Deepening Rooting Depths Improve Plant Water and Carbon Status of a Xeric Tree during Summer Drought
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Determination of Water Potential
2.3. Maximum Photochemical Efficiency and Gas Exchange
2.4. Water Relation Parameters
2.5. Non-Structural Carbohydrates Concentrations
2.6. Data Analysis and Statistics
3. Results
3.1. Summer Drought and Root Depth
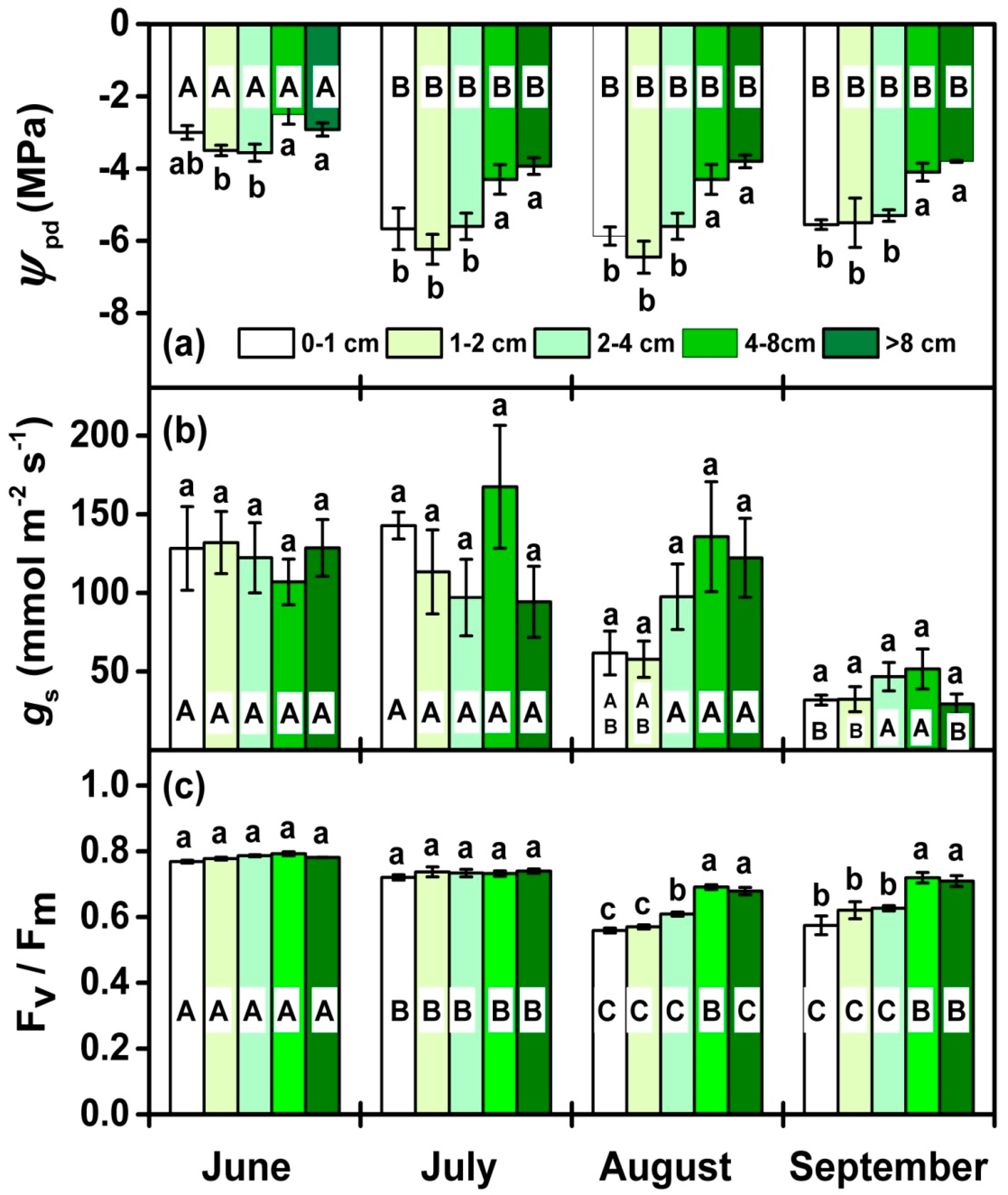
3.2. Physiological Performance During the Growing Season
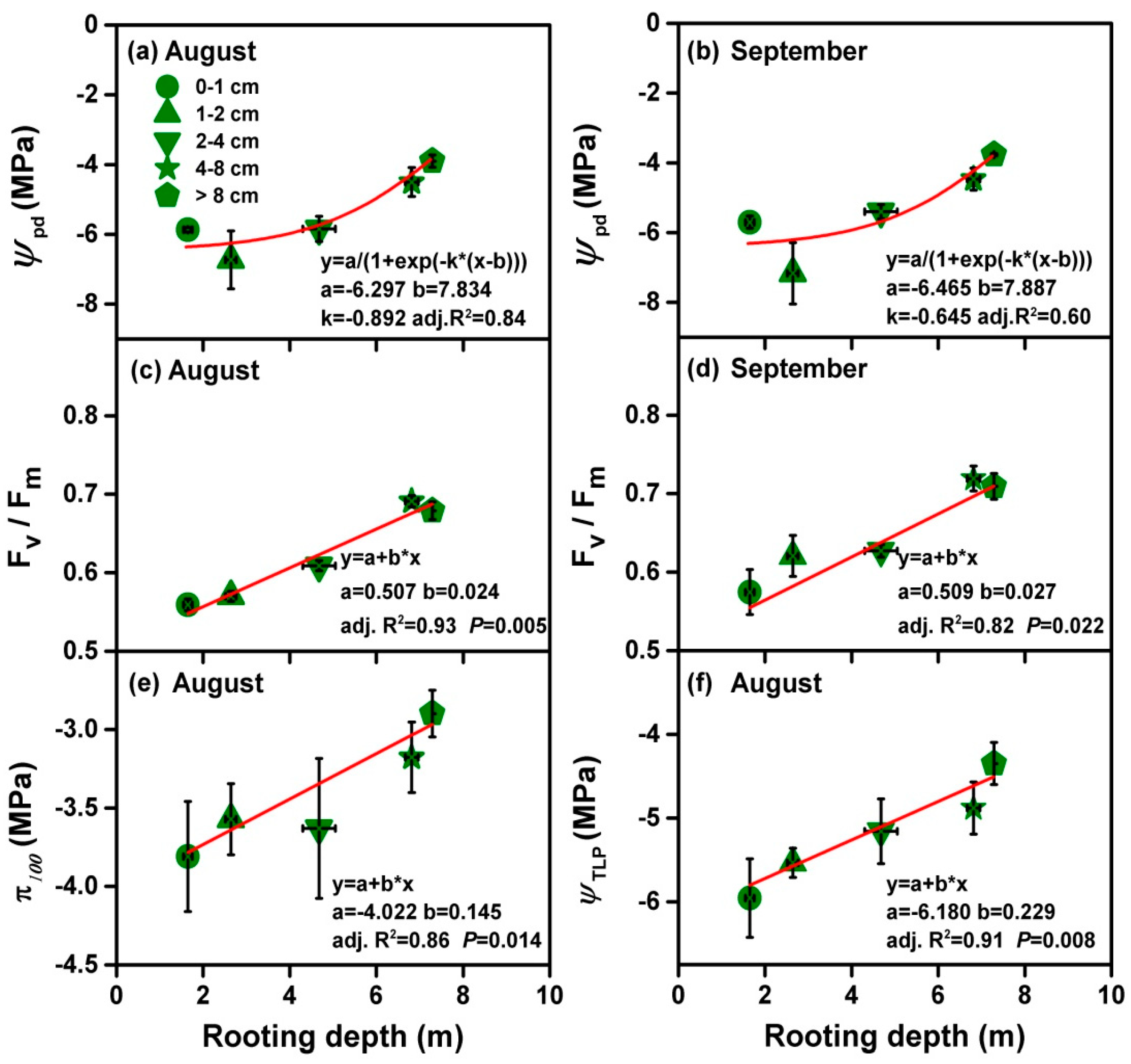
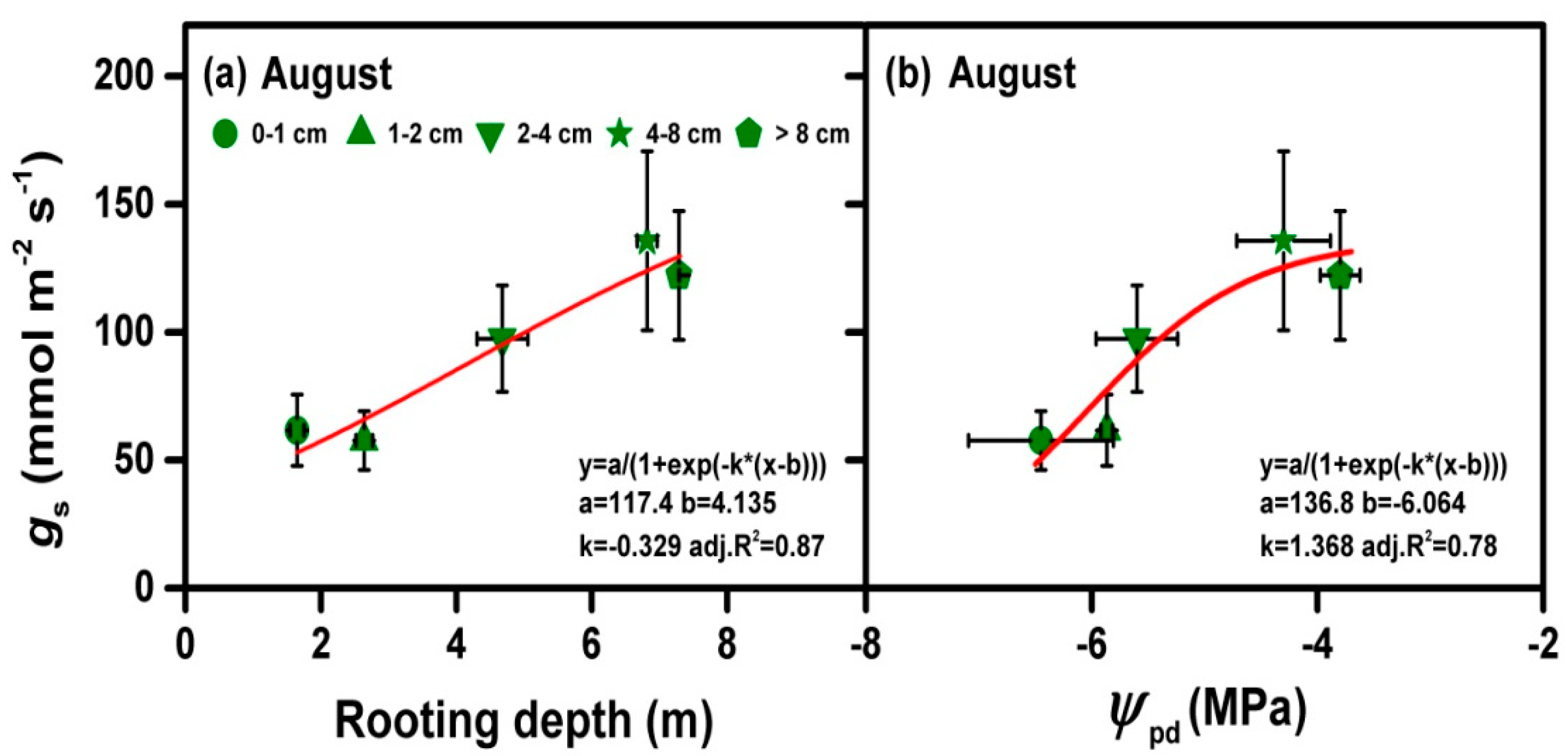
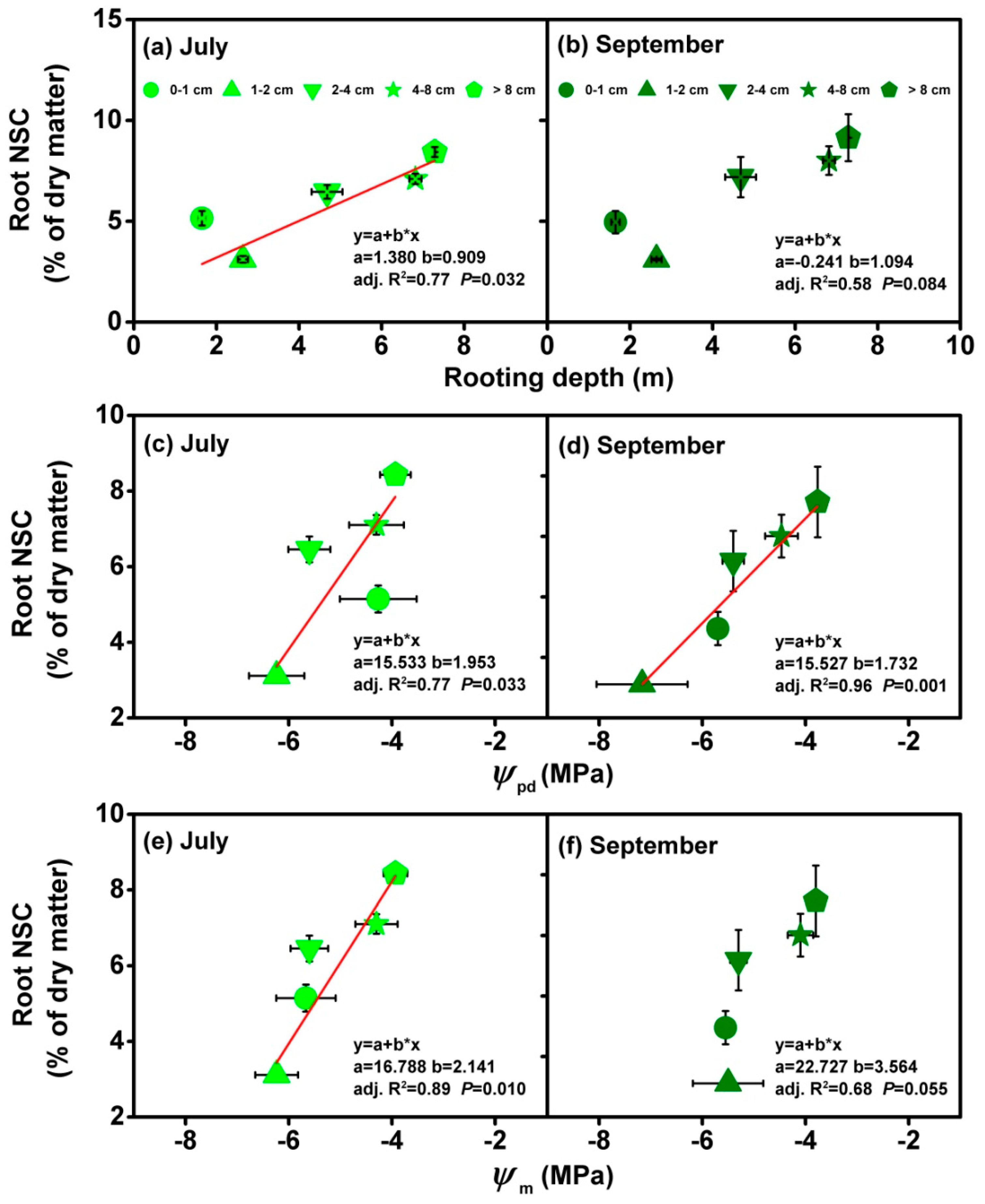
3.3. Relationship between Rooting Depth and Physiological Traits
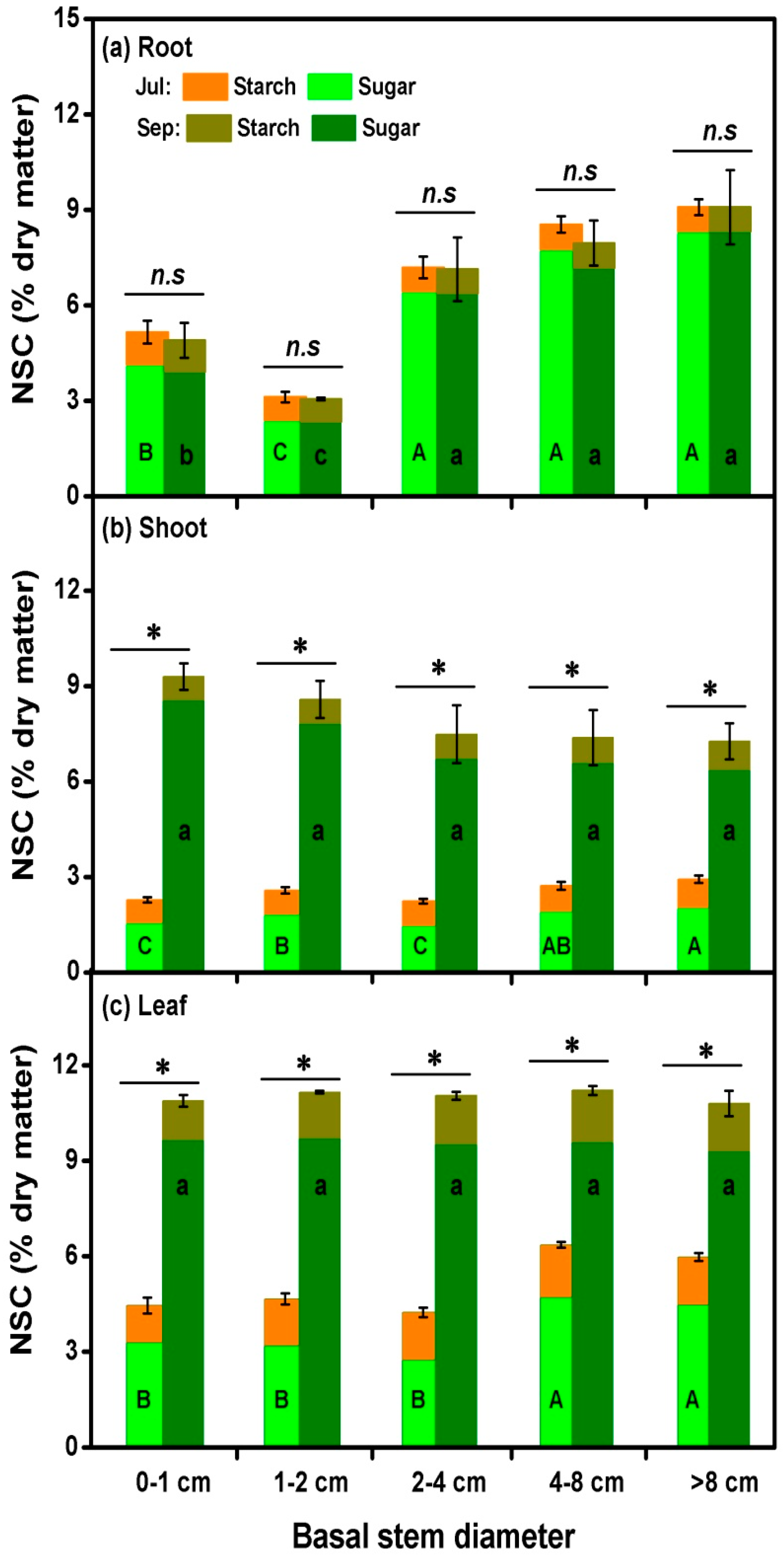
3.4. Non-Structural Carbohydrates Variation
4. Discussion
4.1. Physiological Performance
4.2. The Important of Rooting Depth
4.3. NSC Variation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sheffield, J.; Wood, E.F. Projected changes in drought occurrence under future global warming from multi-model, multi-scenario, IPCC AR4 simulations. Clim. Dyn. 2008, 31, 79–105. [Google Scholar] [CrossRef]
- Adams, H.D.; Guardiola-Claramonte, M.; Barron-Gafford, G.A.; Villegas, J.C.; Breshears, D.D.; Zou, C.B.; Troch, P.A.; Huxman, T.E. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proc. Natl. Acad. Sci. USA 2009, 106, 7063–7066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Mantgem, P.J.; Stephenson, N.L.; Byrne, J.C.; Daniels, L.D.; Franklin, J.F.; Fule, P.Z.; Harmon, M.E.; Larson, A.J.; Smith, J.M.; Taylor, A.H.; et al. Widespread increase of tree mortality rates in the Western United States. Science 2009, 323, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Breshears, D.D.; Lopez-Hoffman, L.; Graumlich, L.J. When ecosystem services crash: Preparing for big, fast, patchy climate change. Ambio 2011, 40, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.M.; Allen, R.B.; Coomes, D.A.; Duncan, R.P. Size-specific tree mortality varies with neighbourhood crowding and disturbance in a montane nothofagus forest. PLoS ONE 2011, 6, e26670. [Google Scholar] [CrossRef] [PubMed]
