Regeneration in the Understory of Declining Overstory Trees Contributes to Soil Respiration Homeostasis along Succession in a Sub-Mediterranean Beech Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Area and Design of the Study
2.2. Soil Respiration
2.3. Soil Physico-Chemical Properties and Root Density
2.4. Data Analyses
3. Results
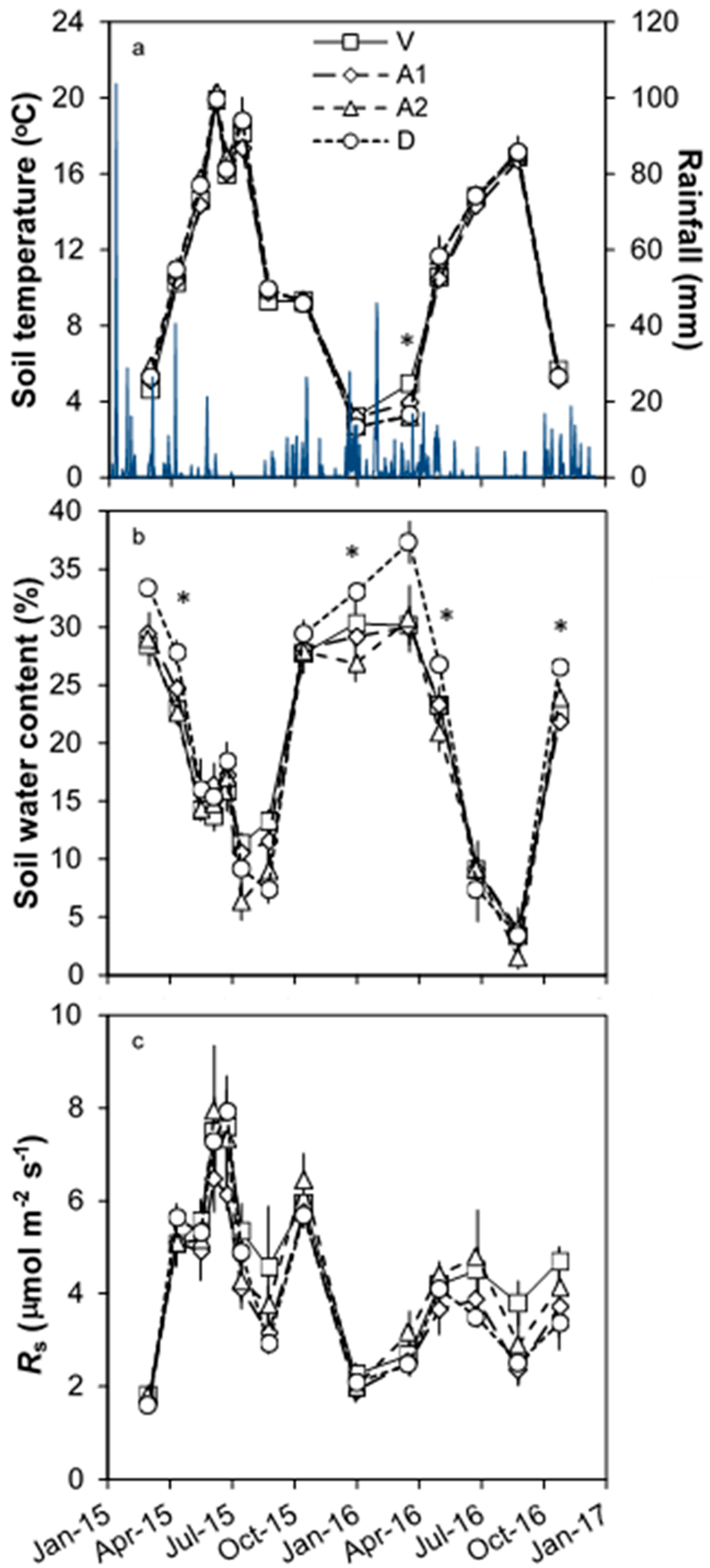
3.1. Environmental Conditions
3.2. Temporal Changes in Rs
3.3. Spatial Changes in Soil Physico-Chemical Variables and Rs
4. Discussion
4.1. Does Rs Change with Tree Decline in a Mature Beech Forest?
4.2. How SWC and Ts Govern Temporal Changes in Rs?
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bond-Lamberty, B.P.; Thomson, A.M. Global Database of Soil Respiration Data, Version 3.0. Data Set; Oak Ridge National Laboratory Distributed Active Archive Center: Oak Ridge, TN, USA, 2014. Available online: http://daac.ornl.gov (accessed on 9 July 2018). [CrossRef]
- Curiel Yuste, J.; Nagy, M.; Janssens, I.A.; Carrara, A.; Ceulemans, R. Soil respiration in a mixed temperate forest and its contribution to total ecosystem respiration. Tree Physiol. 2005, 25, 609–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, E.A.; Richardson, A.D.; Savage, K.E.; Hollinger, D.Y. A distinct seasonal pattern of the ratio of soil respiration to total ecosystem respiration in a spruce- dominated forest. Glob. Chang. Biol. 2006, 12, 230–239. [Google Scholar] [CrossRef]
- Salomón, R.; Rodríguez-Calcerrada, J.; Staudt, M. Carbon losses from respiration and emission of volatile organic compounds—The overlooked side of tree carbon budgets. In Oaks Physiological Ecology, Exploring the Functional Diversity of Genus Quercus L.; Gil-Pelegrín, E., Peguero-Pina, J.J., Sancho-Knapik, D., Eds.; Tree Physiology 7; Springer International Publishing: Basel, Switzerland, 2017; pp. 327–359. [Google Scholar]
- Tedeschi, V.; Rey, A.; Manca, G.; Valentini, R.; Jarvis, P.G.; Borghetti, M. Soil respiration in a Mediterranean oak forest at different developmental stages after coppicing. Glob. Chang. Biol. 2006, 12, 110–121. [Google Scholar] [CrossRef]
- Fernández-Alonso, M.J.; Díaz-Pinés, E.; Ortiz, C.; Rubio, A. Disentangling the effect of tree species and microclimate on heterotrophic and autotrophic soil respiration in a Mediterranean ecotone forest. For. Ecol. Manag. 2018, 430, 533–544. [Google Scholar] [CrossRef]
- Ruiz-Benito, P.; Ratcliffe, S.; Zavala, M.A.; Martínez-Vilalta, J.; Vilà-Cabrera, A.; Lloret, F.; Madrigal-González, J.; Wirth, C.; Greenwood, S.; Kändler, G.; et al. Climate- and successional-related changes in functional composition of European forests are strongly driven by tree mortality. Glob. Chang. Biol. 2017, 23, 4162–4176. [Google Scholar] [CrossRef] [PubMed]
