Floristic Legacies of Historical Land Use in Swedish Boreo-Nemoral Forests: A Review of Evidence and a Case Study on Chimaphila umbellata and Moneses uniflora
Abstract
:1. Introduction
2. Ecological Mechanisms behind Floristic Legacies
3. Some Remarks on Methodological Issues
4. Grassland Species as Floristic Legacies of Historical Land Use in Forests
| Species | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| [78] | [60] | [72] | [74] | [82] | [32] | [83] | [84] | [85] | [41] | |
| Ajuga pyramidalis L. | X | X | X | X | ||||||
| Alchemilla glaucescens Wallr. | X | |||||||||
| Antennaria dioica (L.) Gaertn. | X | |||||||||
| Anthoxanthum odoratum L. | X | X | X | X | ||||||
| Campanula rotundifolia L. | X | X | X | X | X | |||||
| Carex leporina L. | X | X | ||||||||
| Carex pallescens L. | X | X | ||||||||
| Carex pilulifera L. | X | |||||||||
| Carex spicata Huds. | X | |||||||||
| Cerastium fontanum Baumg. | X | |||||||||
| Danthonia decumbens (L.) DC. | X | X | ||||||||
| Euphrasia stricta J.P. Wolff ex J.F. Lehm. | X | |||||||||
| Festuca ovina L. | X | |||||||||
| Festuca rubra L. | X | |||||||||
| Juncus filiformis L. | X | |||||||||
| Lathyrus linifolius * (Reichard) Bässler | X | X | ||||||||
| Leucanthemum vulgare Lam. | X | X | X | X | ||||||
| Lotus corniculatus L. | X | X | X | X | ||||||
| Luzula campestris (L.) DC. | X | X | ||||||||
| Pilosella officinarum Vaill. | X | X | ||||||||
| Pimpinella saxifraga L. | X | X | ||||||||
| Plantago lanceolata L. | X | X | X | |||||||
| Polygala vulgaris L. | X | X | X | X | ||||||
| Primula veris L. | X | X | X | X | X | |||||
| Ranunculus acris L. | X | X | X | X | ||||||
| Ranunculus auricomus L. | X | |||||||||
| Rhinanthus minor L. | X | |||||||||
| Succisa pratensis Moench | X | X | ||||||||
| Trifolium pratense L. | X | X | X | |||||||
| Trifolium repens L. | X | X | ||||||||
| Veronica chamaedrys L. | X | X | X | X | ||||||
| Veronica officinalis * L. | X | X | ||||||||
| Veronica serpyllifolia L. | X | |||||||||
| Viola canina L. | X | |||||||||
5. Forest Species as Floristic Legacies of Historical Land Use in Forests
6. The Case Study
6.1. Description of the Species
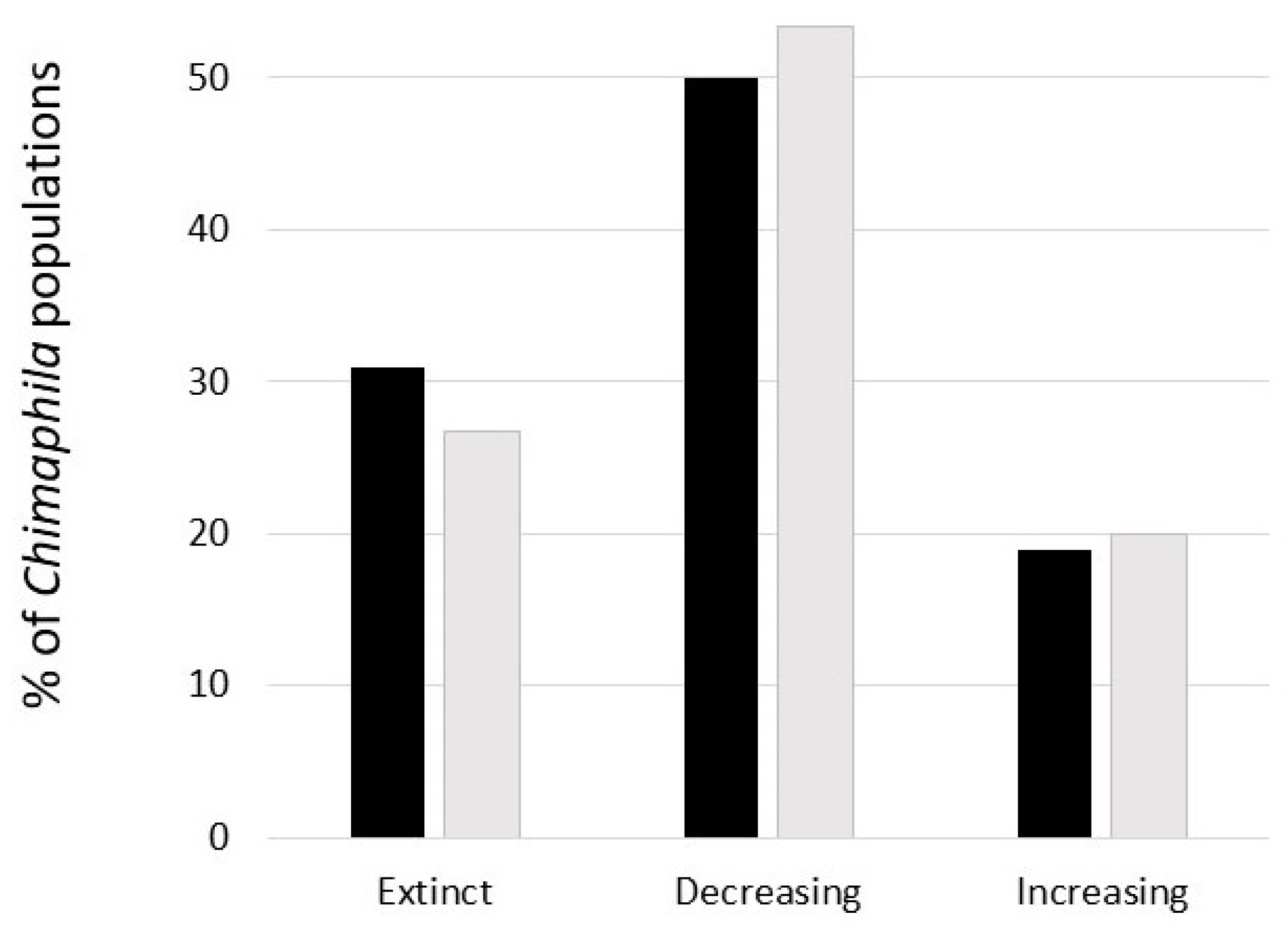
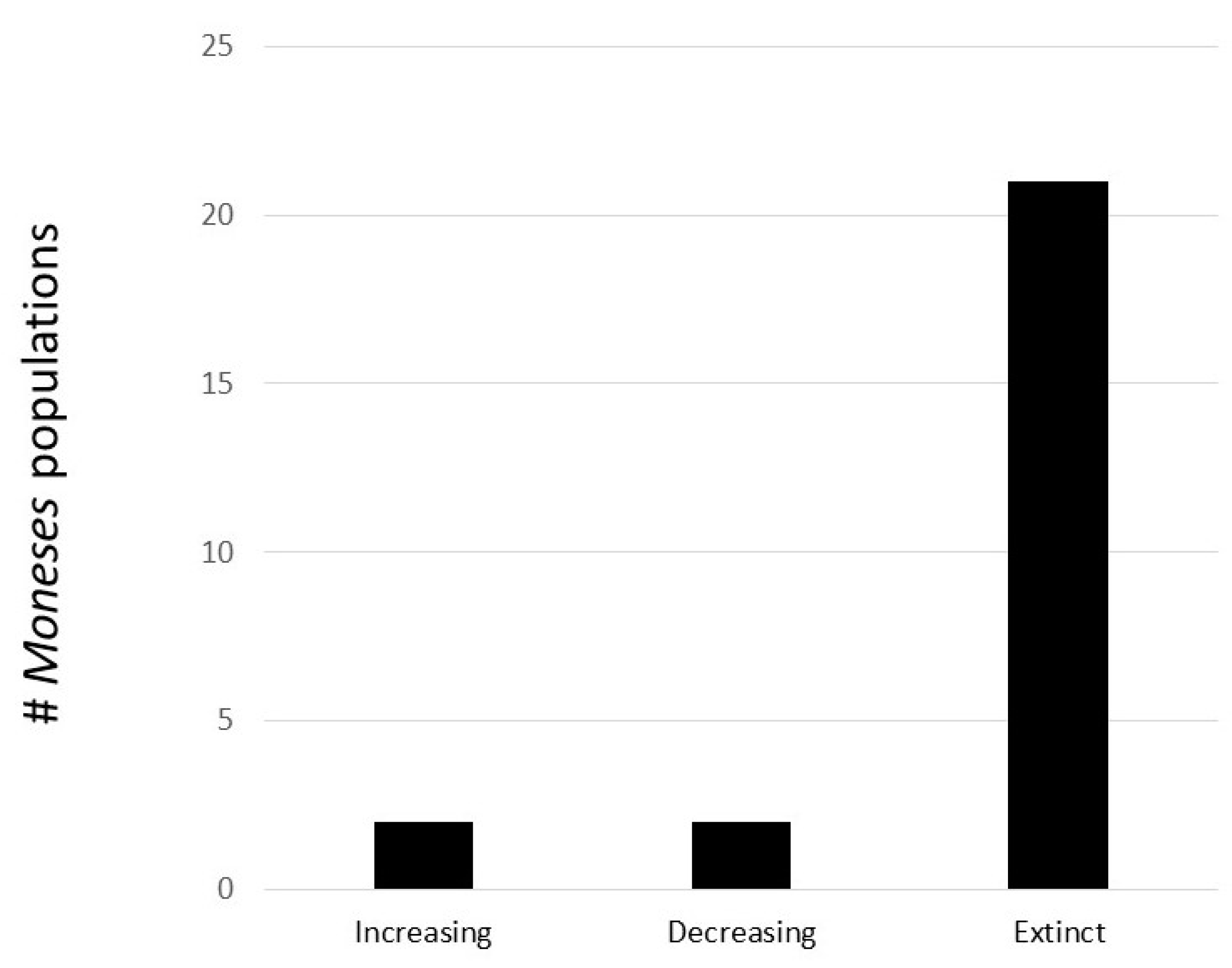
6.2. Population Decline
6.3. Comparison of Life Histories
7. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Populations Persisting 2021 | ||||||
| Site | Name | Coordinates WGS84 | #Ramets 2013 | #Ramets 2021 | Change | |
| Lat | Long | |||||
| Increasing populations | ||||||
| 1 | Bollmora | 59.239331 | 18.235026 | 7 | 10 | 1.43 |
| 2 | Munsö Ekerö | 59.414600 | 17.584391 | 339 | 561 | 1.65 |
| 3 | Snöbergen | 59.544097 | 17.968348 | 13 | 15 | 1.15 |
| 4 | Lapp | 60.115844 | 18.659704 | 51 | 133 | 2.61 |
| 5 | Billudden | 60.637188 | 17.461401 | 1208 | 3578 | 2.96 |
| 6 | Notören | 60.567220 | 17.441189 | 437 | 788 | 1.80 |
| Average change | 1.93 | |||||
| Decreasing populations | ||||||
| 7 | Tullgarn | 58.978209 | 17.601575 | 1776 | 592 | 0.33 |
| 8 | Svarvartorp Ekerö | 59.303610 | 17.701306 | 96 | 11 | 0.11 |
| 9 | Södergården | 59.184542 | 18.366396 | 98 | 75 | 0.76 |
| 10 | Lanan | 59.178118 | 18.323817 | 147 | 64 | 0.44 |
| 11 | Lilla Gräskärret 1 | 59.676369 | 17.558602 | 900 | 647 | 0.72 |
| 12 | Lilla Gräskärret 2 | 59.677099 | 17.557752 | 218 | 48 | 0.22 |
| 13 | Bålstaåsen | 59.575964 | 17.521484 | 140 | 133 | 0.95 |
| 14 | Rosersberg | 59.580045 | 17.892885 | 45 | 4 | 0.09 |
| 15 | Tilskogen | 59.609193 | 17.751514 | 80 | 48 | 0.60 |
| 16 | Österändan | 59.834283 | 18.817657 | 18 | 10 | 0.55 |
| 17 | Långängen | 60.121074 | 18.628193 | 133 | 74 | 0.56 |
| 18 | Hummelfjärden | 60.333915 | 18.422524 | 60 | 21 | 0.35 |
| 19 | Vendelheden | 60.158122 | 17.510899 | 23 | 15 | 0.65 |
| 20 | Billudden/Udden | 60.657148 | 17.508934 | 515 | 286 | 0.56 |
| 21 | Kapplasse | 60.577296 | 17.825108 | 557 | 341 | 0.61 |
