Methane Emissions from Subtropical and Tropical Mangrove Ecosystems in Taiwan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Methane Flux Measurement
2.3. Soil and Water Sampling
2.4. Statistical Analyses
3. Results
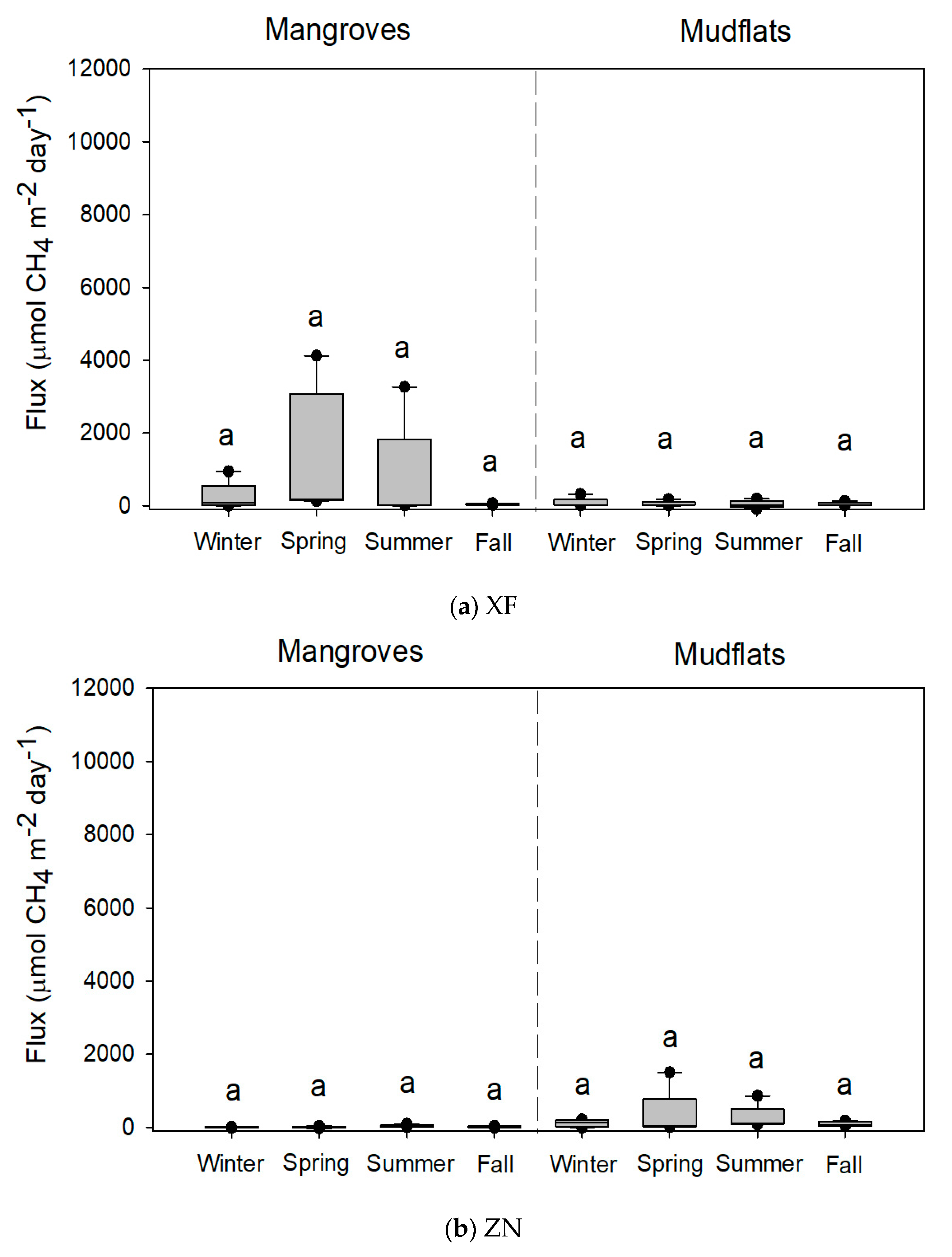
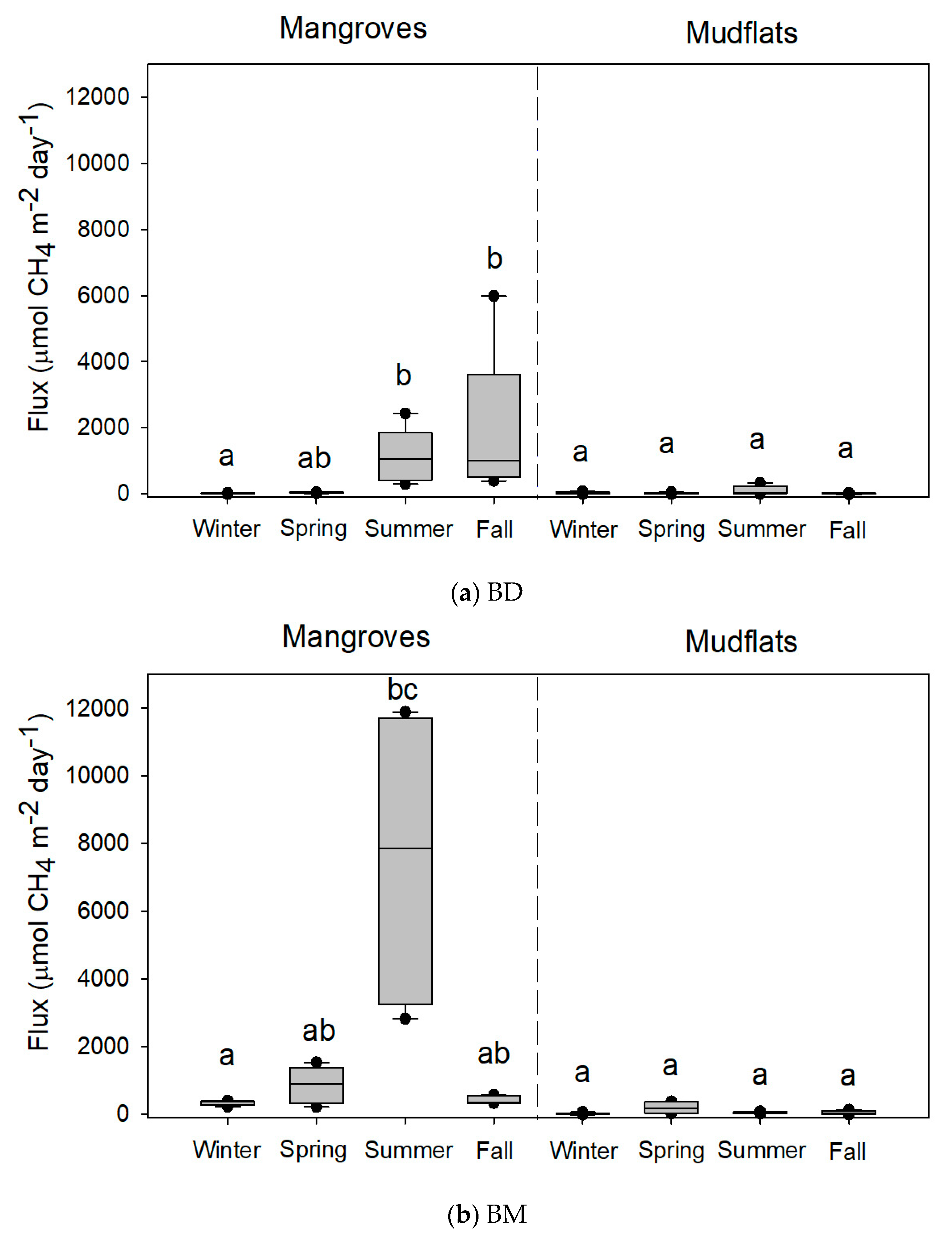
3.1. CH4 Flux and Soil Parameters
3.2. Comparisons of Methane Flux and Soil Parameters under Various Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, B.B.; Dushoff, J. Mangrove filtration of anthropogenic nutrients in the Rio Coco Solo, Panama. Manag. Environ. Qual. Int. J. 2004, 15, 131–142. [Google Scholar] [CrossRef]
- Vermaat, J.E.; Thampanya, U. Mangroves mitigate tsunami damage: A further response. Estuar. Coast. Shelf Sci. 2006, 69, 1–3. [Google Scholar] [CrossRef]
- Guannel, G.; Arkema, K.; Ruggiero, P.; Verutes, G. The power of three: Coral reefs, seagrasses and mangroves protect coastal regions and increase their resilience. PLoS ONE 2016, 11, e0158094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochard, J.P.; Hamilton, S.; Barbier, E.B. Mangroves shelter coastal economic activity from cyclones. Proc. Natl. Acad. Sci. USA 2019, 116, 12232–12237. [Google Scholar] [CrossRef] [Green Version]
- Barbier, E.B. Valuing the environment as input: Review of applications to mangrove-fishery linkages. Ecol. Econ. 2000, 35, 47–61. [Google Scholar] [CrossRef]
- Diele, K.; Koch, V.; Saint-Paul, U. Population structure, catch composition and CPUE of the artisanally harvested mangrove crab Ucides cordatus (Ocypodidae) in the Caeté estuary, North Brazil: Indications for overfishing? Aquat. Living Resour. 2005, 18, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Mumby, P.J.; Edwards, A.J.; Arias-González, J.E.; Lindeman, K.C.; Blackwell, P.G.; Gall, A.; Gorczynska, M.I.; Harborne, A.R.; Pescod, C.L.; Renken, H.; et al. Mangroves enhance the biomass of coral reef fish communities in the Caribbean. Nature 2004, 427, 533–536. [Google Scholar] [CrossRef] [Green Version]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef] [Green Version]