- Yermakov, Z.; Rothstein, D.E. Changes in soil carbon and nitrogen cycling along a 72-year wildfire chronosequence in Michigan jack pine forests. Oecologia 2006, 149, 690–700. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, D.; Zhou, G.; Liu, J. Soil respiration associated with forest succession in subtropical forests in Dinghushan Biosphere Reserve. Soil Biol. Biochem. 2009, 41, 991–999. [Google Scholar] [CrossRef]
- Curiel Yuste, J.; Flores-Rentería, D.; García-Angulo, D.; Hereş, A.-M.; Bragă, C.; Petritan, A.-M.; Petritan, I.C. Cascading effects associated with climate-change-induced conifer mortality in mountain temperate forests result in hot-spots of soil CO2 emissions. Soil Biol. Biochem. 2019, 133, 50–59. [Google Scholar] [CrossRef]
- Avila, J.M.; Gallardo, A.; Ibáñez, B.; Gómez-Aparicio, L. Quercus suber dieback alters soil respiration and nutrient availability in Mediterranean forests. J. Ecol. 2016, 104, 1441–1452. [Google Scholar] [CrossRef]
- Högberg, P.; Nordgren, A.; Buchmann, N.; Taylor, A.F.S.; Ekblad, A.; Högberg, M.N.; Nyberg, G.; Ottosson-Lofvenius, M.; Read, D.J. Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 2001, 411, 789–792. [Google Scholar] [CrossRef]
- Epron, D.; Ngao, J.; Dannoura, M.; Bakker, M.R.; Zeller, B.; Bazot, S.; Bosc, A.; Plain, C.; Lata, J.C.; Priault, P.; et al. Seasonal variations of belowground carbon transfer assessed by in situ 13 CO2 pulse labelling of trees. Biogeosciences 2011, 8, 1153–1168. [Google Scholar] [CrossRef]
- Drake, J.E.; Darby, B.A.; Giasson, M.-A.; Kramer, M.A.; Phillips, R.P.; Finzi, A.C. Stoichiometry constrains microbial response to root exudation—Insights from a model and a field experiment in a temperate forest. Biogeosciences 2013, 10, 821–838. [Google Scholar] [CrossRef]
- Pangle, R.E.; Seiler, J. Influence of seedling roots, environmental factors and soil characteristics on soil CO2 efflux rates in a 2-year-old loblolly pine (Pinus taeda L.) plantation in the Virginia Piedmont. Environ. Pollut. 2002, 116, 85–96. [Google Scholar] [CrossRef]
- Søe, A.R.B.; Buchmann, N. Spatial and temporal variations in soil respiration in relation to stand structure and soil parameters in an unmanaged beech forest. Tree Physiol. 2005, 25, 1427–1436. [Google Scholar] [CrossRef]
- Ngao, J.; Epron, D.; Delpierre, N.; Bréda, N.; Granier, A.; Longdoz, B. Spatial variability of soil CO2 efflux linked to soil parameters and ecosystems characteristics in a temperate beech forest. Agric. For. Meteorol. 2012, 154, 136–146. [Google Scholar] [CrossRef]
- Salomón, R.; Valbuena-Carabaña, M.; Rodríguez-Calcerrada, J.; Aubrey, D.; McGuire, M.A.; Teskey, R.; Gil, L.; González-Doncel, I. Xylem and soil CO2 fluxes in a Quercus pyrenaica Willd. coppice: Root respiration increases with clonal size. Ann. For. Sci. 2015, 72, 1065–1078. [Google Scholar] [CrossRef]