- Steppe, K.; Niinemets, Ü.; Teskey, R.O. Tree size- and age-related changes in leaf physiology and their influence on carbon gain. In Size- and Age-Related Changes in Tree Structure and Function; Meinzer, F.C., Lachenbruch, B., Dawson, T.E., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 235–253. [Google Scholar]
- Luo, Y.; Chen, H.Y.H. Competition, species interaction and ageing control tree mortality in boreal forests. J. Ecol. 2011, 99, 1470–1480. [Google Scholar] [CrossRef]
- McDowell, N.G.; Ryan, M.G.; Zeppel, M.J.B.; Tissue, D.T. Improving our knowledge of drought-induced forest mortality through experiments, observations, and modeling. New Phytol. 2013, 200, 289–293. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, A.Y.; An, Y.N.; Lian, P.Y.; Wu, D.D.; Zhu, J.J.; Meinzer, F.C.; Hao, G.Y. Hydraulics play an important role in causing low growth rate and dieback of aging Pinus sylvestris var. mongolica trees in plantations of Northeast China. Plant Cell Environ. 2018, 41, 1500–1511. [Google Scholar] [CrossRef]
- Zang, C.; Pretzsch, H.; Rothe, A. Size-dependent responses to summer drought in Scots pine, Norway spruce and common oak. Trees Struct. Funct. 2012, 26, 557–569. [Google Scholar] [CrossRef]
- He, J.S.; Zhang, Q.B.; Bazzaz, F.A. Differential drought responses between saplings and adult trees in four co-occurring species of New England. Trees 2005, 19, 442–450. [Google Scholar] [CrossRef]
- Rohner, B.; Bigler, C.; Wunder, J.; Brang, P.; Bugmann, H. Fifty years of natural succession in Swiss forest reserves: Changes in stand structure and mortality rates of oak and beech. J. Veg. Sci. 2012, 23, 892–905. [Google Scholar] [CrossRef]
- Reynolds, J.H.; Ford, E.D. Improving competition representation in theoretical models of self-thinning: A critical review. J. Ecol. 2005, 93, 362–372. [Google Scholar] [CrossRef]
- Weiner, J. Asymmetric competition in plant populations. Trends Ecol. Evol. 1990, 5, 360–364. [Google Scholar] [CrossRef]
