Bat Assemblages Are Shaped by Land Cover Types and Forest Age: A Case Study from Eastern Ukraine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Bat Species
2.3. Bat Mist-Netting Protocol and Field Data Collection
2.4. Bat Assemblage Structure and Indices of Abundance
2.5. The Land Cover Types as Explanatory Variables
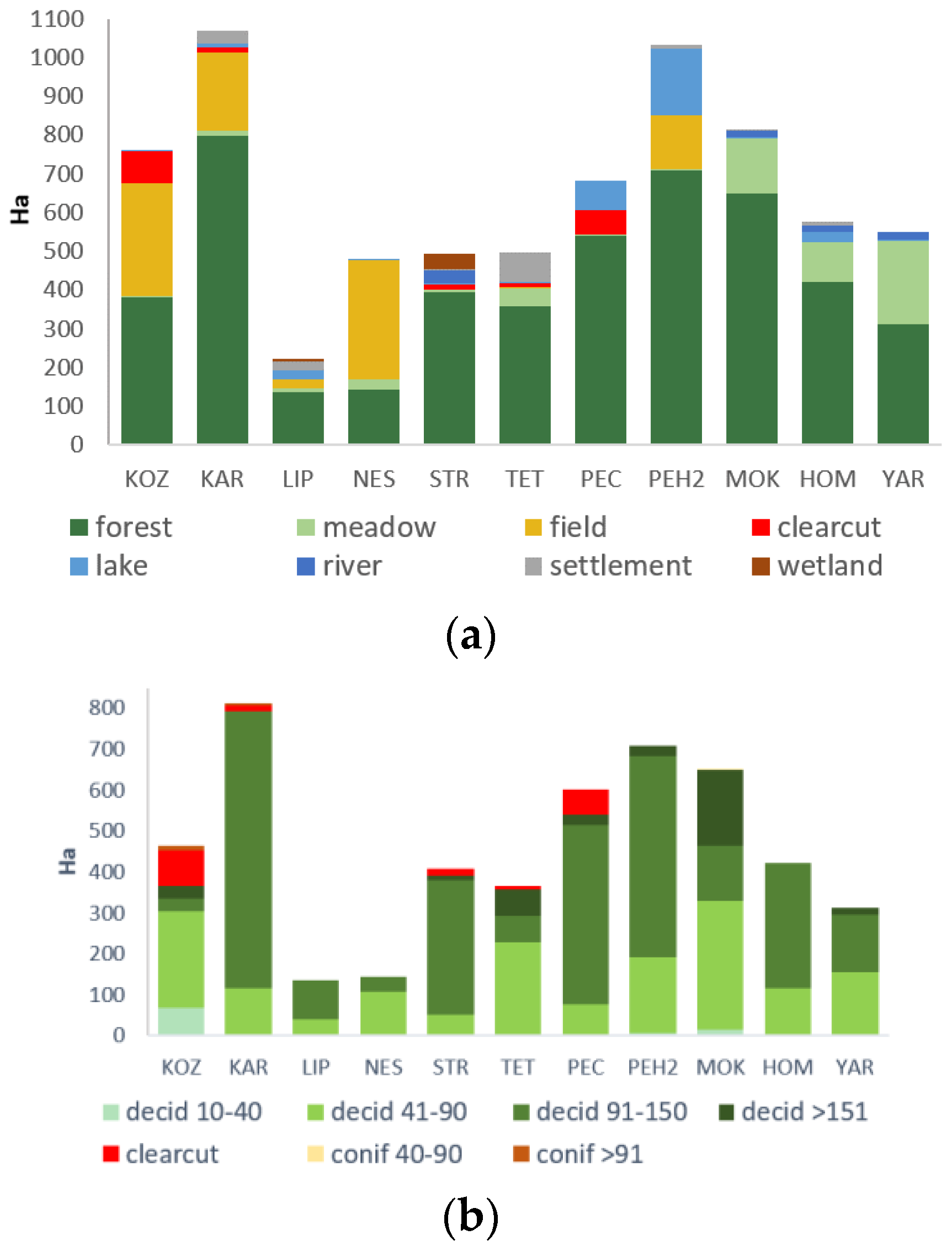
2.5.1. Land Cover Types