| 22 | Slada 1 | 60.532624 | 18.017672 | 20 | 6 | 0.30 |
| Average change | 0.48 | |||||
| Populations extinct 2021 | ||||||
| Site | Name | Coordinates WGS84 | #Ramets 2013 | |||
| Lat | Long | |||||
| 24 | Åva träsk | 59.162262 | 18.342956 | 5 | ||
| 25 | Ekillabadet | 59.606646 | 17.508787 | 4 | ||
| 26 | Hebbo | 59.515183 | 17.533661 | 314 | ||
| 27 | Jomale telemast | 60.497423 | 18.379444 | 19 | ||
| 28 | Vendelheden 2 | 60.157306 | 17.508011 | 25 | ||
| 29 | Slada 2 | 60.534323 | 18.013214 | 125 | ||
| 30 | Skånsta | 59.491324 | 18.324522 | 106 | ||
| Site | Name | Year | Coordinates WGS84 | Earlier Records | Re-Survey 2022 | Change | ||
|---|---|---|---|---|---|---|---|---|
| Lat | Long | #P | #FR/R | #FR/VR | ||||
| 1 | Idbäcken | 2015 | 60.595086 | 17.591258 | 2 | 700 R | 301 FR + 996 VR | Increasing |
| 2 | Billudden | 2017 | 60.640076 | 17.465936 | 1 | 50 FR | 355 FR + 750 VR | Increasing |
| 3 | Stigenberg | 2015 | 60.590743 | 17.477950 | 3 | 1340 R | 50 FR + 164 VR | Decreasing |
| 4 | Pärlmossen | 2015 | 60.500548 | 17.473303 | 1 | 450 R | 15 FR + 37 VR | Decreasing |
| 5 | Skirkällan | 2018 | 59.311823 | 16.671995 | 5 | 477 R | 0 | Extinct |
| 6 | Saldalen | 2015 | 59.155962 | 16.363755 | 8 | 59 FR | 0 | Extinct * |
| 7 | Strandstuviken 1 | 2016 | 58.719304 | 17.081295 | 1 | >10 FR | 0 | Extinct |
| 8 | Strandstuviken 2 | 2019 | 58.719269 | 17.081241 | 2 | 12 FR | 0 | Extinct |
| 9 | Örstigsnäs | 2016 | 58.727422 | 17.091121 | 1 | 5 R | 0 | Extinct |
| 10 | Dyvikskärret | 2016 | 58.935340 | 17.585522 | 2 | 19 FR | 0 | Extinct |
| 11 | Åtorpsmossen | 2016 | 58.978506 | 17.589190 | 1 | 150 R | 0 | Extinct * |
| 12 | Tullgarnskogen | 2016 | 58.976070 | 17.568387 | 1 | 2 R | 0 | Extinct |
| 13 | Svartbäcken | 2017 | 59.164998 | 18.204130 | 1 | 1 FR | 0 | Extinct |
| 14 | Tistelkullen | 2015 | 59.745384 | 18.335471 | 1 | 2 FR | 0 | Extinct |
| 15 | Kvarntorpet | 2015 | 59.744616 | 18.335733 | 1 | 5 FR | 0 | Extinct |
| 16 | Ladängssjön | 2015 | 59.860466 | 18.528562 | 1 | 20 FR | 0 | Extinct |
| 17 | Stornotsand | 2018 | 60.190373 | 18.778001 | 1 | 15 FR | 0 | Extinct |
| 18 | Finkarbo | 2015 | 60.523351 | 17.544233 | 2 | 50 FR | 0 | Extinct * |
| 19 | Postmästarhage | 2015 | 60.543124 | 17.474053 | 3 | 200 FR | 0 | Extinct * |
| 20 | Mullbro mossar | 2016 | 60.543450 | 17.476038 | 1 | 200 FR | 0 | Extinct |
| 21 | Mararna | 2015 | 60.618052 | 17.598409 | 8 | 908 R | 0 | Extinct |
| 22 | Stadsskogen | 2015 | 59.838504 | 17.622772 | 2 | 2 FR | 0 | Extinct |
| 23 | Byholma | 2014 | 60.115404 | 18.809443 | 1 | 11 FR | 0 | Extinct |
| 24 | Backby | 2019 | 60.178772 | 18.770642 | 1 | 2 FR | 0 | Extinct |
| 25 | Boda | 2017 | 60.194603 | 18.735252 | 1 | 5 FR | 0 | Extinct |
Appendix B
- B1.
- The age of flowering in ramets of Moneses uniflora.
References
- Josefsson, T.; Gunnarson, B.; Liedgren, L.; Bergman, I.; Östlund, L. Historical human influence on forest composition and structure in boreal Fennoscandia. Can. J. For. Res. 2010, 40, 872–884. [Google Scholar] [CrossRef]
- Iles, L. The role of metallurgy in transforming global forests. J. Archaeol. Method Th. 2016, 23, 1219–1241. [Google Scholar] [CrossRef]
- Jamrichová, E.; Hédl, R.; Kolář, J.; Tóth, P.; Bobek, P.; Hajnalová, M.; Procházka, J.; Kadlec, J.; Szabó, P. Human impact on open temperate woodlands during the middle Holocene in Central Europe. Rev. Palaeobot. Palynol. 2017, 245, 55–68. [Google Scholar] [CrossRef]
- Roberts, P.; Hunt, C.; Arroyo-Kalin, M.; Evans, D.; Boivin, N. The deep human prehistory of global tropical forests and its relevance for modern conservation. Nat. Plants 2017, 3, 17093. [Google Scholar] [CrossRef] [PubMed]
- Dupouey, J.L.; Dambrine, E.; Laffite, D.; Moares, C. Irreversible impact of past land use on forest soils and biodiversity. Ecology 2002, 83, 2978–2984. [Google Scholar] [CrossRef]