- Sendall, K.M.; Reich, P.B.; Lusk, C.H. Size-related shifts in carbon gain and growth responses to light differ among rainforest evergreens of contrasting shade tolerance. Oecologia 2018, 187, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Tiemuerbieke, B.; Min, X.J.; Zang, Y.X.; Xing, P.; Ma, J.Y.; Sun, W. Water use patterns of co-occurring C3 and C4 shrubs in the Gurbantonggut desert in northwestern China. Sci. Total Environ. 2018, 634, 341–354. [Google Scholar] [CrossRef]
- Markesteijn, L.; Poorter, L. Seedling root morphology and biomass allocation of 62 tropical tree species in relation to drought- and shade-tolerance. J. Ecol. 2009, 97, 311–325. [Google Scholar] [CrossRef]
- Bucci, S.J.; Scholz, F.G.; Goldstein, G.; Meinzer, F.C.; Arce, M.E. Soil water availability and rooting depth as determinants of hydraulic architecture of Patagonian woody species. Oecologia 2009, 160, 631–641. [Google Scholar] [CrossRef]
- Schenk, H.J.; Jackson, R.B. Rooting depths, lateral root spreads and below-ground/above-ground allometries of plants in water-limited ecosystems. J. Ecol. 2002, 90, 480–494. [Google Scholar] [CrossRef]
- Scholz, F.G.; Bucci, S.J.; Arias, N.; Meinzer, F.C.; Goldstein, G. Osmotic and elastic adjustments in cold desert shrubs differing in rooting depth: Coping with drought and subzero temperatures. Oecologia 2012, 170, 885–897. [Google Scholar] [CrossRef]
- Fan, Y.; Miguez-Macho, G.; Jobbágy, E.G.; Jackson, R.B.; Otero-Casal, C. Hydrologic regulation of plant rooting depth. Proc. Natl. Acad. Sci. USA 2017, 114, 10572–10577. [Google Scholar] [CrossRef] [Green Version]
- Hacke, U.G.; Sperry, J.S.; Ewers, B.E.; Ellsworth, D.S.; Schäfer, K.V.R.; Oren, R. Influence of soil porosity on water use in Pinus taeda. Oecologia 2000, 124, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.B.G.; Bras, R.L. Plant rooting strategies in water-limited ecosystems. Water Resour. Res. 2007, 43. [Google Scholar] [CrossRef]
- Padilla, F.M.; Pugnaire, F.I. Rooting depth and soil moisture control Mediterranean woody seedling survival during drought. Funct. Ecol. 2007, 21, 489–495. [Google Scholar] [CrossRef]