- Serrano, O.; Lovelock, C.E.; Atwood, T.B.; Macreadie, P.I.; Canto, R.; Phinn, S.; Arias-Ortiz, A.; Bai, L.; Baldock, J.; Bedulli, C.; et al. Australian vegetated coastal ecosystems as global hotspots for climate change mitigation. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Li, C.; Mosier, A.; Wassmann, R.; Cai, Z.; Zheng, X.; Huang, Y.; Tsuruta, H.; Boonjawat, J.; Lantin, R. Modeling greenhouse gas emissions from rice-based production systems: Sensitivity and upscaling. Glob. Biogeochem. Cycles 2004, 18. [Google Scholar] [CrossRef]
- Alongi, D.M.; Wattayakorn, G.; Pfitzner, J.; Tirendi, F.; Zagorskis, I.; Brunskill, G.J.; Clough, B.F. Organic carbon accumulation and metabolic pathways in sediments of mangrove forests in southern Thailand. Mar. Geol. 2001, 179, 85–103. [Google Scholar] [CrossRef]
- Nóbrega, G.N.; Ferreira, T.O.; Neto, M.S.; Queiroz, H.M.; Artur, A.G.; Mendonça, E.D.S.; Silva, E.D.O.; Otero, X.L. Edaphic factors controlling summer (rainy season) greenhouse gas emissions (CO2 and CH4) from semiarid mangrove soils (NE-Brazil). Sci. Total Environ. 2016, 542, 685–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, D.; Dalal, R.C.; Rennenberg, H.; Schmidt, S. Seasonal variation in nitrous oxide and methane emissions from subtropical estuary and coastal mangrove sediments, Australia. Plant Biol. 2011, 13, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, B.; Yu, D.; Tam, N.F.; Ye, Y.; Chen, S. Soil greenhouse gas emissions reduce the contribution of mangrove plants to the atmospheric cooling effect. Environ. Res. Lett. 2016, 11, 124019. [Google Scholar] [CrossRef]
- Bouillon, S.; Borges, A.V.; Castañeda-Moya, E.; Diele, K.; Dittmar, T.; Duke, N.C.; Kristensen, E.; Lee, S.Y.; Marchand, C.; Middelburg, J.J.; et al. Mangrove production and carbon sinks: A revision of global budget estimates. Glob. Biogeochem. Cycles 2008, 22. [Google Scholar] [CrossRef] [Green Version]
