Foliar and Wood Traits Covary along a Vertical Gradient within the Crown of Long-Lived Light-Demanding Species of the Congo Basin Semi-Deciduous Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species and Location
2.2. Sample Collection
2.3. Traits Measurements
2.4. Statistical Analysis
3. Results
3.1. Trait Variation and Plasticity within the Crown
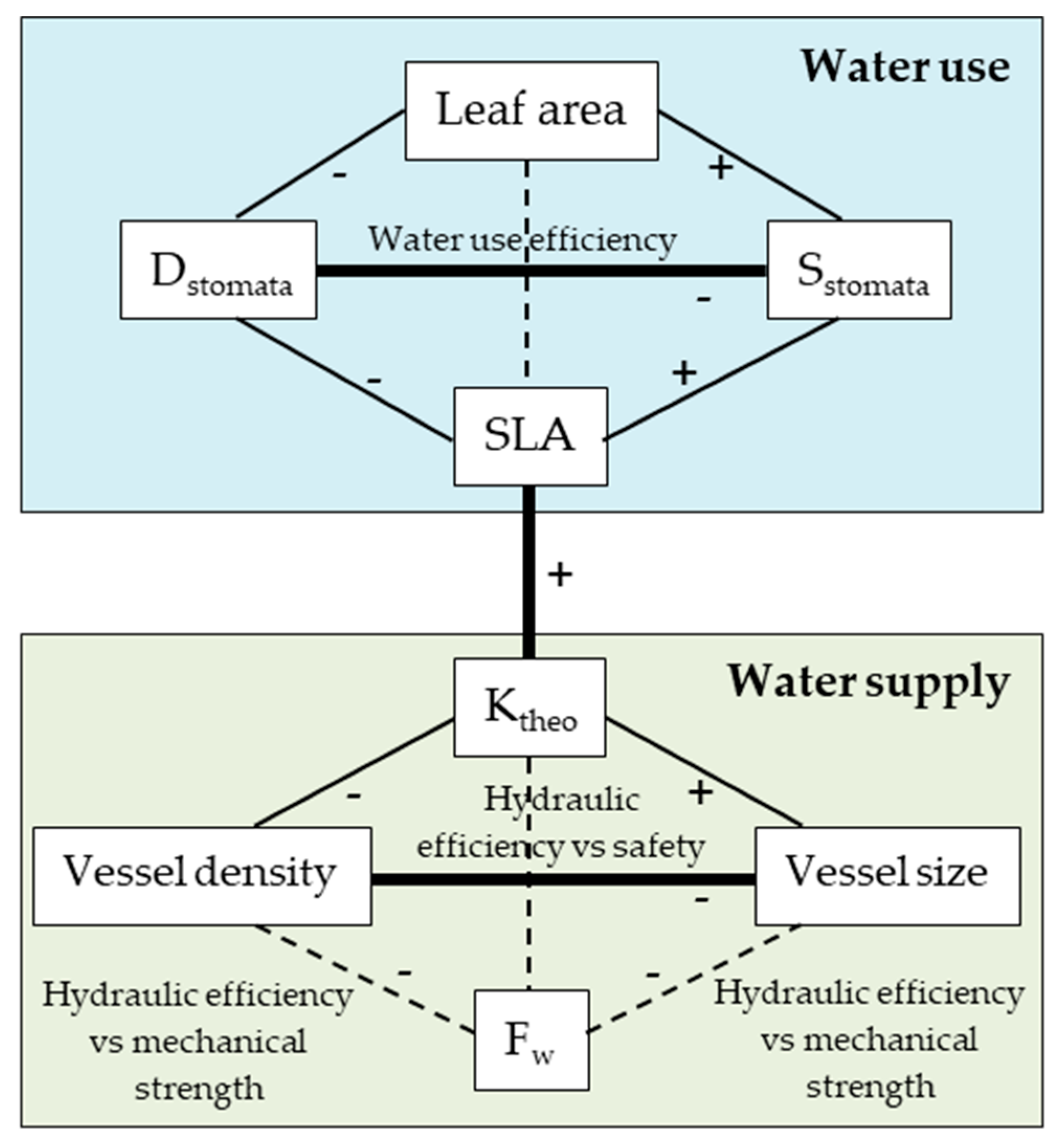
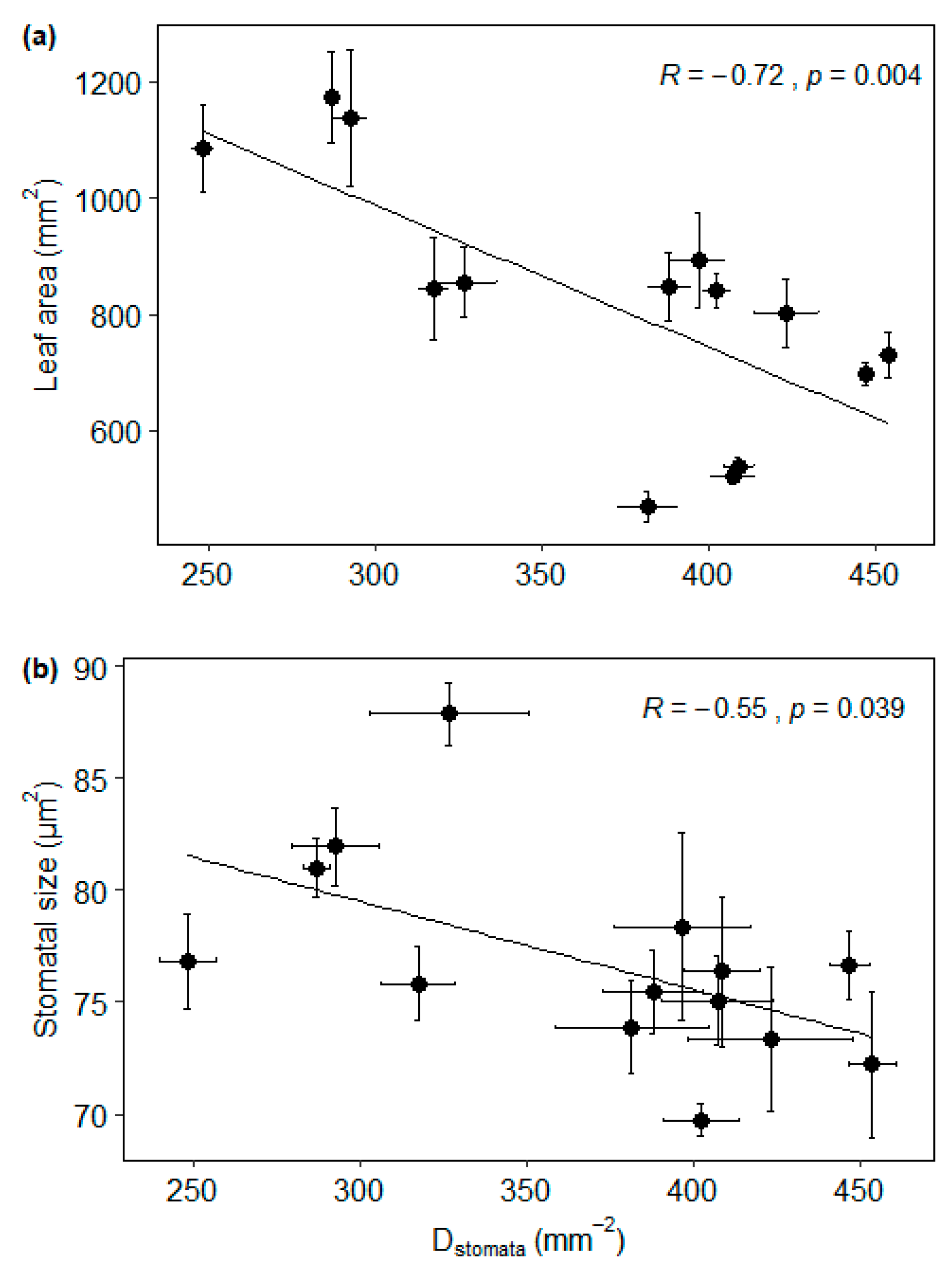
3.2. Trait Associations
3.3. The Effect of Sample Location Height on Trait Associations
4. Discussion
4.1. Trait Variation and Plasticity within the Crown
4.2. Trait Associations and the Effect of Sample Location Height
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beeckman, H. Wood Anatomy and Tait-based ecology. IAWA J. 2016, 37, 127–151. [Google Scholar] [CrossRef]
- Lachenbruch, B.; Mcculloh, K.A. Traits, properties, and performance: How woody plants combine hydraulic and mechanical functions in a cell, tissue, or whole plant. New Phytol. 2014, 204, 747–764. [Google Scholar] [CrossRef] [PubMed]
- Violle, C.; Navas, M.L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Weiher, E.; van der Werf, A.; Thompson, K.; Roderick, M.; Garnier, E.; Eriksson, O. Challenging Theophrastus: A common core list of plant traits for functional ecology. J. Veg. Sci. 1999, 10, 609–620. [Google Scholar] [CrossRef]
- Jung, V.; Violle, C.; Mondy, C.; Hoffmann, L.; Muller, S. Intraspecific variability and trait-based community assembly. J. Ecol. 2010, 98, 1134–1140. [Google Scholar] [CrossRef]
- Savage, J.A.; Cavender-Bares, J. Habitat specialization and the role of trait lability in structuring diverse willow (genus Salix) communities. Ecology 2012, 93, 138–150. [Google Scholar] [CrossRef] [Green Version]
- Greenwood, S.; Ruiz-Benito, P.; Martinez-Vilalta, J.; Lloret, F.; Kitzberger, T.; Allen, D.C.; Fensham, R.; Laughlin, D.C.; Kattge, J.; Bönisch, G.; et al. Tree mortality across biomes is promoted by drought intensity, lower wood density and higher specific leaf area. Ecol. Lett. 2017, 20, 539–553. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Valencia, R.; Ackerly, D.D. Functional traits and niche-based tree community assembly in an Amazonian forest. Science 2008, 322, 580–582. [Google Scholar] [CrossRef] [Green Version]