- Canadell, J.; Zedler, P.H. Underground structures of woody plants in mediterranean ecosystems of Australia, California, and Chile. In Ecology and Biogeography of Mediterranean Ecosystems in Chile, California, and Australia; Arroyo, M.T.K., Zedler, P.H., Fox, M.D., Eds.; Springer: New York, NY, USA, 1995; pp. 177–210. [Google Scholar]
- Nicotra, A.; Babicka, N.; Westoby, M. Seedling root anatomy and morphology: An examination of ecological differentiation with rainfall using phylogenetically independent contrasts. Oecologia 2002, 130, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, W.; Liu, B.; Liu, H. Physiological responses of Haloxylon ammodendron to rainfall pulses in temperate desert regions, Northwestern China. Trees 2014, 28, 709–722. [Google Scholar] [CrossRef]
- Zhou, H.F.; Zheng, X.J.; Zhou, B.; Dai, Q.; Li, Y. Sublimation over seasonal snowpack at the southeastern edge of a desert in central Eurasia. Hydrol. Process. 2012, 26, 3911–3920. [Google Scholar] [CrossRef]
- Fan, L.L.; Tang, L.S.; Wu, L.F.; Ma, J.; Li, Y. The limited role of snow water in the growth and development of ephemeral plants in a cold desert. J. Veg. Sci. 2014, 25, 681–690. [Google Scholar] [CrossRef]
- Xu, G.Q.; McDowell, N.G.; Li, Y. A possible link between life and death of a xeric tree in desert. J. Plant Physiol. 2016, 194, 35–44. [Google Scholar] [CrossRef]
- Dai, Y.; Zheng, X.J.; Tang, L.S.; Li, Y. Stable oxygen isotopes reveal distinct water use patterns of two Haloxylon species in the Gurbantonggut Desert. Plant Soil 2015, 389, 73–87. [Google Scholar] [CrossRef]
- Cho, J.I.; Lim, H.M.; Siddiqui, Z.S.; Park, S.H.; Kim, A.R.; Kwon, T.R.; Lee, S.K.; Park, S.C.; Jeong, M.J.; Lee, G.S. Over-expression of PsGPD, a mushroom glyceraldehyde-3-phosphate dehydrogenase gene, enhances salt tolerance in rice plants. Biotechnol. Lett. 2014, 36, 1641–1648. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C. Measurement of plant water status by the pressure chamber technique. Irrig. Sci. 1988, 9, 289–308. [Google Scholar] [CrossRef]