- Li, S.B.; Chen, P.H.; Huang, J.S.; Hsueh, M.L.; Hsieh, L.Y.; Lee, C.L.; Lin, H.J. Factors regulating carbon sinks in mangrove ecosystems. Glob. Chang. Boil. 2018, 24, 4195–4210. [Google Scholar] [CrossRef]
- IPCC. Climate Change: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; 1535p. [Google Scholar]
- Cameron, C.; Hutley, L.B.; Friess, D.A. Estimating the full greenhouse gas emissions offset potential and profile between rehabilitating and established mangroves. Sci. Total Environ. 2019, 665, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Sasmito, S.D.; Taillardat, P.; Clendenning, J.N.; Cameron, C.; Friess, D.A.; Murdiyarso, D.; Hutley, L.B. Effect of land-use and land-cover change on mangrove blue carbon: A systematic review. Glob. Chang. Boil. 2019, 25, 4291–4302. [Google Scholar] [CrossRef]
- Faunce, C.H.; Layman, C.A. Sources of variation that affect perceived nursery function of mangroves. In Nagelkerken I (ed) Ecological Connectivity Among Tropical Coastal Ecosystems; Media, B.V., Ed.; Springer: New York, NY, USA, 2009; pp. 401–421. [Google Scholar]
- Pai, S.C.; Yang, C.C.; Riley, J.P. Formation kinetics of the pink azo dye in the determination of nitrite in natural waters. Anal. Chim. Acta 1990, 232, 345–349. [Google Scholar] [CrossRef]
- Jenkins, D.; Medsker, L.L. Brucine Method for the Determination of Nitrate in Ocean, Estuarine, and Fresh Waters. Anal. Chem. 1964, 36, 610–612. [Google Scholar] [CrossRef]
- Pai, S.C.; Tsau, Y.J.; Yang, T.I. pH and buffering capacity problems involved in the determination of ammonia in saline water using the indophenol blue spectrophotometric method. Anal. Chim. Acta 2001, 434, 209–216. [Google Scholar] [CrossRef]
- Murphy, J.A.M.E.S.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: http://www.R-project.org (accessed on 11 February 2020).