- Barba, J.; Curiel Yuste, J.; Martinez-Vilalta, J.; Lloret, F. Drought-induced tree species replacement is reflected in the spatial variability of soil respiration in a mixed Mediterranean forest. For. Ecol. Manag. 2013, 306, 79–87. [Google Scholar] [CrossRef]
- Barba, J.; Lloret, F.; Poyatos, R.; Molowny-Horas, R.; Curiel Yuste, J.C. Multi-temporal influence of vegetation on soil respiration in a drought-affected forest. iForest 2018, 11, 189. [Google Scholar] [CrossRef]
- Barba, J.; Curiel Yuste, J.; Poyatos, R.; Janssens, I.A.; Lloret, F. Strong resilience of soil respiration components to drought-induced die-off resulting in forest secondary succession. Oecologia 2016, 182, 27–41. [Google Scholar] [CrossRef] [Green Version]
- Binkley, D.; Stape, J.L.; Takahashi, E.N.; Ryan, M.G. Tree-girdling to separate root and heterotrophic respiration in two Eucalyptus stands in Brazil. Oecologia 2006, 148, 447–454. [Google Scholar] [CrossRef]
- Levy-Varon, J.H.; Schuster, W.S.F.; Griffin, K.L. Rapid rebound of soil respiration following partial stand disturbance by tree girdling in a temperate deciduous forest. Oecologia 2014, 174, 1415–1424. [Google Scholar] [CrossRef]
- Epron, D.; Le Dantec, V.; Dufrene, E.; Granier, A. Seasonal dynamics of soil carbon dioxide efflux and simulated rhizosphere respiration in a beech forest. Tree Physiol. 2001, 21, 145–152. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–58. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, S.; Liu, Q.; Jiang, J. Influences of soil moisture on litter respiration in the semiarid loess plateau. PLoS ONE 2014, 9, e114558. [Google Scholar] [CrossRef]
- Reichstein, M.; Rey, A.; Freibauer, A.; Tenhunen, J.; Valentini, R.; Banza, J.; Casals, P.; Cheng, Y.; Grünzweig, J.M.; Irvine, J.; et al. Modeling temporal and large-scale spatial variability of soil respiration from soil water availability, temperature and vegetation productivity indices. Glob. Biogeochem. Cy. 2003, 4, 1104. [Google Scholar] [CrossRef]
- Curiel Yuste, J.; Janssens, I.A.; Carrara, A.; Meiresonne, L.; Ceulemans, R. Interactive effects of temperature and precipitation on soil respiration in a temperate maritime pine forest. Tree Physiol. 2003, 23, 1263–1270. [Google Scholar]
- Sardans, J.; Peñuelas, J. Drought decreases soil enzyme activity in a Mediterranean Quercus ilex L. forest. Soil Biol. Biochem. 2005, 37, 455–461. [Google Scholar] [CrossRef]
- Raich, J.W.; Potter, C.S.; Bhagawati, D. Interannual variability in global soil respiration, 1980–1994. Glob. Chang. Biol. 2002, 8, 800–812. [Google Scholar] [CrossRef]