- Erickson, C.L. The domesticated landscape of the Bolivian Amazon. In Time and Complexity in Historical Ecology; Balée, W., Erickson, C.L., Eds.; Columbia University Press: New York, NY, USA, 2006; pp. 235–278. [Google Scholar]
- Svensson, E.; Bodin, S.; Hulling, H.; Pettersson, S. The crofter and the iron works: The material culture of structural crisis, identity and making of a living on the edge. Int. J. Hist. Archaeol. 2009, 13, 183–205. [Google Scholar] [CrossRef]
- Carso, J.F.; Watling, J.; Mayle, F.E.; Whitney, B.S.; Iriarte, J.; Prümers, H.; Soto, J.D. Pre-Columbian land use in the ring-ditch region of the Bolivian Amazon. Holocene 2015, 25, 1285–1300. [Google Scholar] [CrossRef] [Green Version]
- Bellemare, J.; Motzkin, G.; Foster, D.R. Legacies of the agricultural past in the forested present: An assessment of historical land-use effect on rich mesic forests. J. Biogeogr. 2002, 29, 1401–1420. [Google Scholar] [CrossRef] [Green Version]
- Hermy, M.; Verheyen, K. Legacies of the past in the present-day forest biodiversity: A review of past land-use effects on forest plant species composition and diversity. Ecol. Res. 2007, 22, 361–371. [Google Scholar] [CrossRef]
- Plue, J.; Hermy, M.; Verheyen, K.; Thuillier, P.; Saguez, R.; Decocq, G. Persistent changes in forest vegetation and seed bank 1600 years after human occupation. Landsc. Ecol. 2008, 23, 673–688. [Google Scholar] [CrossRef]
- Bodin, S.C.; Molino, J.-F.; Odonne, G.; Bremond, L. Unraveling pre-Columbian occupation patterns in the tropical forests of French Guiana using an anthracological approach. Veg. Hist. Archaeobot. 2020, 29, 567–580. [Google Scholar] [CrossRef]
- Sjörs, H. The background: Geology, climate and zonation. In Swedish Plant Geography; Rydin, H., Snoeijs, P., Diekmann, M., Eds.; Acta Phytogeographica Suecica: Uppsala, Sweden, 1999; pp. 5–14. [Google Scholar]
- Östlund, L.; Zackrisson, O.; Axelsson, A.-L. The history and transformation of a Scandinavian boreal forest landscape since the 19th century. Can. J. For. Res. 1997, 27, 1198–1206. [Google Scholar] [CrossRef]
- Lindbladh, M.; Bradshaw, R. The origin of present forest composition and pattern in southern Sweden. J. Biogeogr. 1998, 25, 463–477. [Google Scholar] [CrossRef]
- Ericsson, S.; Östlund, L.; Axelsson, A.-L. A forest of grazing and logging: Deforestation and reforestation history of a boreal landscape in central Sweden. New For. 2000, 19, 227–240. [Google Scholar] [CrossRef]
- Eriksson, O. What is biological cultural heritage and why should we care about it? An example from Swedish rural landscapes and forests. Nat. Conserv. 2018, 28, 1–32. [Google Scholar] [CrossRef]
- Eriksson, O.; Cousins, S.A.O. Historical landscape perspectives on grasslands in Sweden and the Baltic region. Land 2014, 3, 300–321. [Google Scholar] [CrossRef]
- Agnoletti, M.; Santoro, A. Cultural values and sustainable forest management: The case of Europe. J. For. Res. 2015, 20, 438–444. [Google Scholar] [CrossRef]
- Berglund, B.E. The Cultural Landscape during 6000 years in Southern Sweden. Ecol. Bull. 1991, 41, 1–495. [Google Scholar]
- Myrdal, J.; Morell, M. (Eds.) The Agrarian History of Sweden. From 4000 BC to AD 2000; Nordic Academic Press: Lund, Sweden, 2011. [Google Scholar]
- Lagerås, P. The Ecology of Expansion and Abandonment: Medieval and Post-Medieval Land-Use and Settlement Dynamics in a Landscape Perspective; Swedish National Heritage Board: Stockholm, Sweden, 2007. [Google Scholar]
- Emanuelsson, M.; Segerström, U. Medieval slash-and-burn cultivation: Strategic or adapted land use in the Swedish mining district? Environ. Hist. 2002, 8, 173–196. [Google Scholar] [CrossRef]
- Vestbö-Franzén, Å. Farming by fire in north-eastern Småland. Bebygg. Tidskr. 2019, 77, 8–21. [Google Scholar]
- Granström, A.; Niklasson, M. Potentials and limitations for human control over historic fire regimes in boreal forest. Philos. Trans. R. Soc. B 2008, 383, 2353–2358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slotte, H. Harvesting of leaf-hay shaped the Swedish landscape. Landsc. Ecol. 2001, 16, 691–702. [Google Scholar] [CrossRef]