- Allen, D.E.; Dalal, R.C.; Rennenberg, H.; Meyer, R.L.; Reeves, S.; Schmidt, S. Spatial and temporal variation of nitrous oxide and methane flux between subtropical mangrove sediments and the atmosphere. Soil Biol. Biochem. 2007, 39, 622–631. [Google Scholar] [CrossRef]
- Chauhan, R.; Datta, A.; Ramanathan, A.L.; Adhya, T.K. Factors influencing spatio-temporal variation of methane and nitrous oxide emission from a tropical mangrove of eastern coast of India. Atmos. Environ. 2015, 107, 95–106. [Google Scholar] [CrossRef]
- Chen, G.C.; Nora, F.Y.; Tamb, Y.Y. Spatial and seasonal variations of atmospheric N2O and CO2 fluxes from a subtropical mangrove swamp and their relationships with soil characteristics. Soil Biol. Biochem. 2012, 48, 175–181. [Google Scholar] [CrossRef]
- Purvaja, R.; Ramesh, R. Natural and anthropogenic methane emission from coastal wetlands of South India. Environ. Manag. 2001, 27, 547–555. [Google Scholar] [CrossRef]
- Chen, G.C.; Tam, N.F.Y.; Ye, Y. Summer fluxes of atmospheric greenhouse gases N2O, CH4 and CO2 from mangrove soil in South China. Sci. Total Environ. 2010, 408, 2761–2767. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Buchholz, J.; Rennenberg, H. Emission of methane and nitrous oxide by Australian mangrove ecosystems. Plant Biol. 2003, 5, 423–431. [Google Scholar] [CrossRef]
- Lekphet, S.; Nitisoravut, S.; Adsavakulchai, S. Estimating methane emissions from mangrove area in Ranong Province, Thailand. Songklanakarin J. Sci. Technol. 2005, 27, 153–163. [Google Scholar]
- Huang, B.; Yu, K.; Gambrell, R.P. Effects of ferric iron reduction and regeneration on nitrous oxide and methane emissions in a rice soil. Chemosphere 2009, 74, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Livesley, S.J.; Andrusiak, S.M. Temperate mangrove and salt marsh sediments are a small methane and nitrous oxide source but important carbon store. Estuar. Coast. Shelf Sci. 2012, 97, 19–27. [Google Scholar] [CrossRef]
- Rosentreter, J.A.; Maher, D.T.; Erler, D.V.; Murray, R.; Eyre, B.D. Factors controlling seasonal CO2 and CH4 emissions in three tropical mangrove-dominated estuaries in Australia. Estuar. Coast. Shelf Sci. 2018, 215, 69–82. [Google Scholar] [CrossRef]
- Yu, K.; Faulkner, S.P.; Patrick, W.H., Jr. Redox potential characterization and soil greenhouse gas concentration across a hydrological gradient in a Gulf coast forest. Chemosphere 2006, 62, 905–914. [Google Scholar] [CrossRef]
- Kristensen, E.; Bouillon, S.; Dittmar, T.; Marchand, C. Organic carbon dynamics in mangrove ecosystems: A review. Aquat. Bot. 2008, 89, 201–219. [Google Scholar] [CrossRef] [Green Version]