- Arndt, S.K.; Irawan, A.; Sanders, G.J. Apoplastic water fraction and rehydration techniques introduce significant errors in measurements of relative water content and osmotic potential in plant leaves. Physiol. Plant. 2015, 155, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Schulte, P.J.; Hinckley, T.M. A Comparison of Pressure-Volume Curve Data Analysis Techniques. J. Exp. Bot. 1985, 36, 1590–1602. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Berry, J.A.; Smith, D.D.; Sperry, J.S.; Anderegg, L.D.L.; Field, C.B. The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proc. Natl. Acad. Sci. USA 2012, 109, 233–237. [Google Scholar] [CrossRef]
- Nardini, A.; Casolo, V.; Dal Borgo, A.; Savi, T.; Stenni, B.; Bertoncin, P.; Zini, L.; McDowell, N.G. Rooting depth, water relations and non-structural carbohydrate dynamics in three woody angiosperms differentially affected by an extreme summer drought. Plant Cell Environ. 2016, 39, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Nardini, A.; Battistuzzo, M.; Savi, T. Shoot desiccation and hydraulic failure in temperate woody angiosperms during an extreme summer drought. New Phytol. 2013, 200, 322–329. [Google Scholar] [CrossRef]
- Desoyza, A.G.; Franc, A.C.; Virginia, R.A.; Reynolds, J.E.; Whitford, W.G. Effects of plant size on photosynthesis and water relations in the desert shrub Prosopis glandulosa (Fabaceae). Am. J. Bot. 1996, 83, 99–105. [Google Scholar] [CrossRef]
- Ding, H.; Pretzsch, H.; Schuetze, G.; Roetzer, T. Size-dependence of tree growth response to drought for Norway spruce and European beech individuals in monospecific and mixed-species stands. Plant Biol. 2017, 19, 709–719. [Google Scholar] [CrossRef]
- Donovan, L.A.; Pappert, R.A. Ecophysiological differences among growth stages of Quercus laevis in a sandhill oak community. J. Torrey Bot. Soc. 1998, 125, 3–10. [Google Scholar] [CrossRef]
- Colangelo, M.; Camarero, J.J.; Borghetti, M.; Gazol, A.; Gentilesca, T.; Ripullone, F. Size matters a lot: drought-affected Italian Oaks are smaller and show lower growth prior to tree death. Front. Plant Sci. 2017, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Bond, B.J. Age-related changes in photosynthesis of woody plants. Trends Plant Sci. 2000, 5, 349–353. [Google Scholar] [CrossRef]
