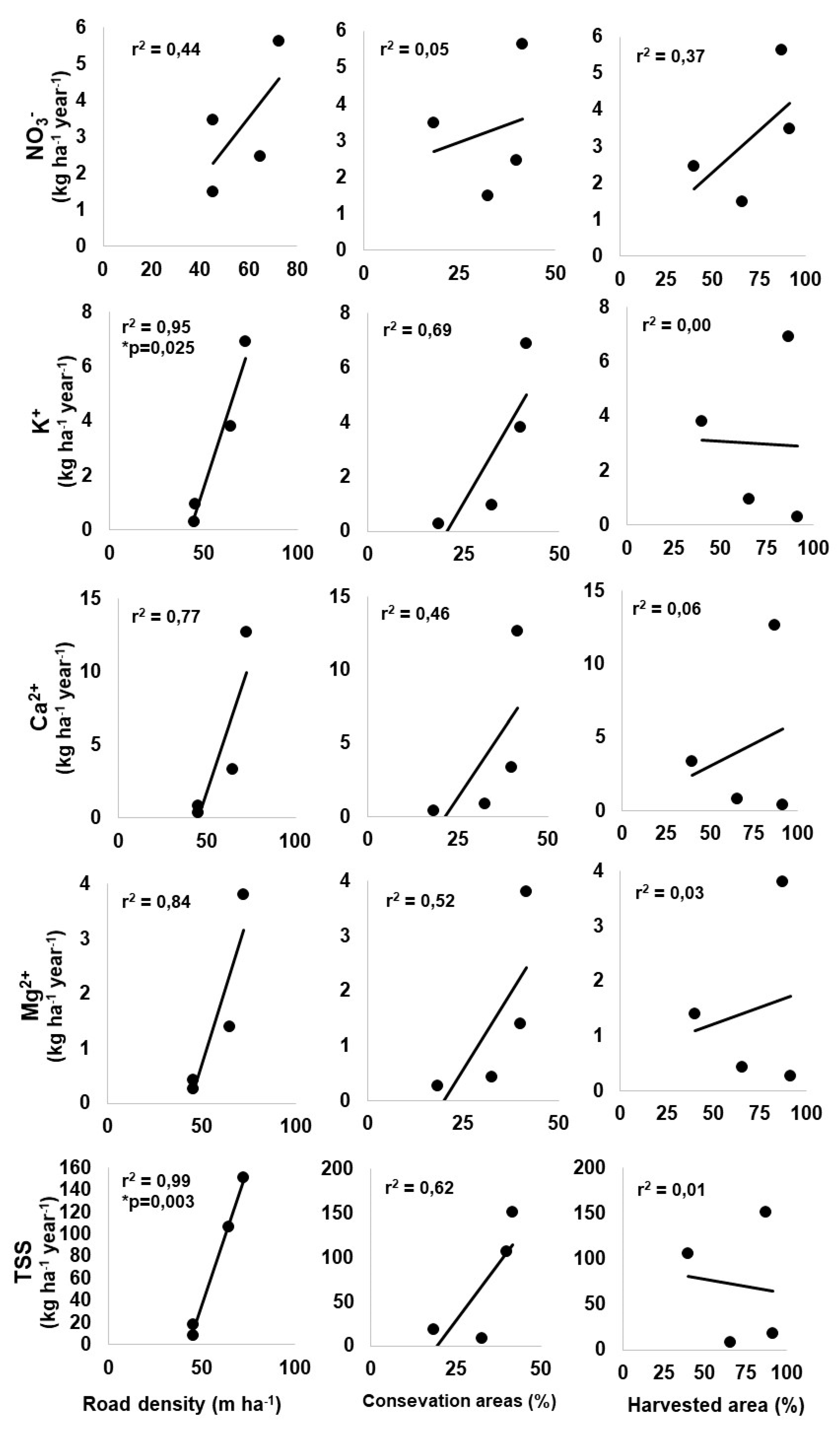
3.4. Relationship between Forest Management and Exportation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baillie, B.R.; Neary, D.G. Water quality in New Zealand’s planted forests: A review. N. Z. J. For. Sci. 2015, 45, 7. [Google Scholar] [CrossRef]
- Binkley, D.; Burnham, H.; Allen, H.L. Water quality impacts of forest fertilization with nitrogen and phosphorus. For. Ecol. Manag. 1999, 121, 191–213. [Google Scholar] [CrossRef]
- Sun, G.; Riedel, M.; Jackson, R.; Kolka, R.; Amatya, D.; Shepard, J. Influences of management of Southern forests on water quantity and quality. South. For. Sci. Past Present. Futur. 2004, GTR SRS-75, 195–224. [Google Scholar]
- Grace III, J.M. Forest operations and wtaer quality in the South. Trans. ASAE 2005, 48, 871–880. [Google Scholar] [CrossRef]
- Da Silva, D.M.L.; Ometto, J.P.H.B.; de Araújo Lobo, G.; de Paula Lima, W.; Scaranello, M.A.; Mazzi, E.; da Rocha, H.R. Can land use changes alter carbon, nitrogen and major ion transport in subtropical brazilian streams? Sci. Agric. 2007, 64, 317–324. [Google Scholar] [CrossRef]
- Van Dijk, A.I.J.M.; Keenan, R.J.; Ecology, F. Planted forests and water in perspective. For. Ecol. Manag. 2007, 251, 1–9. [Google Scholar] [CrossRef]
- Diaz-Chavez, R.; Berndes, G.; Neary, D.; Elia Neto, A.; Fall, M. Water quality assessment of bioenergy production. Biofuels Bioprod. Biorefin. 2011, 5, 445–463. [Google Scholar] [CrossRef]
- Boggs, J.; Sun, G.; Jones, D.; Mcnulty, S.G. Effect of soils on water quantity and quality in Piedmont forested headwater watersheds of North Carolina. J. Am. Water Resour. Assoc. 2013, 49, 132–150. [Google Scholar] [CrossRef]
- Anderson, C.J.; Lockaby, B.G. Research gaps related to forest management and stream sediment in the United States. Environ. Manag. 2011, 47, 303–313. [Google Scholar] [CrossRef]
- Payn, T.; Carnus, J.M.; Freer-Smith, P.; Kimberley, M.; Kollert, W.; Liu, S.; Orazio, C.; Rodriguez, L.; Silva, L.N.; Wingfield, M.J. Changes in planted forests and future global implications. For. Ecol. Manag. 2015, 352, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Jürgensen, C.; Kollert, W.; Lebedys, A. Assessment of industrial roundwood production from planted forests. FAO Plant. For. Trees Work. Pap. 2014, FP/48/E, 40. [Google Scholar]