- Hæggström, C.-A. Vegetation and soil of the wooded meadows in Nåtö, Åland. Acta Bot. Fenn. 1983, 120, 1–66. [Google Scholar]
- Eriksson, O. Origin and development of managed meadows in Sweden. A review. Rural Landsc. Soc. Environ. Hist. 2020, 7, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Segerström, U.; Emanuelsson, M. Extensive forest grazing and hay-making on mires: Vegetation changes in south-central Sweden due to land use since the Medieval times. Veg. Hist. Archaeobot. 2002, 11, 181–190. [Google Scholar] [CrossRef]
- Elveland, J. Wet hay-meadows in northern Sweden—Their history and present status. Svensk Bot. Tidskr. 2015, 109, 292–336. [Google Scholar]
- Dahlström, A. Pastures, Livestock Number and Grazing Pressure 1620–1850. Ecological Aspects of Grazing History in South-Central Sweden. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2006. (In Swedish with English Summary). [Google Scholar]
- Dahlström, A.; Cousins, S.A.O.; Eriksson, O. The history (1620–2003) of land use, people and livestock, and the relationship to present plant species diversity in a rural landscape in Sweden. Environ. Hist. 2006, 12, 191–212. [Google Scholar] [CrossRef]
- Kardell, Ö. Swedish forestry, forest pasture grazing by livestock and game browsing pressure since 1900. Environ. Hist. 2016, 22, 561–587. [Google Scholar] [CrossRef]
- Westin, A.; Lennartsson, T.; Ljung, T. Skogsbeten Och Bondeskogar: Historia, Ekologi, Natur—Och Kulturmiljövård; Centrum för biologisk mångfald: Uppsala, Sweden, 2022. (In Swedish) [Google Scholar]
- Nilsson, S.G. The changing structure and tree composition in the traditionally grazed forests in the parish Stensbrohult. Svensk Bot. Tidskr. 2006, 100, 393–412. [Google Scholar]
- Emanuelsson, U. The Rural Landscape of Europe: How Man Has Shaped European Nature; The Swedish Research Council Formas: Stockholm, Sweden, 2009. [Google Scholar]
- Arpi, G. Den Svenska Järnhanteringens Träkolsförsörjning 1830–1950; Jernkontorets Bergshistoriska Skriftserie N:r 14: Stockholm, Sweden, 1951. (In Swedish) [Google Scholar]
- National Atlas of Sweden. Swedish Mining and Metalworking; Norstedts Förlagsgrupp: Stockholm, Sweden, 2011. [Google Scholar]
- Gadd, C.-J. The agricultural revolution in Sweden, 1700–1870. In The Agrarian History of Sweden. From 4000 BC to AD 2000; Myrdal, J., Morell, M., Eds.; Nordic Academic Press: Lund, Sweden, 2011; pp. 118–164. [Google Scholar]
- Svensson, E.; Amundsen, H.R.; Holm, I.; Hulling, H.; Johansson, A.; Löfgren, J.; Nilsson, P.; Nilsson, S.; Pettersson, S.; Stensby, V. Empowering marginal lifescapes: The heritage of crofters in between the past and the present. Int. J. Herit. Stud. 2018, 24, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, O.; Glav Lundin, L. ‘Gooseberry is the only thing left’—A study of declining biological cultural heritage at abandoned crofts in the province of Södermanland, Sweden. Int. J. Herit. Stud. 2020, 26, 1061–1076. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, O.; Arnell, M.; Lindholm, K.-J. Historical ecology of Scandinavian infield systems. Sustainability 2021, 13, 817. [Google Scholar] [CrossRef]
- Wilson, J.B.; Peet, R.K.; Dengler, J.; Pärtel, M. Plant species richness: The world records. J. Veg. Sci. 2012, 23, 796–802. [Google Scholar] [CrossRef]
- Cousins, S.A.O.; Auffret, A.G.; Lindgren, J.; Tränk, L. Regional-scale land-cover change during the 20th century and its consequences for biodiversity. Ambio 2015, 44 (Suppl. S1), 17–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swedish Board of Agriculture. Plan för Odlingslandskapets Biologiska Mångfald 2019. Available online: https://www2.jordbruksverket.se/download/18.36d57baa168c704154d46f04/1549611543321/ra19_1.pdf (accessed on 7 October 2021).