- Bao, J.T.; Wang, J.; Li, X.R.; Zhang, Z.S.; Su, J.Q. Age-related changes in photosynthesis and water relations of revegetated Caragana korshinskii in the Tengger desert, Northern China. Trees Struct. Funct. 2015, 29, 1749–1760. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Bazzaz, F.A. Changes in drought response strategies with ontogeny in Quercus rubra: Implications for scaling from seedlings to mature trees. Oecologia 2000, 124, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Casper, B.B.; Forseth, I.N.; Wait, D.A. A stage-based study of drought response in Cryptantha flava (Boraginaceae): Gas exchange, water use efficiency, and whole plant performance. Am. J. Bot. 2006, 93, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Flores, R.; Winkel, T.; Anh, N.T.T.; Joffre, R. Root foraging capacity depends on root system architecture and ontogeny in seedlings of three Andean Chenopodium species. Plant Soil 2014, 380, 415–428. [Google Scholar] [CrossRef]
- Wu, X.; Zheng, X.J.; Li, Y.; Xu, G.Q. Varying responses of two Haloxylon species to extreme drought and groundwater depth. Environ. Exp. Bot. 2019, 158, 63–72. [Google Scholar] [CrossRef]
- Hartmann, H.; Adams, H.D.; Hammond, W.M.; Hoch, G.; Landhäusser, S.M.; Wiley, E.; Zaehle, S. Identifying differences in carbohydrate dynamics of seedlings and mature trees to improve carbon allocation in models for trees and forests. Environ. Exp. Bot. 2018, 152, 7–18. [Google Scholar] [CrossRef]
- Rosas, T.; Galiano, L.; Ogaya, R.; Peñuelas, J.; Martínez-Vilalta, J. Dynamics of non-structural carbohydrates in three Mediterranean woody species following long-term experimental drought. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef]
- Woodruff, D.R.; Meinzer, F.C. Water stress, shoot growth and storage of non-structural carbohydrates along a tree height gradient in a tall conifer. Plant Cell Environ. 2011, 34, 1920–1930. [Google Scholar] [CrossRef]