- Kosugi, Y.; Mitani, T.; Itoh, M.; Noguchi, S.; Tani, M.; Matsuo, N.; Takanashi, S.; Ohkubo, S.; Nik, A.R. Spatial and temporal variation in soil respiration in a Southeast Asian tropical rainforest. Agric. For. Meteorol. 2007, 147, 35–47. [Google Scholar] [CrossRef]
- Misson, L.; Rocheteau, A.; Rambal, S.; Ourcival, J.-M.; Limousin, J.-M.; Rodríguez, R. Functional changes in the control of carbon fluxes after 3 years of increased drought in a Mediterranean evergreen forest? Glob. Chang. Biol. 2010, 16, 2461–2475. [Google Scholar] [CrossRef]
- Matías, L.; Castro, J.; Zamora, R. Effect of simulated climate change on soil respiration in a Mediterranean-type ecosystem: Rainfall and habitat type are more important than temperature on the soil carbon pool. Ecosystems 2012, 15, 299–310. [Google Scholar] [CrossRef]
- Campo, J.; Merino, A. Variations in soil carbon sequestration and their determinants along a precipitation gradient in seasonally dry tropical forest ecosystems. Glob. Chang. Biol. 2016, 22, 1942–1956. [Google Scholar] [CrossRef]
- Almagro, M.; López, J.; Querejeta, J.I.; Martínez-Mena, M. Temperature dependence of soil CO2 efflux is strongly modulated by seasonal patterns of moisture availability in a Mediterranean ecosystem. Soil Biol. Biochem. 2009, 41, 594–605. [Google Scholar] [CrossRef]
- Kutsch, W.L.; Staack, A.; Wötzel, J.; Middelhoff, U.; Kappen, L. Field measurements of root respiration and total soil respiration in an alder forest. New Phytol. 2001, 150, 157–168. [Google Scholar] [CrossRef]
- Domínguez, M.T.; Sowerby, A.; Smith, A.R.; Robinson, D.A.; Van Baarsel, S.; Mills, R.T.E.; Marshall, M.R.; Koller, E.; Lebron, I.; Hall, J.; et al. Sustained impact of drought on wet shrublands mediated by soil physical changes. Biogeochemistry 2015, 122, 151–163. [Google Scholar] [CrossRef]
- Aussenac, G. Interactions between forest stands and microclimate: Ecophysiological aspects and consequences for silviculture. Ann. For. Sci. 2000, 57, 287–301. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands undersevere drought: A review of ecophysiological responses, adaptation processes and long-term conse-quences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Rodríguez-Calcerrada, J.; Mutke, S.; Alonso, J.; Gil, L.; Pardos, J.A.; Aranda, I. Influence of overstory density on understory light, soil moisture, and survival of two underplanted oak species in a Mediterranean montane Scots pine forest. For. Syst. 2008, 17, 31–38. [Google Scholar] [CrossRef]
- Gil, L.; Alonso Nager, J.; Aranda García, I.; González Doncel, I.; Gonzalo Jiménez, J.; López de Heredia, U.; Millerón, M.; Nanos, N.; Perea García-Calvo, R.; Rodríguez-Calcerrada, J.; et al. El Hayedo de Montejo—Una Gestión Sostenible; Dirección General de Medio Ambiente: Madrid, Spain, 2010.