- Kanninen, M. Plantation forests: Global perspectives. In Ecosystem Goods and Services from Plantation Forests; Bauhus, J., van de Meer, P., Kanninen, M., Eds.; Earthscan: London, UK, 2010; p. 240. ISBN 9781849711685. [Google Scholar]
- IBA (Indústria Brasileira de Árvores). Report 2017; IBA: São Paulo, Brazil, 2017. [Google Scholar]
- De Moraes Gonçalves, J.L.; Alvares, C.A.; Higa, A.R.; Silva, L.D.; Alfenas, A.C.; Stahl, J.; de Barros Ferraz, S.F.; de Paula Lima, W.; Brancalion, P.H.S.; Hubner, A.; et al. Integrating genetic and silvicultural strategies to minimize abiotic and biotic constraints in Brazilian eucalypt plantations. For. Ecol. Manag. 2013, 301, 6–27. [Google Scholar] [CrossRef]
- Binkley, D.; Brown, T.C. Forest Practices As Nonpoint Sources of Pollution in North America. Water Resour. Bull. 1993, 29, 729–740. [Google Scholar] [CrossRef]
- Carroll, G.D.; Schoenholtz, S.H.; Young, B.W.; Dibble, E.D. Effectiveness of Forestry Streamside Management. Water Air Soil Pollut. Focus 2004, 4, 275–296. [Google Scholar] [CrossRef]
- Feller, M.C. Forest harvesting and streamwater inorganic chemistry in western North America: A review. J. Am. Water Resour. Assoc. 2005, 41, 785–811. [Google Scholar] [CrossRef]
- Aust, W.M.; Blinn, C.R. Forestry best management practices for timber harvesting and site preparation in the eastern United States: An overview of water quality and productivity research during the past 20 years (1982-2002). Water Air Soil Pollut. Focus 2004, 4, 5–36. [Google Scholar] [CrossRef]
- Neary, D. Long-Term Forest Paired Catchment Studies: What Do They Tell Us That Landscape-Level Monitoring Does Not? Forests 2016, 7, 164. [Google Scholar] [CrossRef]
- Campbell, I.C.; Doeg, T.J. Impact of Timber Harvesting and Production on Stream; a Review. Mar. Freshw. Res. 1989, 40, 519. [Google Scholar] [CrossRef]
- Gomi, T.; Moore, R.D.; Hassan, M.A. Suspended Sediment Dynamics in Small Forest Streams of the Pacific Northwest. J. Am. Water Resour. Assoc. 2005, 41, 877–898. [Google Scholar] [CrossRef]