- O’Dwyer, R.; Marquer, L.; Trondman, A.-K.; Jönsson, A.M. Spatially continuous land-cover reconstructions through the Holocene in southern Sweden. Ecosystems 2021, 24, 1450–1467. [Google Scholar] [CrossRef]
- Hedwall, P.-O.; Brunet, J.; Nordin, A.; Berg, J. Changes in the abundance of keystone forest floor species in response to changes of forest structure. J. Veg. Sci. 2013, 24, 296–306. [Google Scholar] [CrossRef]
- Lindbladh, M.; Axelsson, A.-L.; Hultberg, T.; Brunet, J.; Felton, A. From broadleaves to spruce: The borealization of southern Sweden. Scand. J. For. Res. 2014, 29, 686–696. [Google Scholar] [CrossRef]
- Hedwall, P.-O.; Uria-Diez, J.; Brunet, J.; Gustafsson, L.; Axelsson, A.-L.; Strengbom, J. Interactions between local and global drivers determine long-term trends in boreal forest understorey vegetation. Global Ecol. Biogeogr. 2021, 30, 1765–1780. [Google Scholar] [CrossRef]
- Sundberg, S. Boreal plant decline in southern Sweden during the twentieth century. New J. Bot. 2014, 4, 76–84. [Google Scholar] [CrossRef]
- Ljung, T.; Lennartsson, T.; Westin, A. Inventering Av Biologiskt Kulturarv; Riksantikvarienämbetet: Stockholm, Sweden, 2015. [Google Scholar]
- Szabó, P.; Hedl, R. Advancing the integration of history and ecology for conservation. Conserv. Biol. 2011, 25, 680–687. [Google Scholar] [CrossRef]
- Vellend, M.; Brown, C.D.; Kharouba, H.M.; McCune, J.L.; Myers-Smith, I.H. Historical ecology: Using unconventional data sources to test for effects of global environmental change. Am. J. Bot. 2013, 100, 1294–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perring, M.P.; de Frenne, P.; Baeten, L.; Maes, S.L.; Depauw, L.; Blondeel, H.; Carón, M.M.; Verheyen, K. Global environmental change effects on ecosystems: The importance of land-use legacies. Global Change Biol. 2016, 22, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Svedjemyr, O. The flora at abandoned crofts in the parish of Malexander, Östergötland, Sweden. Svensk Bot. Tidskr. 1986, 80, 9–15. [Google Scholar]
- Koerner, W.; Dupouey, J.L.; Dambrine, E.; Benoît, M. Influence of past land use on the vegetation and soils of present day forest in the Vosges mountains, France. J. Ecol. 1997, 85, 351–358. [Google Scholar] [CrossRef]
- Dambrine, E.; Dupouey, J.-L.; Laüt, L.; Humbert, L.; Thinon, M.; Beaufils, T.; Richard, H. Present forest biodiversity patterns in France related to former Roman agriculture. Ecology 2007, 88, 1430–1439. [Google Scholar] [CrossRef]
- Criscuoli, I.; Alberti, G.; Baronti, S.; Favilli, F.; Martinez, C.; Calzolari, C.; Pusceddu, E.; Rumpel, C.; Viola, R.; Miglietta, F. Carbon sequestration and fertility after centennial time scale incorporation of charcoal into soil. PLoS ONE 2014, 3, e91114. [Google Scholar] [CrossRef]
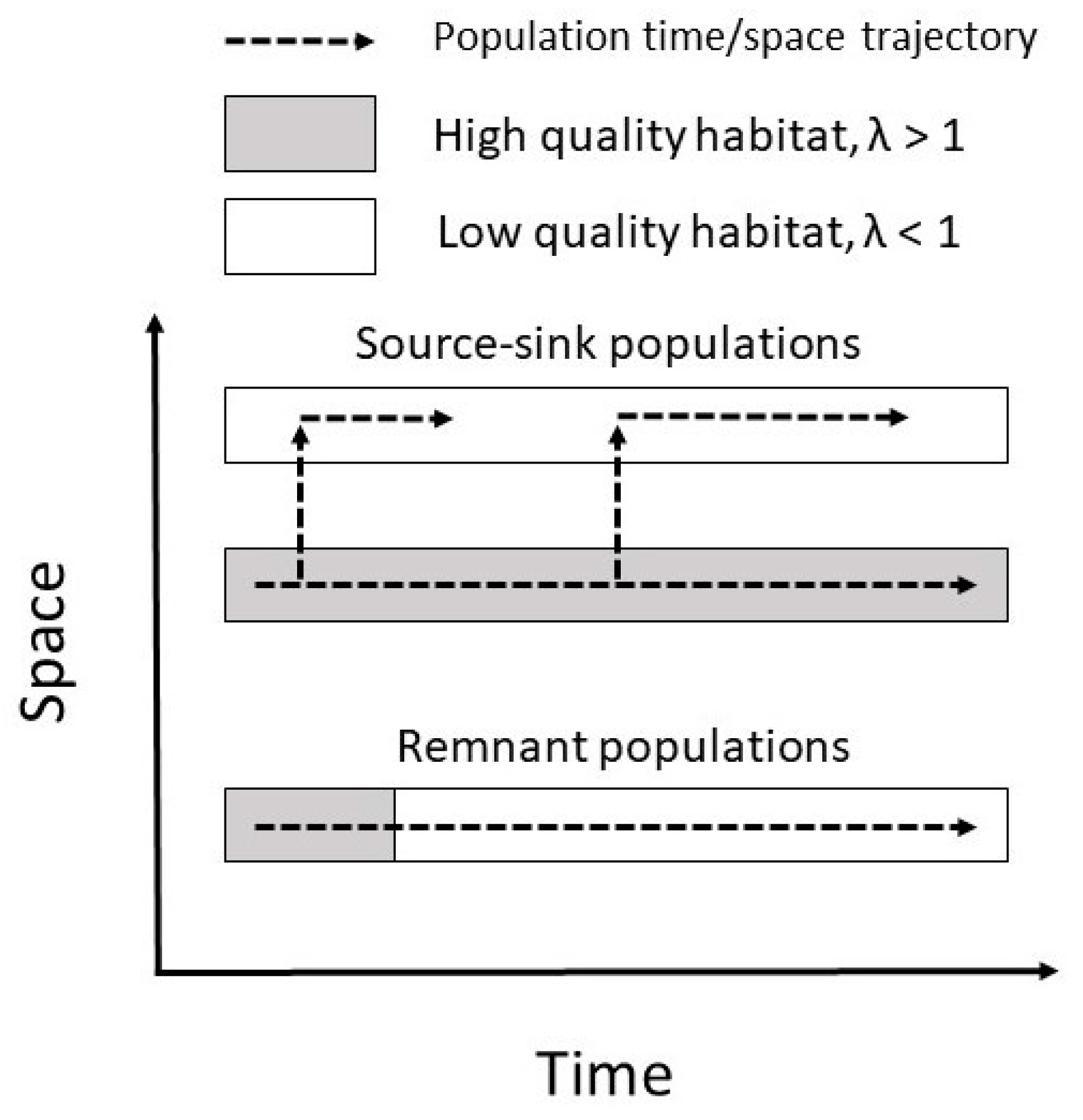
- Eriksson, O. Regional dynamics in plants: A review of evidence for remnant, source-sink and metapopulations. Oikos 1996, 77, 248–258. [Google Scholar] [CrossRef]
- Milberg, P.; Bergman, K.-O.; Jonason, D.; Karlsson, J.; Westerberg, L. Land-use history influence the vegetation in coniferous production forests in southern Sweden. For. Ecol. Manag. 2019, 440, 23–30. [Google Scholar] [CrossRef]
- Pulliam, H.R. Sources, sinks, and population regulation. Am. Nat. 1988, 132, 652–661. [Google Scholar] [CrossRef]
- Hanski, I.; Gilpin, M. Metapopulation dynamics: Brief history and conceptual domain. Biol. J. Linn. Soc. 1991, 42, 3–16. [Google Scholar] [CrossRef]
- De Witte, L.C.; Stöcklin, J. Longevity of clonal plants: Why it matters and how to measure it. Ann. Bot. 2010, 106, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Hylander, K.; Ehrlén, J. The mechanisms causing extinction debts. Trends Ecol. Evol. 2013, 28, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Chase, J.M.; Leibold, M.A. Ecological Niches: Linking Classical and Contemporary Approaches; University of Chicago Press: Chicago, IL, USA, 2003. [Google Scholar]
- Soberón, J.; Nakamura, M. Niches and distributional areas: Concepts, methods, and assumptions. Proc. Natl. Acad. Sci. USA 2009, 106, 19644–19650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchinson, G.E. Concluding remarks. Cold Spring Harb. Sym. 1957, 22, 415–427. [Google Scholar] [CrossRef]
- Sillett, K.B.; Foster, S.A. Ontogenetic niche shifts in two populations of juvenile threespine stickleback, Gasterosteus aculeatus, that differ in pelvic spine morphology. Oikos 2000, 91, 468–476. [Google Scholar] [CrossRef]
- Eriksson, O. Ontogenetic niche shifts and their implications for recruitment in three clonal Vaccinium shrubs: Vaccinium myrtillus, Vaccinium vitis-idaea, and Vaccinium oxycoccos. Can. J. Bot. 2002, 80, 635–641. [Google Scholar] [CrossRef]
- Grubb, P.J. The maintenance of species-richness in plant communities: The importance of the regeneration niche. Biol. Rev. 1977, 52, 107–145. [Google Scholar] [CrossRef]
- Herben, T.; Münzbergová, Z.; Mildén, M.; Ehrlén, J.; Cousins, S.A.O.; Eriksson, O. Long-term spatial dynamics of Succisa pratensis in a changing rural landscape: Linking dynamical modelling with historical maps. J. Ecol. 2006, 94, 131–143. [Google Scholar] [CrossRef]
- Dahlgren, J.; Ehrlén, J. Incorporating environmental change over succession in an integral projection model of population dynamics of a forest herb. Oikos 2011, 120, 1183–1190. [Google Scholar] [CrossRef]