- Krithika, K.; Purvaja, R.; Ramesh, R. Fluxes of methane and nitrous oxide from an Indian mangrove. Curr. Sci. 2008, 94, 218–224. [Google Scholar]
- Kristensen, E.; Flindt, M.R.; Ulomi, S.; Borges, A.V.; Abril, G.; Bouillon, S. Emission of CO2 and CH4 to the atmosphere by sediments and open waters in two Tanzanian mangrove forests. Mar. Ecol. Prog. Ser. 2008, 370, 53–67. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Guan, W.; Xue, D.; Liu, L.; Peng, C.; Liao, B.; Hu, J.; Yang, Y.; Wang, X.; Zhou, G. Comparison of methane emissions among invasive and native mangrove species in Dongzhaigang, Hainan Island. Sci. Total Environ. 2019, 697, 133945. [Google Scholar] [CrossRef]
- Chen, G.C.; Ulumuddin, Y.I.; Chen, S.Y.; Chen, B.; Ye, Y.; Ou, D.Y.; Ma, Z.Y.; Huang, H.; Wang, J.K. Rich soil carbon and nitrogen but low atmospheric greenhouse gas fluxes from North Sulawesi mangrove swamps in Indonesia. Sci. Total Environ. 2014, 487, 91–96. [Google Scholar] [CrossRef]



| Site | XF | ZN | BD | BM | |
|---|---|---|---|---|---|
| Monthly rainfall (mm) | February (Winter) | 41 | 30.5 | 9.5 | 9.5 |
| April (Spring) | 185 | 169.5 | 78 | 72 | |
| July (Summer) | 47 | 67.5 | 334 | 545 | |
| October (Fall) | 9 | 17 | 0 | 0 | |
| Mean temperature (°C) | February (Winter) | 16.3 | 17.1 | 20.2 | 20.3 |
| April (Spring) | 22.1 | 23.1 | 24.6 | 24.5 | |
| July (Summer) | 28.6 | 29.4 | 28.8 | 28.6 | |
| October (Fall) | 23.7 | 24.8 | 25.6 | 25.6 | |
| Mean tidal range (cm) | 366 | 388 | 181 | 136 | |
| Mean immersion time during flood tides (hours/day) | 11.5 | 12.0 | 11.3 | 13.5 | |
| Major mangrove species | Kandelia obovata | Kandelia obovata | Avicennia marina | Avicennia marina | |
| Presence of pneumatophores | No | No | Yes | Yes | |
| Mangrove classification (Faunce and Layman [21]) | High tide fringe mangroves | Low tide riverine mangroves | Riverine mangroves | High tide fringe mangroves | |
| Total area of mangrove forests and mudflats (ha) | 9.37 | 19.59 | 30.2 | 5.48 | |
| Area ratio of mangroves to mudflats | 7.37 | 2.85 | 1.27 | 0.37 | |
| Width of extended mudflats (m) | 13–20 | 7–10 | 25–70 | 60–90 | |
| Mean tree height (m) | 5.1 | 5.0 | 4.0 | 3.2 | |
| Mean tree density (trees m−1) | 2.4 | 1.9 | 0.9 | 0.6 | |
| Mean diameter at breast height (DBH) (cm) | 5.6 | 5.9 | 5.4 | 6.2 | |
| Soil texture | Fine sand | Very fine sand | Fine sand | Very fine sand | |
| PO43− (µM) | Range | 1.30–72.30 | 2.89–18.30 | 0.91–5.23 | 1.46–6.93 |
| Mean ± standard error | 29.94 ± 5.28 | 8.33 ± 1.16 | 3.07 ± 0.30 | 3.40 ± 0.39 | |
| NO3− (µM) | Range | 41.48–753.84 | 2.64–17.09 | 0.07–8.97 | 3.24–22.85 |