- Neary, D.G.; Ice, G.G.; Jackson, C.R. Linkages between forest soils and water quality and quantity. For. Ecol. Manag. 2009, 258, 2269–2281. [Google Scholar] [CrossRef]
- Bruijnzeel, L.A. (De)forestation and dry season flow in the tropics: a closer look. J. Trop. For. Sci. 1989, 1, 229–243. [Google Scholar]
- Wohl, E.; Barros, A.; Brunsell, N.; Chappell, N.A.; Coe, M.; Giambelluca, T.; Goldsmith, S.; Harmon, R.; Hendrickx, J.M.H.; Juvik, J.; et al. The hydrology of the humid tropics. Nat. Publ. Gr. 2012, 2, 655–662. [Google Scholar] [CrossRef]
- David, J.S.; Henriques, M.O.; David, T.S.; Tome, J.; Ledger, D.C.; Tomé, J.; Ledger, D.C. Clearcutting effects on streamflow in coppiced Eucalyptus globulus stands in Portugal. J. Hydrol. 1994, 162, 143–154. [Google Scholar] [CrossRef]
- Ramos-Scharrón, C.E.; LaFevor, M.C. The role of unpaved roads as active source areas of precipitation excess in small watersheds drained by ephemeral streams in the Northeastern Caribbean. J. Hydrol. 2016, 533, 168–179. [Google Scholar] [CrossRef]
- Jones, J.A.; Grant, G.E. Peak flow response to clear-cutting and roads in small and large basin, western Cascades, Oregon. Water Resour. Res. 1996, 32, 959–974. [Google Scholar] [CrossRef]
- Hibbert, A.R. Forest Treatment effects on water yield. In International Symposium on Forest Hydrology, Penn State University, Statclich; Pergamon Press: New York, NY, USA, 1965; pp. 527–543. [Google Scholar]
- Bosch, J.M.; Hewlett, J.D. A review of catchment experiments to determine the effect of vegetation changes on water yield and evapotranspiration. J. Hydrol. 1982, 55, 3–23. [Google Scholar] [CrossRef]
- Brown, A.E.; Zhang, L.; McMahon, T.A.; Western, A.W.; Vertessy, R.A. A review of paired catchment studies for determining changes in water yield resulting from alterations in vegetation. J. Hydrol. 2005, 310, 28–61. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.J.L.; Sentelhas, P.C.; De Moraes Gonçalves, J.L.; Sparovek, G.; De Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Taxonomy, A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; United States Department of Agriculture: Washington, WA, USA, 1999.