- Curiel Yuste, J.; Barba, J.; Fernández-Gonzalez, J.A.; Fernández-López, M.; Mattana, S.; Nolis, P.; Lloret, F. Changes in soil bacterial community triggered by drought-induced gap succession preceded changes in soil C stocks and quality. Ecol. Evol. 2012, 2, 3016–3031. [Google Scholar] [CrossRef] [Green Version]
- Buckee, G.K. Determination of total nitrogen in Barley, Malt and Beer by Kjeldahl procedures and the Dimas combustion method. J. Inst. Brew. 1994, 100, 57–64. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Gelman, A.; Hill, J. Data Analysis Using Regression and Multilevel/Hierarchical Models; Cambridge University Press: New York, NY, USA, 2007. [Google Scholar]
- Warton, D.I.; Duursma, R.A.; Falster, D.S.; Taskinen, S. SMATR 3—An R package for estimation and inference about allometric lines. Methods Ecol. Evol. 2012, 3, 257–259. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DepRoy, S. Linear and Nonlinear Mixed Effects Models. In R Package Version 3; R Foundation for Statistical Computing: Vienna, Austria, 2009; pp. 1–96. [Google Scholar]
- Barton, K. MuMIn: Multi-model inference. In R Package Version 3; R Foundation for Statistical Computing: Vienna, Austria, 2014; pp. 1–96. [Google Scholar]
- Nave, L.E.; Gough, C.M.; Maurer, K.D.; Bohrer, G.; Hardiman, B.S.; Le Moine, J.; Munoz, A.B.; Nadelhoffer, K.J.; Sparks, J.P.; Strahm, B.D.; et al. Disturbance and the resilience of coupled carbon and nitrogen cycling in a north temperate forest. J. Geophys. Res. Biogeosci. 2011, 116, G04016. [Google Scholar] [CrossRef]
- Pereira-Blanco, E. Response of Fine Root Respiration to Variations in Biotic and Abiotic Factors in A Mixed Mediterranean Forest Affected by Drought Induced Secondary Succession. Master’s Thesis, Digital Repository, Autonomous University of Barcelona, Bellaterra, Spain, 2014; pp. 1–27. [Google Scholar]
- McCarthy, J. Gap dynamics of forest trees: A review with particular attention to boreal forests. Environ. Rev. 2001, 9, 1–59. [Google Scholar] [CrossRef]
- Moore, D.J.; Trahan, N.A.; Wilkes, P.; Quaife, T.; Stephens, B.B.; Elder, K.; Desai, A.R.; Negron, J.; Monson, R.K. Persistent reduced ecosystem respiration after insect disturbance in high elevation forests. Ecol. Lett. 2013, 16, 731–737. [Google Scholar] [CrossRef] [Green Version]
- Borkhuu, B.; Peckham, S.D.; Ewers, B.E.; Norton, U.; Pendall, E. Does soil respiration decline following bark beetle induced forest mortality? Evidence from a lodgepole pine forest. Agric. For. Meteorol. 2015, 214, 201–207. [Google Scholar] [CrossRef]
- Ngao, J.; Longdoz, B.; Granier, A.; Epron, D. Separating autotrophic and heterotrophic respiration in two young beech stands: Impact of corrections for root decomposition in trenched plots. Plant Soil 2007, 301, 99–110. [Google Scholar] [CrossRef]
- Curiel Yuste, J.; Janssens, I.A.; Carrara, A.; Ceulemans, R. Annual Q10 of soil respiration reflects plant phenological patterns as well as temperature sensitivity. Glob. Chang. Biol. 2004, 161–169. [Google Scholar] [CrossRef]