| Mean ± standard error | 300.24 ± 43.51 | 8.20 ± 1.04 | 4.18 ± 0.57 | 11.39 ± 1.07 | |
| NO2− (µM) | Range | 5.57–97.82 | 0.66–35.22 | 0.12–2.09 | 1.99–6.79 |
| Mean ± standard error | 30.06 ± 6.10 | 13.92 ± 2.28 | 0.74 ± 0.14 | 3.74 ± 0.27 | |
| NH4+ (µM) | Range | 26.10–100.84 | 93.21–203.75 | 2.42–17.66 | 10.62–38.99 |
| Mean ± standard error | 56.08 ± 4.81 | 129.94 ± 6.12 | 8.59 ± 0.87 | 24.16 ± 1.76 | |
| Site | Season | Methane Fluxes | Temperature | ORP | pH | Salinity | Bulk Density | Water Content | Organic Matter |
|---|---|---|---|---|---|---|---|---|---|
| (µmol-CH4 m−2 day−1) | (°C) | (mV) | (g cm−3) | (%) | (%) | ||||
| XF | Winter | 241.6 ± 178.1 a | 17.5 ± 0.3 a | 218.2 ± 16.2 a | 6.4 ± 0.1 a | 0.4 ± 0.1 a | 1.5 ± 0.0 b | 25.9 ± 1.5 ab | 3.9 ± 0.5 a |
| Spring | 1326.6 ± 786.9 a | 20.5 ± 0.1 ab | 171.6 ± 9.5 a | 6.2 ± 0.0 a | 0.9 ± 0.1 ab | 1.0 ± 0.0 a | 32.9 ± 1.5 b | 5.2 ± 0.4 a | |
| Summer | 742.5 ± 635.3 a | 28.6 ± 0.2 c | 160.6 ± 22.9 a | 6.4 ± 0.1 a | 0.9 ± 0.1 ab | 1.0 ± 0.1 a | 34.4 ± 1.4 b | 5.5 ± 0.4 a | |
| Fall | 42.4 ± 9.1 a | 25.1 ± 0.5 bc | 183.6 ± 9.9 a | 6.2 ± 0.1 a | 1.2 ± 0.1 b | 1.2 ± 0.0 ab | 22.7 ± 1.2 a | 5.5 ± 1.0 a | |
| ZN | Winter | 1.3 ± 3.4 a | 17.4 ± 0.1 a | 139.2 ± 29.8 ab | 6.6 ± 0.1 ab | 2.4 ± 0.3 a | 1.6 ± 0.0 b | 23.7 ± 1.0 ab | 4.1 ± 0.4 a |
| Spring | 10.0 ± 9.5 a | 22.8 ± 0.4 ab | 152.2 ± 2.7 b | 6.6 ± 0.1 ab | 2.2 ± 0.2 a | 1.2 ± 0.0 a | 31.1 ± 0.8 b | 4.0 ± 0.2 a | |
| Summer | 39.3 ± 13.3 a | 27.5 ± 0.1 c | −3.6 ± 20.5 a | 6.4 ± 0.1 a | 3.1 ± 0.3 a | 1.3 ± 0.0 a | 29.2 ± 0.9 b | 3.8 ± 0.2 a | |
| Fall | 14.5 ± 9.3 a | 25.7 ± 0.5 bc | 133.6 ± 10.6 ab | 7.0 ± 0.1 b | 3.1 ± 0.2 a | 1.4 ± 0.0 ab | 19.7 ± 0.6 a | 4.3 ± 0.4 a | |
| BD | Winter | 10.1 ± 5.5 a | 23.0 ± 0.0 a | −274.4 ± 28.0 a | 7.1 ± 0.1 b | 2.9 ± 0.1 ab | 1.1 ± 0.2 b | 43.5 ± 7.5 a | 5.3 ± 1.1 a |
| Spring | 32.0 ± 5.9 ab | 27.9 ± 0.2 bc | −285.0 ± 9.2 a | 6.7 ± 0.0 ab | 4.5 ± 0.3 c | 1.1 ± 0.1 b | 58.0 ± 2.1 a | 7.2 ± 0.4 a | |
| Summer | 1116.6 ± 372.3 b | 30.5 ± 0.4 c | −340.8 ± 13.9 a | 6.6 ± 0.1 ab | 2.5 ± 0.3 a | 0.5 ± 0.1 a | 57.6 ± 2.3 a | 8.4 ± 0.9 a | |
| Fall | 1847.8 ± 1045.4 b | 24.5 ± 0.1 ab | −290.2 ± 24.0 a | 6.6 ± 0.2 a | 3.8 ± 0.2 abc | 0.8 ± 0.1 ab | 37.8 ± 5.5 a | 6.5 ± 0.5 a | |
| BM | Winter | 337.6 ± 31.7 a | 20.1 ± 0.3 a | 33.0 ± 37.4 a | 6.7 ± 0.0 a | 3.6 ± 0.4 ab | 1.3 ± 0.0 ab | 25.5 ± 3.6 a | 4.3 ± 0.4 b |
| Spring | 865.0 ± 244.5 ab | 27.6 ± 0.2 bc | −8.4 ± 10.8 a | 6.7 ± 0.1 a | 5.6 ± 0.8 b | 2.2 ± 0.0 b | 30.8 ± 1.2 a | 2.6 ± 0.2 a | |
| Summer | 7606.6 ± 2304.8 bc | 30.1 ± 0.4 c | −151.4 ± 54.1 a | 7.0 ± 0.1 a | 3.1 ± 0.1 a | 1.0 ± 0.0 a | 38.2 ± 2.7 a | 3.5 ± 0.2 ab | |