- Newman, M.C.; Dixon, P.M.; Looney, B.B.; Pinder, J.E. Estimating mean and variance for environmental samples with below detection limit observations. J. Am. Water Resour. Assoc. 1989, 25, 905–916. [Google Scholar] [CrossRef]
- Siemion, J.; Burns, D.A.; Murdoch, P.S.; Germain, R.H. The relation of harvesting intensity to changes in soil, soil water, and stream chemistry in a northern hardwood forest, Catskill Mountains, USA. For. Ecol. Manag. 2011, 261, 1510–1519. [Google Scholar] [CrossRef]
- Farley, K.A.; Piñeiro, G.; Palmer, S.M.; Jobbágy, E.G.; Jackson, R.B. Stream acidification and base cation losses with grassland afforestation. Water Resour. Res. 2009, 45, 1–11. [Google Scholar] [CrossRef]
- De Moraes Gonçalves, J.L.; Stape, J.L.; Laclau, J.P.; Bouillet, J.P.; Ranger, J. Assessing the effects of early silvicultural management on long-term site productivity of fast-growing eucalypt plantations: The Brazilian experience. South. For. 2008, 70, 105–118. [Google Scholar] [CrossRef]
- Pulito, A.P.; De Moraes Gonçalves, J.L.; Smethurst, P.J.; Junior, J.C.A.; Alvares, C.A.; Rocha, J.H.T.; Hübner, A.; de Moraes, L.F.; Miranda, A.C.; Kamogawa, M.Y.; et al. Available nitrogen and responses to nitrogen fertilizer in brazilian eucalypt plantations on soils of contrasting texture. Forests 2015, 6, 973–991. [Google Scholar] [CrossRef]
- Laclau, J.P.; Ranger, J.; de Moraes Gonçalves, J.L.; Maquère, V.; Krusche, A.V.; M’Bou, A.T.; Nouvellon, Y.; Saint-André, L.; Bouillet, J.P.; de Cassia Piccolo, M.; et al. Biogeochemical cycles of nutrients in tropical Eucalyptus plantations. Main features shown by intensive monitoring in Congo and Brazil. For. Ecol. Manag. 2010, 259, 1771–1785. [Google Scholar] [CrossRef]
- Câmara, C.D.; De Paula Lima, W. Clearcutting of a 50 years old growth Eucalyptus saligna plantation: Impacts on water balance and water quality in an experimental catchment. Sci. For. 2000, 57, 41–58. [Google Scholar]
- Megahan, W.F.; King, J.H. Erosion, sedimentation, and cumula- tive effects in the northern Rocky Mountains. In A Century of Forest and Wildland Watershed Lessons; Ice, G.G., Stednick, D., Eds.; The Society of American Foresters: Bethesda, MD, USA, 2004; pp. 201–222. [Google Scholar]
- McBroom, M.W.; Beasley, R.S.; Chang, M.; Ice, G.G. Storm runoff and sediment losses from forest clearcutting and stand re-establishment with best management practices in East Texas, USA. Hydrol. Process. 2008, 22, 1509–1522. [Google Scholar] [CrossRef]