- Maguire, A.J.; Kobe, R.K. Drought and shade deplete nonstructural carbohydrate reserves in seedlings of five temperate tree species. Ecol. Evol. 2015, 5, 5711–5721. [Google Scholar] [CrossRef] [PubMed]
- Bachofen, C.; Moser, B.; Hoch, G.; Ghazoul, J.; Wohlgemuth, T. No carbon “bet hedging” in pine seedlings under prolonged summer drought and elevated CO2. J. Ecol. 2018, 106, 31–46. [Google Scholar] [CrossRef]
- Galvez, D.A.; Landhäusser, S.M.; Tyree, M.T. Root carbon reserve dynamics in aspen seedlings: Does simulated drought induce reserve limitation? Tree Physiol. 2011, 31, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Würth, M.K.R.; Peláez-Riedl, S.; Wright, S.J.; Körner, C. Non-structural carbohydrate pools in a tropical forest. Oecologia 2005, 143, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Hoch, G. Height-related growth declines in ponderosa pine are not due to carbon limitation. Plant Cell Environ. 2009, 32, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.A.; Clearwater, M.J.; Haines, D.F.; Klein, T.; Mencuccini, M.; Sevanto, S.; Turgeon, R.; Zhang, C. Allocation, stress tolerance and carbon transport in plants: How does phloem physiology affect plant ecology? Plant Cell Environ. 2016, 39, 709–725. [Google Scholar] [CrossRef]
- Sala, A.; Piper, F.; Hoch, G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol. 2010, 186, 274–281. [Google Scholar] [CrossRef]
- Granda, E.; Julio Camarero, J. Drought reduces growth and stimulates sugar accumulation: New evidence of environmentally driven non-structural carbohydrate use. Tree Physiol. 2017, 37, 997–1000. [Google Scholar] [CrossRef]
- Piper, F.I.; Fajardo, A.; Hoch, G. Single-provenance mature conifers show higher non-structural carbohydrate storage and reduced growth in a drier location. Tree Physiol. 2017, 37, 1001–1010. [Google Scholar] [CrossRef]
- Epron, D.; Bahn, M.; Derrien, D.; Lattanzi, F.A.; Pumpanen, J.; Gessler, A.; Högberg, P.; Maillard, P.; Dannoura, M.; Gérant, D.; et al. Pulse-labelling trees to study carbon allocation dynamics: A review of methods, current knowledge and future prospects. Tree Physiol. 2012, 32, 776–798. [Google Scholar] [CrossRef]