- Lehtilä, K.; Dahlgren, J.; García, M.B.; Leimu, R.; Syrjänen, K.; Ehrlén, J. Forest succession and population viability of grassland plants: Long repayment of extinction debt in Primula veris. Oecologia 2016, 181, 125–135. [Google Scholar] [CrossRef]
- Eriksson, O. Functional roles of remnant populations in communities and ecosystems. Global Ecol. Biogeogr. 2000, 9, 443–449. [Google Scholar] [CrossRef]
- Leuridan, B.; Froeyman, A. On lawfulness in history and historiography. Hist. Theory 2012, 51, 172–192. [Google Scholar] [CrossRef]
- Ekstam, U.; Forshed, N. Om Hävden Upphör: Kärlväxter Som Indikatorarter i Ängs—Och Hagmarker; Naturvårdsverket: Stockholm, Sweden, 1992. (In Swedish) [Google Scholar]
- Jonason, D.; Ibbe, M.; Milberg, P.; Tunér, A.; Westerberg, L.; Bergman, K.-O. Vegetation in clear-cuts depends on previous land use: A century-old grassland legacy. Ecol. Evol. 2014, 4, 4287–4295. [Google Scholar] [CrossRef] [PubMed]
- Palmgren, A. Studier öfver löfängsområdena på Åland. III Statistisk undersökning av floran. Acta Soc. Pro. Fauna Flora Fenn. 1916, 42, 477–633. (In Swedish) [Google Scholar]
- Lindborg, R.; Eriksson, O. Historical landscape connectivity affects present plant species richness. Ecology 2004, 85, 1840–1845. [Google Scholar] [CrossRef]
- Helm, A.; Hanski, I.; Pärtel, M. Slow response of plant species richness to habitat loss and fragmentation. Ecol. Lett. 2006, 9, 72–77. [Google Scholar] [CrossRef]
- Johansson, V.A.; Cousins, S.A.O.; Eriksson, O. Remnant populations and plant functional traits in abandoned semi-natural grasslands. Folia Geobot. 2011, 46, 165–179. [Google Scholar] [CrossRef]
- Lindborg, R.; Cousins, S.A.O.; Eriksson, O. Plant species response to land use change—Campanula rotundifolia, Primula veris and Rhinanthus minor. Ecography 2005, 28, 29–36. [Google Scholar] [CrossRef]
- Dahlström, A.; Borgegård, S.-O.; Rydin, H. The vascular flora after 50 and 90 years of succession in abandoned infields in Kilsbergen, south-central Sweden. Svensk Bot. Tidskr. 1998, 91, 211–226. [Google Scholar]
- Eriksson, O.; Glav Lundin, L. Legacies of historic charcoal production affect the forest flora in a Swedish mining district. Nord. J. Bot. 2021, 39, e003312. [Google Scholar] [CrossRef]
- Angelstam, P.; Andersson, K.; Isacson, M.; Gavrilov, D.V.; Axelsson, R.; Bäckström, M.; Degerman, E.; Elbakidze, M.; Kazakova-Apkarimova, E.Y.; Satrz, L.; et al. Learning about the history of landscape use for the future: Consequences for ecological and social systems in Swedish Bergslagen. Ambio 2013, 42, 146–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindbladh, M. The influence if former land-use on vegetation and biodiversity in the boreo-nemoral zone of Sweden. Ecography 1999, 22, 485–498. [Google Scholar] [CrossRef]
- Eliasson, P.; Nilsson, S.G. You should hate young oaks and young noblemen—The environmental history of oaks in eighteenth- and nineteenth-century Sweden. Environ. Hist. 2002, 7, 659–677. [Google Scholar] [CrossRef]
- Eriksson, S.; Skånes, H.; Hammer, M.; Lönn, M. Current distribution of older and deciduous forests as legacies from historical use patterns in a Swedish boreal landscape (1725–2007). For. Ecol. Manag. 2010, 260, 1095–1103. [Google Scholar] [CrossRef]
- Arnell, M.; Cousins, S.A.O.; Eriksson, O. Does historical land use affect the regional distribution of fleshy-fruited wood plants? PLoS ONE 2019, 14, e0225791. [Google Scholar] [CrossRef] [Green Version]
- Berg, Å.; Gärdenfors, U.; Hallingbäck, T.; Norén, M. Habitat preferences of red-listed fungi and bryophytes in woodland key habitats in southern Sweden—Analyses of data from a national survey. Biodivers. Conserv. 2002, 11, 1479–1503. [Google Scholar] [CrossRef]
- Nilsson, S.G.; Hedin, J.; Niklasson, M. Biodiversity and its assessment in boreal and nemoral forests. Scand. J. For. Res. 2001, 3, 10–26. [Google Scholar] [CrossRef] [Green Version]
- Jansson, G.; Angelstam, P. Threshold levels of habitat composition for the presence of the long-tailed tit (Aegithalos caudatus) in a boreal landscape. Landsc. Ecol. 1999, 14, 283–290. [Google Scholar] [CrossRef]
- Wiktander, U.; Olsson, O.; Nilsson, S.G. Seasonal variation in home-range size, and habitat area requirement of the lesser spotted woodpecker (Dendrocopos minor) in southern Sweden. Biol. Conserv. 2001, 100, 387–395. [Google Scholar] [CrossRef]
- Ranius, T.; Eliasson, P.; Johansson, P. Large-scale occurrence patterns of red-listed lichens and fungi on old oaks are influenced both by current and historical habitat density. Biodivers. Conserv. 2008, 17, 2371–2381. [Google Scholar] [CrossRef] [Green Version]
- Johansson, V.; Snäll, T.; Ranius, T. Estimates of connectivity reveal non-equilibrium epiphytes occurrence patterns almost 180 years after habitat decline. Oecologia 2013, 172, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Sjöbeck, M. Vång och utmark i Skånes skogsbygd. In Sveriges Natur; Svenska Naturskyddsföreningens årsbok; Svenska Naturskyddsföreningen: Stockholm, Sweden, 1966; pp. 149–182. (In Swedish) [Google Scholar]
- Greiser, C.; Hylander, K.; Meineri, E.; Luoto, M.; Ehrlén, J. Climate limitation at the cold edge: Contrasting perspectives from species distribution modelling and a transplant experiment. Ecography 2020, 43, 637–647. [Google Scholar] [CrossRef]