- Visser, M.D.; Bruijning, M.; Wright, S.J.; Muller-Landau, H.C.; Jongejans, E.; Comita, L.S.; de Kroon, H. Functional traits as predictors of vital rates across the life cycle of tropical trees. Funct. Ecol. 2016, 30, 168–180. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Lloret, F.; Breshears, D.D. Drought-induced forest decline: Causes, scope and implications. Biol. Lett. 2012, 8, 689–691. [Google Scholar] [CrossRef]
- Skelton, R.P.; West, A.G.; Dawson, T.E. Predicting plant vulnerability to drought in biodiverse regions using functional traits. Proc. Natl. Acad. Sci. USA 2015, 112, 5744–5749. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Niu, H.; Wang, S.; Zhu, X.; Luo, C.; Li, Y.; Zhao, X. Gene or environment? Species-specific control of stomatal density and length. Ecol. Evol. 2012, 2, 1065–1070. [Google Scholar] [CrossRef]
- Ladjal, M.; Huc, R.; Ducrey, M. Drought effects on hydraulic conductivity and xylem vulnerability to embolism in diverse species and provenances of Mediterranean cedars. Tree Physiol. 2005, 25, 1109–1117. [Google Scholar] [CrossRef] [Green Version]
- Lovisolo, C.; Schubert, A. Effects of water stress on vessel size and xylem hydraulic conductivity in Vitis vinifera L. J. Exp. Bot. 1998, 49, 693–700. [Google Scholar]
- Savi, T.; Bertuzzi, S.; Branca, S.; Tretiach, M.; Nardini, A. Drought-induced xylem cavitation and hydraulic deterioration: Risk factors for urban trees under climate change? New Phytol. 2015, 205, 1106–1116. [Google Scholar] [CrossRef] [Green Version]
- Paine, C.E.T.; Baraloto, C.; Chave, J.; Hérault, B. Functional traits of individual trees reveal ecological constraints on community assembly in tropical rain forests. Oikos 2011, 120, 720–727. [Google Scholar] [CrossRef]
- Albert, C.H.; Thuiller, W.; Yoccoz, N.G.; Douzet, R.; Aubert, S.; Lavorel, S. A multi-trait approach reveals the structure and the relative importance of intra- vs. interspecific variability in plant traits. Funct. Ecol. 2010, 24, 1192–1201. [Google Scholar] [CrossRef]
- Chacón-Madrigal, E.; Wanek, W.; Hietz, P.; Dullinger, S. Is local trait variation related to total range size of tropical trees? PLoS ONE 2018, 13, e0193268. [Google Scholar] [CrossRef]
- Liu, R.; Liang, S.; Long, W.; Jiang, Y. Variations in Leaf Functional Traits Across Ecological Scales in Riparian Plant Communities of the Lijiang River, Guilin, Southwest China. Trop. Conserv. Sci. 2018, 11, 194008291880468. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; He, N.; Zhang, J.; Li, Y.; Wang, Q.; Sack, L.; Yu, G. Variation of stomatal traits from cold temperate to tropical forests and association with water use efficiency. Funct. Ecol. 2018, 32, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Violle, C.; Enquist, B.J.; McGill, B.J.; Jiang, L.; Albert, C.H.; Hulshof, C.; Jung, V.; Messier, J. The return of the variance: Intraspecific variability in community ecology. Trends Ecol. Evol. 2012, 27, 244–252. [Google Scholar] [CrossRef]