- Kono, Y.; Ishida, A.; Saiki, S.T.; Yoshimura, K.; Dannoura, M.; Yazaki, K.; Kimura, F.; Yoshimura, J.; Aikawa, S.I. Initial hydraulic failure followed by late-stage carbon starvation leads to drought-induced death in the tree Trema orientalis. Commun. Biol. 2019, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.W.; Sillett, S.C.; Jennings, G.M.; Davis, S.D. The limits to tree height. Nature 2004, 428, 851. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, D.R.; Bond, B.J.; Meinzer, F.C. Does turgor limit growth in tall trees? Plant Cell Environ. 2004, 27, 229–236. [Google Scholar] [CrossRef]
- Xu, G.Q.; Li, Y. Rooting depth and leaf hydraulic conductance in the xeric tree Haloxyolon ammodendron growing at sites of contrasting soil texture. Funct. Plant Biol. 2008, 35, 1234–1242. [Google Scholar] [CrossRef]

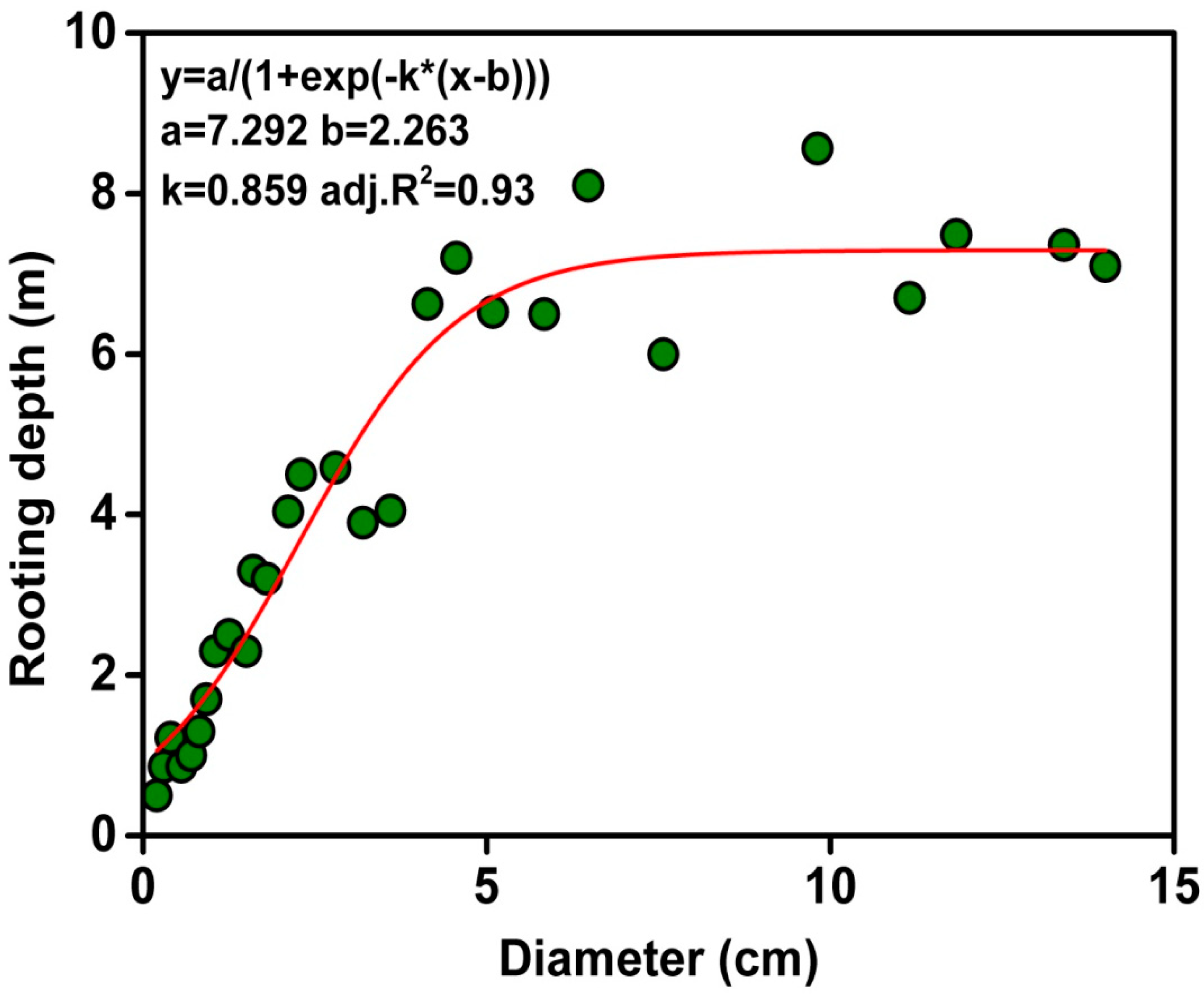

| Diameter Class (cm) | Basal Stem Diameter (cm) | Rooting Depth (m) | Height (m) | Crown Width (m) |
|---|---|---|---|---|
| 0–1 | 0.82 ± 0.10 | 1.65 ± 0.10 | 0.45 ± 0.08 | 0.26 ± 0.03 |
| 1–2 | 1.60 ± 0.09 | 2.64 ± 0.12 | 0.64 ± 0.04 | 0.55 ± 0.07 |
| 2–4 | 2.97 ± 0.26 | 4.68 ± 0.38 | 1.12 ± 0.09 | 0.97 ± 0.12 |
| 4–8 | 5.59 ± 0.39 | 6.82 ± 0.15 | 1.46 ± 0.31 | 1.65 ± 0.21 |
| >8 | 12.12 ± 1.04 | 7.29 ± 0.00 | 1.94 ± 0.25 | 2.23 ± 0.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.-J.; Xu, G.-Q.; Li, Y.; Wu, X. Deepening Rooting Depths Improve Plant Water and Carbon Status of a Xeric Tree during Summer Drought. Forests 2019, 10, 592. https://doi.org/10.3390/f10070592
Zheng X-J, Xu G-Q, Li Y, Wu X. Deepening Rooting Depths Improve Plant Water and Carbon Status of a Xeric Tree during Summer Drought. Forests. 2019; 10(7):592. https://doi.org/10.3390/f10070592
Chicago/Turabian StyleZheng, Xin-Jun, Gui-Qing Xu, Yan Li, and Xue Wu. 2019. "Deepening Rooting Depths Improve Plant Water and Carbon Status of a Xeric Tree during Summer Drought" Forests 10, no. 7: 592. https://doi.org/10.3390/f10070592