| Fall | 424.7 ± 54.5 ab | 23.7 ± 0.2 ab | −148.0 ± 50.3 a | 6.6 ± 0.1 a | 4.2 ± 0.4 ab | 1.2 ± 0.1 ab | 29.5 ± 3.8 a | 3.3 ± 0.2 ab |
| Site | Season | Methane Fluxes | Temperature | ORP | pH | Salinity | Bulk Density | Water Content | Organic Matter |
|---|---|---|---|---|---|---|---|---|---|
| (µmol-CH4 m−2 day−1) | (°C) | (mV) | (g cm−3) | (%) | (%) | ||||
| XF | Winter | 79.1 ± 61.6 | 17.5 ± 0.4 | −15.5 ± 30.5 | 7.1 ± 0.2 | 1.7 ± 0.0 | 1.1 ± 0.0 | 39.9 ± 0.4 | 3.2 ± 0.0 |
| Spring | 56.3 ± 32.1 | 24.7 ± 0.9 | −62.0 ± 36.0 | 6.6 ± 0.0 | 2.9 ± 0.2 | 1.0 ± 0.0 | 36.5 ± 2.1 | 3.9 ± 0.1 | |
| Summer | 45.2 ± 46.7 | 28.6 ± 0.1 | 62.5 ± 86.5 | 6.9 ± 0.1 | 1.8 ± 0.0 | 1.1 ± 0.0 | 37.8 ± 0.9 | 3.8 ± 0.1 | |
| Fall | 48.9 ± 22.4 | 24.5 ± 0.1 | 47.5 ± 15.5 | 7.4 ± 0.2 | 3.4 ± 0.3 | 1.1 ± 0.1 | 31.9 ± 1.0 | 3.7 ± 0.4 | |
| ZN | Winter | 113.5 ± 40.8 | 20.2 ± 0.0 | −19.0 ± 64.0 | 7.0 ± 0.1 | 1.7 ± 0.0 | 1.3 ± 0.1 | 33.2 ± 1.8 | 3.6 ± 0.3 |
| Spring | 334.5 ± 293.2 | 21.6 ± 0.4 | −31.5 ± 45.5 | 6.8 ± 0.1 | 3.7 ± 0.9 | 1.1 ± 0.0 | 34.5 ± 0.1 | 3.6 ± 0.0 | |
| Summer | 257.9 ± 150.8 | 27.9 ± 0.0 | −196.0 ± 4.0 | 7.2 ± 0.0 | 1.7 ± 0.0 | 1.1 ± 0.0 | 36.2 ± 1.9 | 3.4 ± 0.1 | |
| Fall | 93.9 ± 29.1 | 26.3 ± 0.1 | −54.5 ± 1.5 | 7.3 ± 0.1 | 3.3 ± 0.1 | 1.1 ± 0.0 | 30.8 ± 0.6 | 4.4 ± 0.2 | |
| BD | Winter | 17.0 ± 14.0 | 24.7 ± 0.1 | −54.5 ± 15.5 | 7.5 ± 0.1 | 1.9 ± 0.0 | 1.8 ± 0.0 | 18.8 ± 0.3 | 0.7 ± 0.1 |
| Spring | 11.3 ± 10.5 | 29.5 ± 0.1 | 98.5 ± 5.5 | 6.9 ± 0.3 | 4.8 ± 0.0 | 2.8 ± 0.0 | 21.2 ± 0.2 | 0.8 ± 0.1 | |
| Summer | 102.8 ± 61.6 | 34.4 ± 1.7 | −20.0 ± 30.0 | 7.3 ± 0.1 | 1.7 ± 0.0 | 1.4 ± 0.0 | 23.8 ± 2.1 | 1.5 ± 0.1 | |
| Fall | 6.6 ± 7.4 | 22.6 ± 0.1 | −51.0 ± 27.0 | 7.3 ± 0.3 | 2.3 ± 0.0 | 1.5 ± 0.1 | 15.9 ± 0.2 | 0.8 ± 0.1 | |
| BM | Winter | 14.3 ± 13.5 | 21.8 ± 0.2 | −40.0 ± 7.0 | 7.0 ± 0.1 | 4.3 ± 0.2 | 1.0 ± 0.1 | 41.1 ± 2.5 | 4.8 ± 0.2 |
| Spring | 192.7 ± 102.2 | 28.0 ± 0.1 | −281.0 ± 3.0 | 6.8 ± 0.0 | 7.5 ± 0.5 | 2.1 ± 0.1 | 32.4 ± 0.1 | 1.7 ± 0.0 | |
| Summer | 37.4 ± 12.1 | 30.3 ± 0.6 | −73.5 ± 222.5 | 7.3 ± 0.1 | 1.7 ± 0.1 | 1.3 ± 0.0 | 25.3 ± 1.6 | 1.6 ± 0.0 | |
| Fall | 49.0 ± 25.7 | 24.9 ± 0.2 | 22.0 ± 13.0 | 7.1 ± 0.1 | 3.0 ± 0.5 | 1.4 ± 0.1 | 27.4 ± 0.3 | 2.1 ± 0.2 |
| Parameters | XF | ZN | ||
| p-Value | Note | p-Value | Note | |
| CH4 flux | 3.15 × 10−2 | Mangrove > Mudflat | 1.25 × 10−5 | Mangrove < Mudflat |
| Temperature | 1.00 | n.s. | 0.89 | n.s. |
| ORP | 3.04 × 10−2 | Mangrove > Mudflat | 3.04 × 10−2 | Mangrove > Mudflat |
| pH | 2.84 × 10−2 | Mangrove < Mudflat | 0.08 | n.s. |
| Salinity | 2.94 × 10−2 | Mangrove < Mudflat | 1.00 | n.s. |
| Bulk density | 0.88 | n.s. | 0.07 | n.s. |
| Water content | 0.11 | n.s. | 0.06 | n.s. |