- Webb, A.A.; Dragovich, D.; Jamshidi, R. Temporary increases in suspended sediment yields following selective eucalypt forest harvesting. For. Ecol. Manag. 2012, 283, 96–105. [Google Scholar] [CrossRef]
- Boggs, J.; Sun, G.; Mcnulty, S.G.; Jones, D.; Mcnulty, S.G. Effects of timber harvest on water quantity and quality in small watersheds in the Piedmont of North Carolina. J. For. 2016, 114, 27–40. [Google Scholar] [CrossRef]
- FAO. Status of the World’s Soil Resources; FAO: Rome, Italy, 2015. [Google Scholar]
- Vital, A.R.T.; Lima, W.P.; De Camargo, F.R. Efeitos do corte raso de plantação de eucalyptus sobre o balanço hídrico, a qualidade da água e as perdas de solo e de nutrientes em uma microbacia no Vale do Paraíba, SP. Sci. For. Sci. 1999, 55, 5–16. [Google Scholar]
- Brasil. Lei n. 12651, de 25 de maio de 2012. Available online: http://www.planalto.gov.br/ccivil_03/_Ato2011-2014/2012/Lei/L12651.htm (accessed on 29 May 2019).
- Lara, A.; Little, C.; Urrutia, R.; McPhee, J.; Álvarez-Garretón, C.; Oyarzún, C.; Soto, D.; Donoso, P.; Nahuelhual, L.; Pino, M.; et al. Assessment of ecosystem services as an opportunity for the conservation and management of native forests in Chile. For. Ecol. Manag. 2009, 258, 415–424. [Google Scholar] [CrossRef]
- Cook, R.L.; Binkley, D.; Mendes, J.C.T.; Stape, J.L. Soil carbon stocks and forest biomass following conversion of pasture to broadleaf and conifer plantations in southeastern Brazil. For. Ecol. Manag. 2014, 324, 37–45. [Google Scholar] [CrossRef]
- Reiter, M.; Heffner, J.T.; Beech, S.; Turner, T.; Bilby, R.E. Temporal and Spatial Turbidity Patterns Over 30 Years in a Managed Forest of Western Washington. J. Am. Water Resour. Assoc. 2009, 45, 793–808. [Google Scholar] [CrossRef]
- Wang, X.; Burns, D.A.; Yanai, R.D.; Briggs, R.D.; Germain, R.H. Changes in stream chemistry and nutrient export following a partial harvest in the Catskill Mountains, New York, USA. For. Ecol. Manag. 2006, 223, 103–112. [Google Scholar] [CrossRef]
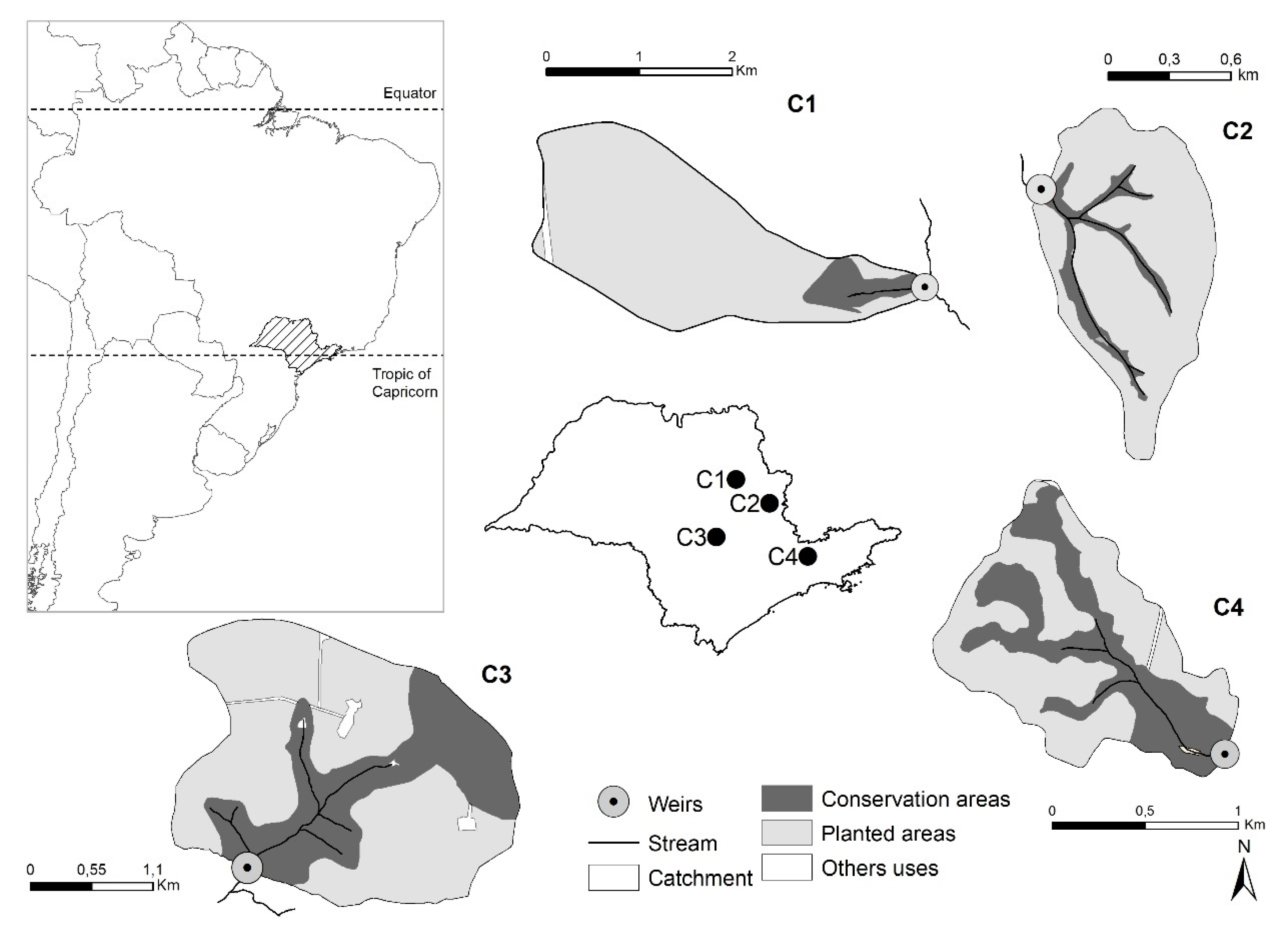
| Characteristic | Catchments | |||||
|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | |||
| Total area (ha) | 470.1 | 86.6 | 533.7 | 125.7 | ||
| Average slope (%) | 6.8 | 14.3 | 9.6 | 22.5 | ||
| Forest age (years) | 6 | 7 | 7 | 6 | ||
| Stream flow | perennial | intermittent | perennial | perennial | ||
| Main soil type (%) | Entisols(1) (82%) | Inceptisols (64%) | Entisols(1) (58%) | Inceptisols (59%) | ||
| Land use (%) | BH(2) | AH(3) | BH(2) | AH(3) | BH(2) | BH(2) |
| Forest plantation | 91.5 | 80.6 | 87.1 | 58.3 | 65.8 | 59.3 |
| Conservation areas | 7.6 | 18.4 | 12.7 | 41.5 | 32.5 | 39.9 |
| Others uses | 0.9 | 0.9 | 0.2 | 0.2 | 1.8 | 0.8 |