- Subke, J.-A.; Bahn, M. On the “temperature sensitivity” of soil respiration: Can we use the immeasurable to predict the unknown? Soil Biol. Biochem. 2010, 42, 1653–1656. [Google Scholar] [CrossRef]
- Buchmann, N. Biotic and abiotic factors controlling soil respiration rates in Picea abies stands. Soil Biol. Biochem. 2000, 32, 1625–1635. [Google Scholar] [CrossRef]
- Curiel Yuste, J.; Baldocchi, D.D.; Gershenson, A.; Godlstein, A.; Misson, L.; Wong, S. Microbial soil respiration and its dependency on carbon inputs, soil temperature and moisture. Glob. Chang. Biol. 2007, 13, 2018–2035. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Hou, E.; Liu, Y.; Wen, D. Altitudinal patterns and controls of plant and soil nutrient concentrations and stoichiometry in subtropical China. Sci. Rep. 2016, 6, 24261. [Google Scholar] [CrossRef]
- Liu, Z.-P.; Shao, M.-A.; Wang, Y.-Q. Spatial patterns of soil total nitrogen and soil total phosphorus across the entire Loess Plateau region of China. Geoderma 2013, 197, 67–78. [Google Scholar] [CrossRef]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The C: N: P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect. Plant Ecol. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Pardo, F.; Gil, L.; Pardos, J.A. Field study of beech (Fagus sylvatica L.) and melojo oak (Quercus pyrenaica Willd.) leaf litter decomposition in the centre of the Iberian Peninsula. Plant Soil 1997, 191, 89–100. [Google Scholar] [CrossRef]
- Jin, V.L.; Haneu, R.L.; Fay, P.A.; Polley, H.W. Soil type and moisture regime control microbial C and N mineralization in grassland soils more than atmospheric CO2-induced changes in litter quality. Soil Biol. Biochem. 2013, 58, 172–180. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persisntent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Wood, T.E.; Detto, M.; Silver, W.L. Sensitivity of soil respiration to variability in soil moisture and temperature in a humid tropical forest. PLoS ONE 2013, 8, e80965. [Google Scholar] [CrossRef]
- Gao, X.; Giorgi, F. Increased aridity in the Mediterranean region under greenhouse gas forcing estimated from high resolution simulations with a regional climate model. Glob. Planet. Chang. 2008, 62, 195–209. [Google Scholar] [CrossRef]
- Comeau, L.-P.; Lai, D.Y.F.; Cui, J.J.; Farmer, J. Separation of soil respiration: A site-specific comparison of partition methods. Soil 2018, 4, 141–152. [Google Scholar] [CrossRef]





| Vigorous | Affected | Severely Affected | Dead | |
|---|---|---|---|---|
| Crown dieback 1 | 6.3 ± 3.8 a | 25 ± 5.4 a | 68.8 ± 8.3 b | 100 ± 0 c |
| Herb. cover 1 | 3.7 ± 1.3 | 3.2 ± 2.0 | 11.1 ± 4.4 | 12.7 ± 4.7 |
| Shrub cover 1 | 4.2 ± 3.2 | 10.4 ± 4.9 | 13.3 ± 8.4 | 12.3 ± 3.9 |
| Stem density (ALL) | 1003 ± 486 | 2045 ± 543 | 2690 ± 592 | 2355 ± 185 |
| Basal area (ALL) | 48.8 ± 9.7 | 36.9 ± 5.7 | 37.7 ± 6.6 | 27.4 ± 9.5 |
| Stem density (REC) | 963 ± 491 | 2021 ± 538 | 2658 ± 592 | 2340 ± 183 |
| Basal area (REC) | 5.3 ± 1.8 | 12 ± 4.1 | 15.6 ± 6.6 | 18 ± 1.3 |
| Soil litter depth | 5.3 ± 0.3 | 5.3 ± 0.3 | 5.3 ± 0.8 | 4.1 ± 0.6 |
| SWC | 18.8 ± 0.2 | 19.4 ± 0.1 | 18.5 ± 0.1 | 21.4 ± 0.3 |