| Organic matter content | 4.08 × 10−2 | Mangrove > Mudflat | 0.31 | n.s. |
| Parameters | BD | BM | ||
| p-Value | Note | p-Value | Note | |
| CH4 flux | 1.01 × 10−3 | Mangrove > Mudflat | 6.76 × 10−7 | Mangrove > Mudflat |
| Temperature | 0.89 | n.s. | 0.67 | n.s. |
| ORP | 3.04 × 10−2 | Mangrove < Mudflat | 0.89 | n.s. |
| pH | 0.06 | n.s. | 0.08 | n.s. |
| Salinity | 0.31 | n.s. | 0.89 | n.s. |
| Bulk density | 2.94 × 10−2 | Mangrove < Mudflat | 0.88 | n.s. |
| Water content | 3.04 × 10−2 | Mangrove > Mudflat | 1 | n.s. |
| Organic matter content | 2.94 × 10−2 | Mangrove > Mudflat | 0.31 | n.s. |
| Parameters | p-Value | Note |
|---|---|---|
| CH4 flux | 1.56 × 10−5 | K < A |
| Temperature | 4.94 × 10−3 | K < A |
| ORP | 4.14 × 10−13 | K > A |
| pH | 9.33 × 10−6 | K < A |
| Salinity | 1.61 × 10−9 | K < A |
| Bulk density | 1.17 × 10−2 | K > A |
| Water content | 1.81 × 10−5 | K < A |
| Organic matter content | 0.84 | n.s. |
| Site | Climate | Dominant Mangrove Species | CH4 Fluxes (μg m−2 h−1) | References |
|---|---|---|---|---|
| XF and ZN | Subtropical | Kandelia obovata | 0.9–884.4 | This study |
| BD and BM | Tropical | Avicennia marina | 6.7–5071.1 | |
| North Sulawesi, Indonesia | Equatorial | Rhizophora apiculate and Bruguiera gymnorrhiza | 0–210.24 | [42] |
| Dar es Salaam, Tanzania | Humid tropical | Sonneratia alba, Avicenniamarina, Ceriops tagal, Rhizophora mucronata | 7–233 | [40] |
| Ceará state, NE-Brazil | Tropical | Rhizophora spp. | 0.7–8.8 | [13] |
| Odisha state, India | Tropical | Avicennia spp. | 80–2300 | [28] |
| Queensland, Australia | Tropical | NA | 26.7–698 | [36] |
| Shenzhen, China | Subtropical monsoonal | Kandelia obovata | 190.6–4390.9 | [31] |
| Moreton Bay, Australia | Subtropical | Avicennia marina | 20–350 | [32] |
| Chelmer, Australia | Subtropical | Avicennia marina | 3.0–17,370.0 | [27] |
| Southeast Queensland, Australia | Subtropical | Avicennia spp. | 47–1570 | [14] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-W.; Kao, Y.-C.; Chou, M.-C.; Wu, H.-H.; Ho, C.-W.; Lin, H.-J. Methane Emissions from Subtropical and Tropical Mangrove Ecosystems in Taiwan. Forests 2020, 11, 470. https://doi.org/10.3390/f11040470
Lin C-W, Kao Y-C, Chou M-C, Wu H-H, Ho C-W, Lin H-J. Methane Emissions from Subtropical and Tropical Mangrove Ecosystems in Taiwan. Forests. 2020; 11(4):470. https://doi.org/10.3390/f11040470
Chicago/Turabian StyleLin, Chiao-Wen, Yu-Chen Kao, Meng-Chun Chou, Hsin-Hsun Wu, Chuan-Wen Ho, and Hsing-Juh Lin. 2020. "Methane Emissions from Subtropical and Tropical Mangrove Ecosystems in Taiwan" Forests 11, no. 4: 470. https://doi.org/10.3390/f11040470