- Anfodillo, T.; Carraro, V.; Carrer, M.; Fior, C.; Rossi, S. Convergent tapering of xylem conduits in different woody species. New Phytol. 2006, 169, 279–290. [Google Scholar] [CrossRef]
- Bourland, N.; Kouadio, L.Y.; Lejeune, P.; Sonké, B.; Philippart, J.; Daïnou, K.; Fétéké, F.; Doucet, J.-L. Ecology of Pericopsis elata (Fabaceae), an Endangered Timber Species in Southeastern Cameroon. Biotropica 2012, 44, 840–847. [Google Scholar]
- Boyemba, F. Ecologie de Pericopsis elata (Harms) Van Meeuwen (Fabaceae), Arbre de Forêt Tropicale Africaine à Répartition Agrégée. Ph.D. Thesis, Université Libre de Bruxelles, Bruxelles, Belgium, 2011, unpublished. [Google Scholar]
- De Ridder, M.; Toirambe, B.; Van den Bulcke, J.; Bourland, N.; Van Acker, J.; Beeckman, H. Dendrochronological potential in a semi-deciduous rainforest: The case of Pericopsis elata in central Africa. Forests 2014, 5, 3087–3106. [Google Scholar] [CrossRef]
- Hall, J.; Swaine, M.D. Distribution and Ecology of Vascular Plants in A Tropical Rain Forest; Werger, M.J., Ed.; Geobotany W. Junk: The Hague, The Netherlands, 1981; ISBN 9789400986527. [Google Scholar]
- White, F. La Végétation de L’afrique; ORSTOM-UNESCO: Paris, France, 1986; ISBN 9232019558. [Google Scholar]
- Oumbe, A.; Wald, L. A parameterisation of vertical profile of solar irradiance for correcting solar fluxes for changes in terrain elevation. In Proceedings of the Earth Observation and Water Cycle Science Conference, Frascati, Italy, 4 April 2010; p. S05. [Google Scholar]
- Jansen, S.; Kitin, P.; De Pauw, H.; Idris, M.; Beeckman, H.; Smets, E. Preparation of wood specimens for transmitted light microscopy and scanning electron microscopy. Belg. J. Bot. 1998, 131, 41–49. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef]
- Tyree, M.T.; Zimmermann, M.H. Xylem Structure and the Ascent of Sap; Timell, T.E., Ed.; Wood Scien.: Karnataka, India; Springer Verlag: Berlin, Germany, 2002; ISBN 9783642640711. [Google Scholar]
- Fortunel, C.; Ruelle, J.; Beauchêne, J.; Fine, P.V.A.; Baraloto, C. Wood specific gravity and anatomy of branches and roots in 113 Amazonian rainforest tree species across environmental gradients. New Phytol. 2014, 202, 79–94. [Google Scholar] [CrossRef]