| SWC summer | 10.7 ± 0.2 | 11.0 ± 0.2 | 9.6 ± 0.2 | 10.7 ± 0.4 |
| Soil bulk density | 0.77 ± 0.01 | 0.78 ± 0.01 | 0.76 ± 0.01 | 0.73 ± 0.01 |
| Soil OM | 9.7 ± 1.0 | 8.3 ± 0.6 | 8.9 ± 0.4 | 8.8 ± 0.6 |
| Soil total carbon | 7.7 ± 0.8 | 6.5 ± 0.5 | 7.2 ± 0.4 | 7.4 ± 0.7 |
| Soil total nitrogen | 0.48 ± 0.05 | 0.41 ± 0.04 | 0.45 ± 0.04 | 0.49 ± 0.06 |
| Soil C/N | 15.8 ± 0.1 | 16.1 ± 0.2 | 16.1 ± 0.1 | 15.2 ± 0.2 |
| Root density | 232 ± 4 | 208 ± 11 | 229 ± 6 | 224 ± 8 |
| Rs | 4.67 ± 0.08 | 3.95 ± 0.06 | 4.49 ± 0.14 | 4.29 ± 0.05 |
| Rs_11 °C | 4.51 ± 0.07 | 3.92 ± 0.07 | 4.31 ± 0.15 | 4.15 ± 0.07 |
| Rs_11 °C, 20% | 5.19 ± 0.06 | 4.41 ± 0.07 | 5.20 ± 0.12 | 4.81 ± 0.04 |
| Estimate | SE | DF | t-Value | p-Value | |
|---|---|---|---|---|---|
| Intercept | 0.226 | 0.184 | 186 | 1.225 | 0.222 |
| Ts | 0.829 | 0.163 | 186 | 5.088 | <0.001 |
| SWC | 0.103 | 0.154 | 186 | 0.665 | 0.507 |
| Ts × SWC | 0.233 | 0.088 | 186 | 2.646 | 0.009 |
| Tree-decline | |||||
| V | - | - | - | - | - a |
| A1 | −0.376 | 0.245 | 12 | −1.536 | 0.151 a |
| A2 | −0.026 | 0.245 | 12 | −0.105 | 0.918 a |
| D | −0.131 | 0.249 | 12 | −0.525 | 0.609 a |
| Season | |||||
| Spring | - | - | - | - | - a |
| Summer | 0.796 | 0.189 | 186 | 4.211 | <0.001 b |
| Fall | 1.828 | 0.478 | 186 | 3.821 | <0.001 c |
| Winter | 0.231 | 1.931 | 186 | 0.12 | 0.905 abc |
| Ts × season | |||||
| Ts × spring | - | - | - | - | - a |
| Ts × summer | 0.093 | 0.225 | 186 | 0.411 | 0.681 a |
| Ts × fall | 1.017 | 0.393 | 186 | 2.589 | 0.01 b |
| Ts × winter | 0.188 | 1.237 | 186 | 0.152 | 0.879 ab |
| SWC × season | |||||
| SWC × spring | - | - | - | - | - a |
| SWC × summer | 1.009 | 0.198 | 186 | 5.09 | <0.001 b |
| SWC × fall | −0.507 | 0.378 | 186 | −1.341 | 0.182 a |
| SWC × winter | 0.117 | 0.43 | 186 | 0.273 | 0.786 ab |
| Ts × tree-decline | |||||
| Ts × V | - | - | - | - | - a |
| Ts × A1 | −0.252 | 0.092 | 186 | −2.738 | 0.007 b |
| Ts × A2 | −0.105 | 0.091 | 186 | −1.157 | 0.249 ab |
| Ts × D | −0.147 | 0.097 | 186 | −1.517 | 0.131 ab |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Calcerrada, J.; Salomón, R.; Barba, J.; Gordaliza, G.G.; Curiel Yuste, J.; Magro, C.; Gil, L. Regeneration in the Understory of Declining Overstory Trees Contributes to Soil Respiration Homeostasis along Succession in a Sub-Mediterranean Beech Forest. Forests 2019, 10, 727. https://doi.org/10.3390/f10090727
Rodríguez-Calcerrada J, Salomón R, Barba J, Gordaliza GG, Curiel Yuste J, Magro C, Gil L. Regeneration in the Understory of Declining Overstory Trees Contributes to Soil Respiration Homeostasis along Succession in a Sub-Mediterranean Beech Forest. Forests. 2019; 10(9):727. https://doi.org/10.3390/f10090727
Chicago/Turabian StyleRodríguez-Calcerrada, Jesús, Roberto Salomón, Josep Barba, Guillermo G. Gordaliza, Jorge Curiel Yuste, Carlos Magro, and Luis Gil. 2019. "Regeneration in the Understory of Declining Overstory Trees Contributes to Soil Respiration Homeostasis along Succession in a Sub-Mediterranean Beech Forest" Forests 10, no. 9: 727. https://doi.org/10.3390/f10090727