- Brunet, J. Environmental and historical factors limiting the distribution of rare forest grasses in south Sweden. For. Ecol. Manag. 1993, 61, 263–275. [Google Scholar] [CrossRef]
- Ljung, T. Fäbodskogen Som Biologiskt Kulturarv; Centrum för biologisk mångfald: Uppsala, Sweden, 2011. [Google Scholar]
- Sundberg, S. Present status for Cephalanthera rubra and Chimaphila umbellata in Sweden. Svensk Bot. Tidskr. 2017, 111, 90–104. [Google Scholar]
- Kiedrzyński, M.; Zielińska, K.M.; Kiedrzyńska, E.; Rewicz, A. Refugial debate: On small sites according to their function and capacity. Evol. Ecol. 2017, 31, 815–827. [Google Scholar] [CrossRef] [Green Version]
- Sandström, A.; Svensson, B.; Milberg, P. An example of how to build conservation evidence from case studies: Fire and raking to enhance Pulsatilla vernalis populations. J. Nat. Conserv. 2017, 36, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Oinonen, E. Sporal regeneration of ground pine (Lycopodium complanatum L.) in southern Finland in the light of the dimensions and the age of its clones. Acta For. Fenn. 1967, 83, 1–85. [Google Scholar] [CrossRef] [Green Version]
- Jonsell, L. (Ed.) Upplands Flora; SBF-förlaget: Uppsala, Sweden, 2010. (In Swedish) [Google Scholar]
- SLU Artdatabanken. Rödlistade Arter i Sverige 2020; The Swedish Red List, in Swedish with English Summary; SLU: Uppsala, Sweden, 2020. [Google Scholar]
- Lallemand, F.; Puttsepp, Ü.; Lang, M.; Luud, A.; Courty, P.-E.; Palancade, C.; Selosse, M.-A. Mixotrophy in Pyroleae (Ericaceae) from Estonian boreal forest does not vary with light or tissue age. Ann. Bot. 2017, 120, 361–371. [Google Scholar] [CrossRef]
- Merckx, V.S.F.T. (Ed.) Mycoheterotrophy: The Biology of Plants Living on Fungi; Springer: New York, NY, USA, 2013. [Google Scholar]
- Johansson, V.A.; Mikusinska, A.; Ekblad, A.; Eriksson, O. Partial mycoheterotrophy in Pyroleae: Nitrogen and carbon stable isotope signatures during development from seedling to adult. Oecologia 2015, 177, 203–211. [Google Scholar] [CrossRef]
- Hynson, N.A.; Preiss, K.; Gebauer, G.; Bruns, T.D. Isotopic evidence of full and partial myco-heterotrophy in the plant tribe Pyroleae (Ericaceae). New Phytol. 2009, 182, 719–726. [Google Scholar] [CrossRef]
- Eriksson, O.; Kainulainen, K. The evolutionary ecology of dust seeds. Perspect. Plant Ecol. Evol. Syst. 2011, 13, 73–87. [Google Scholar] [CrossRef]
- Johansson, V.A.; Eriksson, O. Recruitment limitation, germination of dust seeds, and early development of underground seedlings in six Pyroleae species. Botany 2013, 91, 17–24. [Google Scholar] [CrossRef]
- Warming, E. Om Jordudløbere; In Danish with English Summary; Det Kgl. Danske Videnskabernes Selskabs Skrifter, Naturvidenskabelig og Mathematisk Afdelning, 8, Række II. 6.: Copenhagen, Denmark, 1918. [Google Scholar]
- Lundell, A.; Cousins, S.A.O.; Eriksson, O. Population size and reproduction in the declining endangered forest plant Chimaphila umbellata in Sweden. Folia Geobot. 2015, 50, 13–23. [Google Scholar] [CrossRef]
- Antos, J.A.; Zobel, D.B.; Fischer, D.G. Belowground morphology and population dynamics of two forest understory herbs of contrasting growth forms. Botany 2021, 99, 569–580. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Olesen, J.M. Buzz-pollination and patterns in sexual traits in north European Pyrolaceae. Am. J. Bot. 1993, 80, 900–913. [Google Scholar] [CrossRef]
- Johansson, V.A.; Müller, G.; Eriksson, O. Dust seed production and dispersal in Swedish Pyroleae species. Nord. J. Bot. 2014, 32, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Johansson, V.A.; Bahram, M.; Tedersoo, L.; Kõljalg, U.; Eriksson, O. Specificity of fungal associations of Pyroleae and Monotropa hypopitys during germination and seedling development. Mol. Ecol. 2017, 26, 2591–2604. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-W.; Zhou, J.; Peng, H.; Freudenstein, J.V.; Milne, R.I. Relationships between Tertiary relict and circumboreal woodland floras: A case study in Chimaphila (Ericaeae). Ann. Bot. 2019, 123, 1089–1098. [Google Scholar] [CrossRef]
- Massicote, H.B.; Melwille, L.H.; Tackaberry, L.E.; Peterson, R.L. A comparative study of mycorrhizas in several genera of Pyroleae (Ericaceae) from western Canada. Botany 2008, 86, 610–622. [Google Scholar] [CrossRef]
- Warming, E. Om Skudbygning, Overvintring Og Foryngelse; Aftryck af Naturhistorisk Forenings Festskrift. Kgl. Hof-Bogtrykkeri: Kjøbenhavn, Denmark, 1884. (In Danish) [Google Scholar]
- Warming, E. The structure and biology of arctic flowering plants: Ericineae (Ericaceae, Pirolaceae) 1. Morphology and Biology. Arb. Fra Den Bot. Have I København 1908, 43, 1–71. [Google Scholar]
- Hynson, N.A.; Bidartondo, M.I.; Read, D.J. Are there geographic mosaics of mycorrhizal specificity and partial mycoheterotrophy? A case study in Moneses uniflora (Ericaceae). New Phytol. 2015, 208, 1003–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rydberg, H.; Wanntorp, H.-E. Sörmlands Flora; Botaniska Sällskapet: Stockholm, Sweden, 2001. (In Swedish) [Google Scholar]
- Johansson, V.A.; Lundell, A.; Eriksson, O. Ecology of ericaceous mycoheterotrophs in Sweden. Svensk Bot. Tidskr. 2017, 111, 240–263. [Google Scholar]
- Artportalen. Available online: www.artportalen.se (accessed on 15 March 2022).