- Zanne, A.E.; Westoby, M.; Falster, D.S.; Ackerly, D.D.; Loarie, S.R.; Arnold, S.E.J.; Coomes, D.A. Angiosperm wood structure: Global patterns in vessel anatomy and their relation to wood density and potential conductivity. Am. J. Bot. 2010, 97, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Sterck, F.J.; Zweifel, R.; Sass-Klaassen, U.; Chowdhury, Q. Persisting soil drought reduces leaf specific conductivity in Scots pine (Pinus sylvestris) and pubescent oak (Quercus pubescens). Tree Physiol. 2008, 28, 529–536. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Taylor, S.H.; Franks, P.J.; Hulme, S.P.; Spriggs, E.; Christin, P.A.; Edwards, E.J.; Woodward, F.I.; Osborne, C.P. Photosynthetic pathway and ecological adaptation explain stomatal trait diversity amongst grasses. New Phytol. 2012, 193, 387–396. [Google Scholar] [CrossRef]
- Brown, H.T.; Escombe, F. Static diffusion of gases and liquids in relation to the assimilation of carbon and translocation in plants. Ann. Bot. 1900, 14, 537–542. [Google Scholar] [CrossRef]
- Franks, P.J.; Farquhar, C.D. The Mechanical Diversity of Stomata and Its Significance in Gas-Exchange Control. Plant Physiol. 2006, 143, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Dow, G.J.; Bergmann, D.C.; Berry, J.A. An integrated model of stomatal development and leaf physiology. New Phytol. 2014, 201, 1218–1226. [Google Scholar] [CrossRef]
- Fanourakis, D.; Giday, H.; Milla, R.; Pieruschka, R.; Kjaer, K.H.; Bolger, M.; Vasilevski, A.; Nunes-Nesi, A.; Fiorani, F.; Ottosen, C.O. Pore size regulates operating stomatal conductance, while stomatal densities drive the partitioning of conductance between leaf sides. Ann. Bot. 2015, 115, 555–565. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2018; Volume 1, pp. 1–2630. [Google Scholar]
- Shieh, G. Clarifying the role of mean centring in multicollinearity of interaction effects. Br. J. Math. Stat. Psychol. 2011, 64, 462–477. [Google Scholar] [CrossRef]
- Logan, M. Biostatistical Design and Analysis Using R: A Practical Guide; Wiley-Blackwell: Oxford, UK, 2010; ISBN 9781444335248. [Google Scholar]
- Siefert, A. Incorporating intraspecific variation in tests of trait-based community assembly. Oecologia 2012, 170, 767–775. [Google Scholar] [CrossRef]
- Ordoñez, J.C.; Van Bodegom, P.M.; Witte, J.P.M.; Bartholomeus, R.P.; Van Dobben, H.F.; Aerts, R. Leaf habit and woodiness regulate different leaf economy traits at a given nutrient supply. Ecology 2010, 91, 3218–3228. [Google Scholar] [CrossRef]