| Harvested area | 91.5 | - | 87.1 | - | 65.8 | 40.0 |
| Road density (m ha−1) | 49.6 | 45.2 | 81.5 | 72.3 | 45.4 | 64.6 |
| Catchment | Harvesting Date | Type of Weir | Electronic Equipment | Water Samples | |
|---|---|---|---|---|---|
| Water Level/Precipitation | BH(1) | AH(2) | |||
| C1 | 11/2009 | 90° | Campbell Scientific (models CS540 and CR510)/Texas Electronics (model TR525MR3) | 45 | 48 |
| C2 | 07/2008 | 50° | Campbell Scientific (models CS450 and CR500)/Hydrological Services | 15 | 30 |
| C3 | 10/2008 | 35° | Solinst (model 3001)/Solinst | 46 | 49 |
| C4 | 06/2009 | 20° | Campbell Scientific (models CS450 and CR510)/Hydrological Services | 51 | 52 |
| Catchment | Precipitation (P) (mm) | Water Yield (Q) (mm) | Q:P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BH | AH | %(1) | BH | AH | %(1) | BH | AH | %(1) | |
| C1 | 1700.1 | 1138.6 | −33 | 130.7 | 137.0 | 5 | 0.08 | 0.12 | 57 |
| C2 | 1702.8 | 1414.2 | −17 | 253.1 | 244.9 | −3 | 0.15 | 0.17 | 17 |
| C3 | 983.5 | 1124.1 | 14 | 126.3 | 90.4 | −28 | 0.13 | 0.08 | −37 |
| C4 | 1576.5 | 1428.3 | −9 | 415.1 | 648.1 | 56 | 0.26 | 0.45 | 72 |
| Parameters | Exports by Catchment kg ha−1 year−1 (% of Change) | |||||||
|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | |||||
| BH(1) | AH(2) | BH(1) | AH(2) | BH(1) | AH(2) | BH(1) | AH(2) | |
| Nitrate | 2.8 | 3.5 (22%) | 3.5 | 5.6 (59%) | 1.7 | 1.5 (−14%) | 2.9 | 2.5 (−14%) |
| Potassium | 0.5 | 0.3 (−44%) | 5.1 | 6.9 (36%) | 1.4 | 1.0 (−29%) | 2.7 | 3.8 (41%) |
| Calcium | 0.4 | 0.4 (0%) | 9.6 | 12.7 (33%) | 1.3 | 0.8 (−38%) | 2.5 | 3.3 (32%) |
| Magnesium | 0.3 | 0.3 (0%) | 2.6 | 3.8 (46%) | 0.6 | 0.4 (−33%) | 1.1 | 1.4 (32%) |
| Total suspended solids | 9.0 | 18.3 (104%) | 113.7 | 151.5 (33%) | 11.4 | 8.5 (−25%) | 49.8 | 106.3 (113%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, C.B.; Taniwaki, R.H.; Lane, P.; Lima, W.d.P.; Ferraz, S.F.d.B. Eucalyptus Short-Rotation Management Effects on Nutrient and Sediments in Subtropical Streams. Forests 2019, 10, 519. https://doi.org/10.3390/f10060519
Rodrigues CB, Taniwaki RH, Lane P, Lima WdP, Ferraz SFdB. Eucalyptus Short-Rotation Management Effects on Nutrient and Sediments in Subtropical Streams. Forests. 2019; 10(6):519. https://doi.org/10.3390/f10060519
Chicago/Turabian StyleRodrigues, Carolina Bozetti, Ricardo Hideo Taniwaki, Patrick Lane, Walter de Paula Lima, and Silvio Frosini de Barros Ferraz. 2019. "Eucalyptus Short-Rotation Management Effects on Nutrient and Sediments in Subtropical Streams" Forests 10, no. 6: 519. https://doi.org/10.3390/f10060519