- Rydberg, H. (Linnaeus University). Personal communication, 2021.
- Thompson, J.N. The Coevolutionary Process; University of Chicago Press: Chicago, IL, USA, 1994. [Google Scholar]
- Leake, J.R.; Cameron, D.D. Physiological ecology of mycoheterotrophy. New Phytol. 2010, 185, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Bidartondo, M.; Bruns, T.D. Extreme specificity in epiparasitic Monotropoideae (Ericaceae): Widespread phylogenetic and geographical structure. Mol. Ecol. 2001, 10, 2285–2295. [Google Scholar] [CrossRef] [Green Version]
- Flygare, I.A.; Isacson, M. The tension between modernity and reality. In The Agrarian History of Sweden. From 4000 BC to AD 2000; Myrdal, J., Morell, M., Eds.; Nordic Academic Press: Lund, Sweden, 2011; pp. 214–256. [Google Scholar]
- Lundmark, H.; Östlund, L.; Josefsson, T. Continuity forest or second-generation forest? Historical aerial photos provide evidence of early clear-cutting in northern Sweden. Silva Fenn. 2021, 51, 10460. [Google Scholar] [CrossRef]
- Lundmark, H.; Josefsson, T.; Östlund, L. The history of clear-cutting in northern Sweden: Driving forces and myths in boreal silviculture. For. Ecol. Manag. 2013, 307, 112–122. [Google Scholar] [CrossRef]
- Östlund, L. (Swedish University of Agricultural Sciences). Personal communication, 2022.
- Steen, E. Effects of Grazing on Swedish Vegetation; In Swedish with English summary; Statens Jordbruksförsök Meddelande nr 89: Uppsala, Sweden, 1958. [Google Scholar]
- Plieninger, T.; Hartel, T.; Martín-López, B.; Beaufoy, G.; Bergmeier, E.; Kirby, K.; Montero, M.J.; Moreno, G.; Oteros-Rozas, E.; van Uytvanck, J. Wood-pastures of Europe: Geographic coverage, social-ecologcial values, conservation management, and policy implications. Biol. Conserv. 2015, 190, 70–79. [Google Scholar] [CrossRef]
- Oldén, A.; Raatikainen, K.J.; Tervonen, K.; Halme, P. Grazing and soil pH are biodiversity drivers of vascular plants and bryophytes in boreal wood-pastures. Agri. Ecosyst. Environ. 2016, 222, 171–184. [Google Scholar] [CrossRef]
- Lilleskov, E.A.; Fahey, T.J.; Horton, T.R.; Lovett, G.M. Belowground ectomycorrhizal fungal community change over a nitrogen deposition gradient in Alaska. Ecology 2002, 83, 104–115. [Google Scholar] [CrossRef]
- Davey, M.L.; Skogen, M.J.; Heegaard, E.; Halvorsen, R.; Kauserud, H.; Ohlson, M. Host and tissue variations overshadow the response of boreal moss-associated fungal communities to increased nitrogen load. Mol. Ecol. 2017, 26, 571–588. [Google Scholar] [CrossRef]
- Rajala, T.; Tuomivirta, T.; Pennanen, T.; Mäkipää, R. Habitat models of wood-inhabiting fungi along a decay gradient of Norway spruce logs. Fungal Ecol. 2015, 18, 48–55. [Google Scholar] [CrossRef]
- Mäkipää, R.; Rajala, T.; Schigel, D.; Rinne, K.T.; Pennanen, T.; Abrego, N.; Ovaskainen, O. Interactions between soil- and dead wood-inhabiting fungal communities during the decay of Norway spruce logs. ISME J. 2017, 11, 1964–1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonsson, B.G.; Kruys, N.; Ranius, T. Ecology of species living on dead wood—Lessons from dead wood management. Silva Fenn. 2005, 39, 289–309. [Google Scholar] [CrossRef] [Green Version]
- Whittier, D.P. Red light inhibition of spore germination in Lycopodium clavatum. Am. Fern J. 2008, 98, 194–198. [Google Scholar] [CrossRef]
- Winther, J.L.; Friedman, W.E. Arbuscular mycorrhizal association in Lycopodiaceae. New Phytol. 2008, 177, 790–801. [Google Scholar] [CrossRef]
- Johnson, D.; Ijdo, M.; Genney, D.R.; Anderson, I.C.; Alexander, I.J. How do plants regulate the function, community structure, and diversity of mycorrhizal fungi? J. Exp. Bot. 2005, 56, 1751–1760. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: Oxford, UK, 2008. [Google Scholar]
- Öpik, M.; Moora, M.; Zobel, M.; Saks, Ü.; Wheatley, R.; Wright, F.; Daniell, T. High diversity of arbuscular mycorrhizal fungi in a boreal herb-rich coniferous forest. New Phytol. 2008, 179, 867–876. [Google Scholar] [CrossRef]
- Vellend, M. The behavioral economics of biodiversity conservation scientists. Philos. Top. 2019, 47, 219–237. [Google Scholar] [CrossRef]
- Gustafsson, L.; Baker, S.C.; Bauhus, J.; Beese, W.J.; Brodie, A.; Kouki, J.; Lindenmayer, D.B.; Lõhmus, A.; Martínez Pastur, G.; Messier, C.; et al. Retention forestry to maintain multifunctional forests: A world perspective. BioScience 2012, 62, 633–645. [Google Scholar] [CrossRef] [Green Version]
- Koivula, M.; Vanha-Majamaa, I. Experimental evidence on biodiversity impacts of variable retention forestry, prescribed burning, and deadwood manipulation in Fennoscandia. Ecol. Process. 2020, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.; Strengbom, J.; Kouki, J. Low levels of tree retention do not mitigate the effects of clearcutting on ground vegetation dynamics. For. Ecol. Manag. 2014, 330, 67–74. [Google Scholar] [CrossRef]
- Fedrowitz, K.; Koricheva, J.; Baker, S.C.; Lindenmayer, D.B.; Palik, B.; Rosenvald, R.; Beese, W.; Franklin, J.F.; Kouki, J.; Macdonald, E.; et al. Can retention forestry help conserve biodiversity? A meta-analysis. J. Appl. Ecol. 2014, 51, 1669–1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koorem, K.; Moora, M. Positive association between understory species richness and a dominant shrub species (Corylus avellana) in a boreonemoral spruce forest. For. Ecol. Manag. 2010, 260, 1407–1413. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eriksson, O. Floristic Legacies of Historical Land Use in Swedish Boreo-Nemoral Forests: A Review of Evidence and a Case Study on Chimaphila umbellata and Moneses uniflora. Forests 2022, 13, 1715. https://doi.org/10.3390/f13101715
Eriksson O. Floristic Legacies of Historical Land Use in Swedish Boreo-Nemoral Forests: A Review of Evidence and a Case Study on Chimaphila umbellata and Moneses uniflora. Forests. 2022; 13(10):1715. https://doi.org/10.3390/f13101715
Chicago/Turabian StyleEriksson, Ove. 2022. "Floristic Legacies of Historical Land Use in Swedish Boreo-Nemoral Forests: A Review of Evidence and a Case Study on Chimaphila umbellata and Moneses uniflora" Forests 13, no. 10: 1715. https://doi.org/10.3390/f13101715