- Oladi, R.; Bräuning, A.; Pourtahmasi, K. “Plastic” and “static” behavior of vessel-anatomical features in Oriental beech (Fagus orientalis Lipsky) in view of xylem hydraulic conductivity. Trees-Struct. Funct. 2014, 28, 493–502. [Google Scholar] [CrossRef]
- Giagli, K.; Gričar, J.; Vavrčík, H.; Menšík, L.; Gryc, V. The effects of drought on wood formation in Fagus Sylvatica during two contrasting years. IAWA J. 2016, 37, 332–348. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.M.; Miller, J.R. Determining digital hemispherical photograph exposure for leaf area index estimation. Agric. For. Meteorol. 2005, 133, 166–181. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G. Effects of water stress and nocturnal temperature on carbon allocation in the perennial grass, Leymus chinensis. Physiol. Plant. 2005, 123, 272–280. [Google Scholar] [CrossRef]
- Martínez, J.P.; Silva, H.; Ledent, J.F.; Pinto, M. Effect of drought stress on the osmotic adjustment, cell wall elasticity and cell volume of six cultivars of common beans (Phaseolus vulgaris L.). Eur. J. Agron. 2007, 26, 30–38. [Google Scholar] [CrossRef]
- Hubbard, R.M.; Bond, B.J.; Senock, R.S.; Ryan, M.G. Effects of branch height on leaf gas exchange, branch hydraulic conductance and branch sap flux in open-grown ponderosa pine. Tree Physiol. 2002, 22, 575–581. [Google Scholar] [CrossRef] [Green Version]
- Delzon, S.; Sartore, M.; Burlett, R.; Dewar, R.; Loustau, D. Hydraulic responses to height growth in maritime pine trees. Plant Cell Environ. 2004, 27, 1077–1087. [Google Scholar] [CrossRef]
- Zaehle, S. Effect of height on tree hydraulic conductance incompletely compensated by xylem tapering. Funct. Ecol. 2005, 19, 359–364. [Google Scholar] [CrossRef]
- Whitehead, D. Regulations of stomatal conductance and transpiration in forest canopies. Tree Physiol. 1998, 18, 633–644. [Google Scholar] [CrossRef]
- McDowell, N.; Barnard, H.; Bond, B.J.; Hinckley, T.; Hubbard, R.M.; Ishii, H.; Köstner, B.; Magnani, F.; Marshall, J.D.; Meinzer, F.C.; et al. The relationship between tree height and leaf area: Sapwood area ratio. Oecologia 2002, 132, 12–20. [Google Scholar] [CrossRef]
- Butz, P.; Hölscher, D.; Cueva, E.; Graefe, S. Tree Water Use Patterns as Influenced by Phenology in a Dry Forest of Southern Ecuador. Front. Plant Sci. 2018, 9, 945. [Google Scholar] [CrossRef] [Green Version]
- Sperry, J.S.; Meinzer, F.C.; McCulloh, K.A. Safety and efficiency conflicts in hydraulic architecture: Scaling from tissues to trees. Plant Cell Environ. 2008, 31, 632–645. [Google Scholar] [CrossRef]
- Fichtler, E.; Worbes, M. Wood anatomical variables in tropical trees and their relation to site conditions and individual tree morphology. IAWA J. 2012, 33, 119–140. [Google Scholar] [CrossRef]
- Fan, Z.-X.; Sterck, F.; Zhang, S.-B.; Fu, P.-L.; Hao, G.-Y. Tradeoff between Stem Hydraulic Efficiency and Mechanical Strength Affects Leaf–Stem Allometry in 28 Ficus Tree Species. Front. Plant Sci. 2017, 8, 1619–1629. [Google Scholar] [CrossRef] [Green Version]
- Santiago, L.S.; Goldstein, B.G.; Meinzer, F.C.; Fisher, J.B.; Maehado, K.; Woodruff, D.; Jones, T. Leaf photosynthetic traits scale with hydraulic conductivity and wood density in Panamanian forest canopy trees. Oecologia 2004, 140, 543–550. [Google Scholar] [CrossRef]
- Jacobsen, A.L.; Pratt, R.B.; Ewers, F.W.; Davis, S.D. Cavitation resistance among 26 chaparral species of southern California. Ecol. Monogr. 2007, 77, 99–115. [Google Scholar] [CrossRef]
- Franks, P.J.; Drake, P.L.; Beerling, D.J. Plasticity in maximum stomatal conductance constrained by negative correlation between stomatal size and density: An analysis using Eucalyptus globulus. Plant Cell Environ. 2009, 32, 1737–1748. [Google Scholar] [CrossRef]
- Douhovnikoff, V.; Taylor, S.H.; Hazelton, E.L.G.; Smith, C.M.; O’Brien, J. Maximal stomatal conductance to water and plasticity in stomatal traits differ between native and invasive introduced lineages of Phragmites australis in North America. AoB Plants 2016, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Drake, R.E.; Frey, W.; Bond, G.R.; Goldman, H.H.; Salkever, D.; Miller, A.; Moore, T.A.; Riley, J.; Karakus, M.; Milfort, R. Assisting social security disability insurance beneficiaries with schizophrenia, bipolar disorder, or major depression in returning to work. Am. J. Psychiatry 2013, 170, 1433–1441. [Google Scholar] [CrossRef]
- Aasamaa, K.; Sõber, A.; Rahi, M. Leaf anatomical characteristics associated with shoot hydraulic conductance, stomatal conductance and stomatal sensitivity to changes of leaf water status in temperate deciduous trees. Funct. Plant Biol. 2001, 28, 765–774. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Portsmuth, A.; Tena, D.; Tobias, M.; Matesanz, S.; Valladares, F. Do we underestimate the importance of leaf size in plant economics? Disproportional scaling of support costs within the spectrum of leaf physiognomy. Ann. Bot. 2007, 100, 283–303. [Google Scholar] [CrossRef]


| Museum Code | Longitude | Latitude | Ht (m) | DBH (cm) |
|---|---|---|---|---|
| Tw69042 | 25.42097 | 0.11895 | 41.0 | 99.5 |
| Tw69043 | 25.42125 | 0.12157 | 43.3 | 126.0 |
| Tw69045 | 25.40137 | 0.11743 | 42.0 | 105.3 |
| Tw69046 | 25.39676 | 0.11752 | 40.9 | 142.5 |
| Tw69047 | 25.39537 | 0.14871 | 42.4 | 93.9 |
| Traits | 95% CI Statistics | Plasticity (%) | ||||
|---|---|---|---|---|---|---|
| Mean | n | Range | SE | CV | ||
| Wood Traits | ||||||
| D (µm) | 42.45 | 660 | 21.3–64.3 | 0.3 | 0.18 | 23.4 ± 4.8 |
| A (µm2) | 1303.4 | 660 | 319.3–2798.2 | 18 | 0.36 | 42.1 ± 6.8 |
| Fw (µm) | 3.08 | 660 | 1.15–5.95 | 0.04 | 0.25 | 29.7 ± 12.5 |
| N (mm−1) | 186.72 | 22 | 121.9–256.3 | 6.5 | 0.16 | 26.6 ± 4.4 |
| Dh (µm) | 43.59 | 22 | 33.2–53.3 | 1.13 | 0.12 | 21.6 ± 3.4 |
| Ktheo (kg m−1 MPa s−1) | 0.86 | 22 | 0.25–1.74 | 0.08 | 0.44 | 57.7 ± 12.8 |
| Leaf Traits | ||||||
| LA (mm2) | 703.83 | 119 | 175–1739 | 27.64 | 0.43 | 33.9 ± 13.1 |
| LDM (mg) | 54.76 | 119 | 11.3–152 | 2.52 | 0.50 | 40.5 ± 15.3 |
| SLA (mm.mg−1) | 13.84 | 119 | 6.7–23.6 | 0.33 | 0.26 | 27.3 ± 16.4 |
| Dstomata (mm−2) | 374.16 | 90 | 208–553.1 | 8.95 | 0.23 | 28.9 ± 8.9 |
| Stomatal size (µm) | 76.64 | 69 | 64–91 | 0.75 | 0.08 | 8.1 ± 4.5 |
| gsmax (mole m−2 s−1) | 1.39 | 69 | 0.73–2.03 | 0.04 | 0.22 | 27.3 ± 10.9 |
| Traits | % Variance of Traits (95% CI) | Model | |||
|---|---|---|---|---|---|
| Tree | Hs | Error | R2 | p-Value | |
| Wood traits | |||||
| D (µm) | 8.7 (8.4–9.0) | 36.9 (35.9–37.9) | 54.4 (53.0–55.8) | 0.46 | *** |
| A (µm2) | 10.0 (9.7–10.3) | 36.5 (35.5–37.5) | 53.5 (52.0–55.0) | 0.46 | *** |
| Fw (µm) | 4.8 (4.6–5.0) | 34.5 (33.5–35.3) | 60.8 (59.3–62.5) | 0.39 | *** |
| N | 35.1 (34.1–36.1) | - | 64.9 (63.2–66.6) | 0.35 | *** |
| Dh | 8.1 (7.8–8.4) | - | 91.9 (89.5–94.3) | 0.10 | *** |
| Ktheo | 49.1 (47.7–50.5) | - | 50.9 (49.5–52.3) | 0.49 | ** |
| Leaf traits | |||||
| LA (mm2) | 41.1 (40.0–42.2) | 11.1 (10.8–11.4) | 47.8 (46.6–49.2) | 0.52 | *** |
| LDM (mg) | 53.5 (52.1–54.9) | 14.1 (13.7–14.5) | 32.4 (31.5–33.3) | 0.68 | *** |
| SLA (mm.mg−1) | 41.5 (40.4–42.6) | 35.1 (34.1–36.1) | 23.3 (22.7–23.9) | 0.77 | *** |
| Dstomata (mm−2) | 29.3 (28.5–30.1) | 17.2 (16.6–17.6) | 53.5 (52.1–54.9) | 0.46 | *** |
| Stomatal size (µm) | 32.8 (31.9–33.7) | 9.2 (8.9–9.5) | 58.0 (56.5–59.5) | 0.42 | *** |
| gsmax (mole m−2 s−1) | 25.1 (24.4–25.8) | 17.9 (17.4–18.4) | 57.0 (55.5–58.5) | 0.43 | ** |
| Model | Model Fit | ||||||
|---|---|---|---|---|---|---|---|
| R2 | F | p | |||||
| Leaf | |||||||
| Dstomata ~ SLA × Hs | −13.53 * | −9.61 * | −1.75 ns | 2.90 ns | 0.22 | 5.69 | ** |
| gs ~ SLA × Hs | −0.075 ** | −0.055 ** | −0.015 ns | 0.005 ns | 0.42 | 4.51 | * |
| S ~ Dstomata × Hs | −0.021 ns | −0.029 * | −0.046 *** | −0.055 ** | 0.25 | 6.81 | *** |
| LA ~ Dstomata × Hs | −2.096 * | −1.802 ** | −1.212 ns | −0.918 ns | 0.22 | 5.79 | ** |
| Wood | |||||||
| Ktheo ~ T × Hs | 0.038 ns | −0.235 ns | −0.782 ** | −1.056 * | 0.45 | 4.12 | * |
| VF ~ Ktheo × Hs | 0.039 ns | 0.075 ns | 0.147 *** | 0.182 ** | 0.57 | 7.03 | ** |
| Fw ~ Ktheo × Hs | 0.219 ns | 0.039 ns | −0.321 ns | −0.501 ns | 0.07 | 0.39 | ns |
| Dh ~ VD × Hs | −0.293 * | −0.188 * | 0.021 ns | 0.126 ns | 0.27 | 2.01 | * |
| Ktheo ~ VD × Hs | −0.009 ns | −0.005 ns | 0.004 ns | 0.009 ns | 0.22 | 1.51 | ns |
| Fw ~ VD × Hs | −0.012 ns | −0.008 ns | −0.004 ns | −0.002 ns | 0.15 | 0.90 | ns |
| Leaf vs. wood | |||||||
| Ktheo ~ LDM × Hs | 0.093 ns | 0.029 ns | −0.096 ns | −0.159 ns | 0.12 | 0.73 | ns |
| TVN ~ LDM × Hs | −1.347 ns | 1.634 ns | 7.596 * | 10.577 * | 0.36 | 3.02 | ns |
| Ktheo ~ SLA × Hs | −0.020 ns | 0.038 ns | 0.154 *** | 0.212 ** | 0.59 | 7.75 | ** |
| T ~ SLA × Hs | 0.113 ns | 0.045 ns | −0.093 ns | −0.162 ns | 0.24 | 1.64 | ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kafuti, C.; Bourland, N.; De Mil, T.; Meeus, S.; Rousseau, M.; Toirambe, B.; Bolaluembe, P.-C.; Ndjele, L.; Beeckman, H. Foliar and Wood Traits Covary along a Vertical Gradient within the Crown of Long-Lived Light-Demanding Species of the Congo Basin Semi-Deciduous Forest. Forests 2020, 11, 35. https://doi.org/10.3390/f11010035
Kafuti C, Bourland N, De Mil T, Meeus S, Rousseau M, Toirambe B, Bolaluembe P-C, Ndjele L, Beeckman H. Foliar and Wood Traits Covary along a Vertical Gradient within the Crown of Long-Lived Light-Demanding Species of the Congo Basin Semi-Deciduous Forest. Forests. 2020; 11(1):35. https://doi.org/10.3390/f11010035
Chicago/Turabian StyleKafuti, Chadrack, Nils Bourland, Tom De Mil, Sofie Meeus, Mélissa Rousseau, Benjamin Toirambe, Papy-Claude Bolaluembe, Léopold Ndjele, and Hans Beeckman. 2020. "Foliar and Wood Traits Covary along a Vertical Gradient within the Crown of Long-Lived Light-Demanding Species of the Congo Basin Semi-Deciduous Forest" Forests 11, no. 1: 35. https://doi.org/10.3390/f11010035