2.5.2. Forest Age Structure
2.6. Statistical Analyses
2.6.1. Validation of Sampling Effort
2.6.2. Associations of Bat Assemblages with Land Cover Types and Forest Age
2.6.3. Species Richness and Total Bat Abundance in Relation to Land Cover Types and Forest Age Structure
2.6.4. Breeding Activity
3. Results
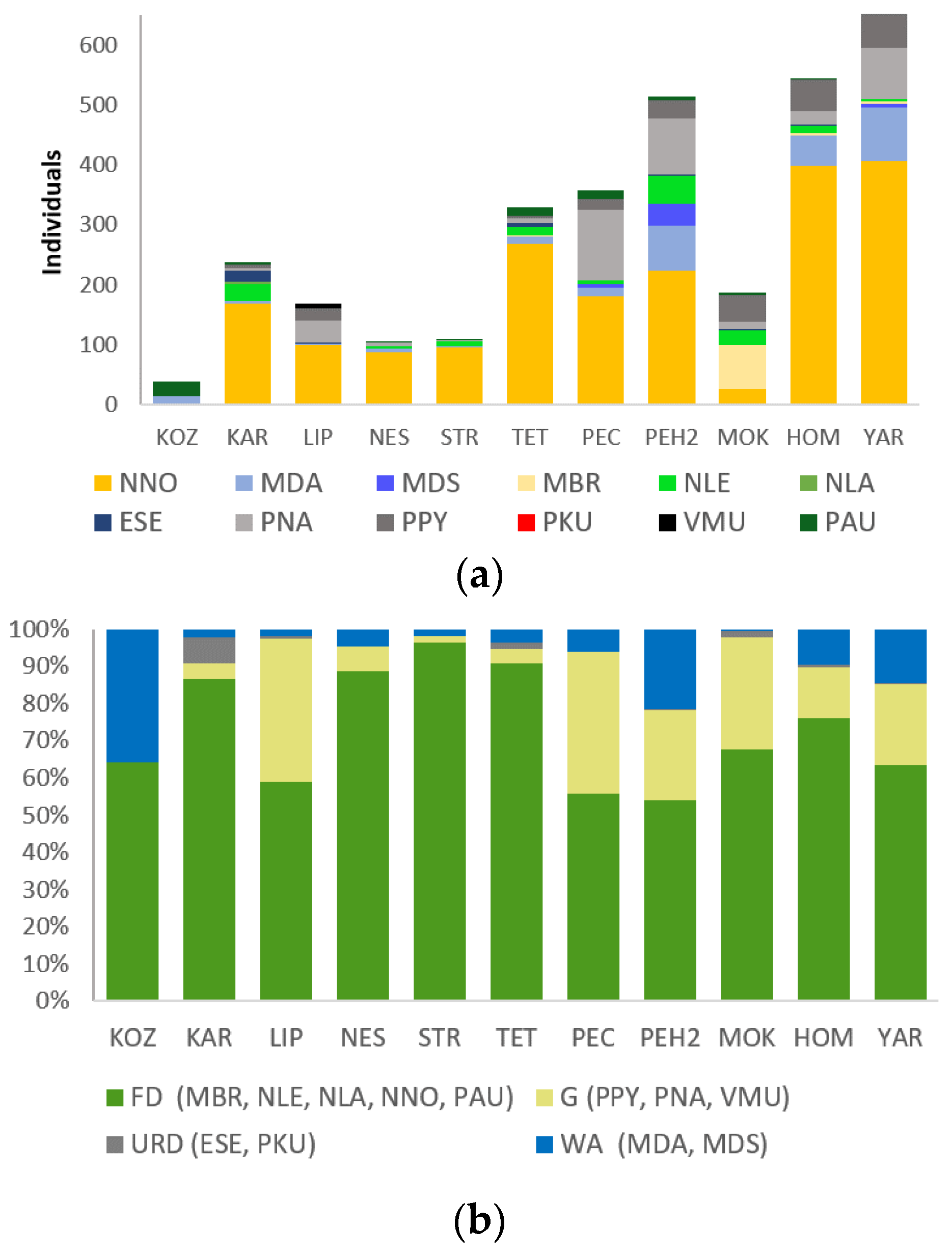
3.1. Mist-Netting Results
3.2. The Validation of Sampling Effort
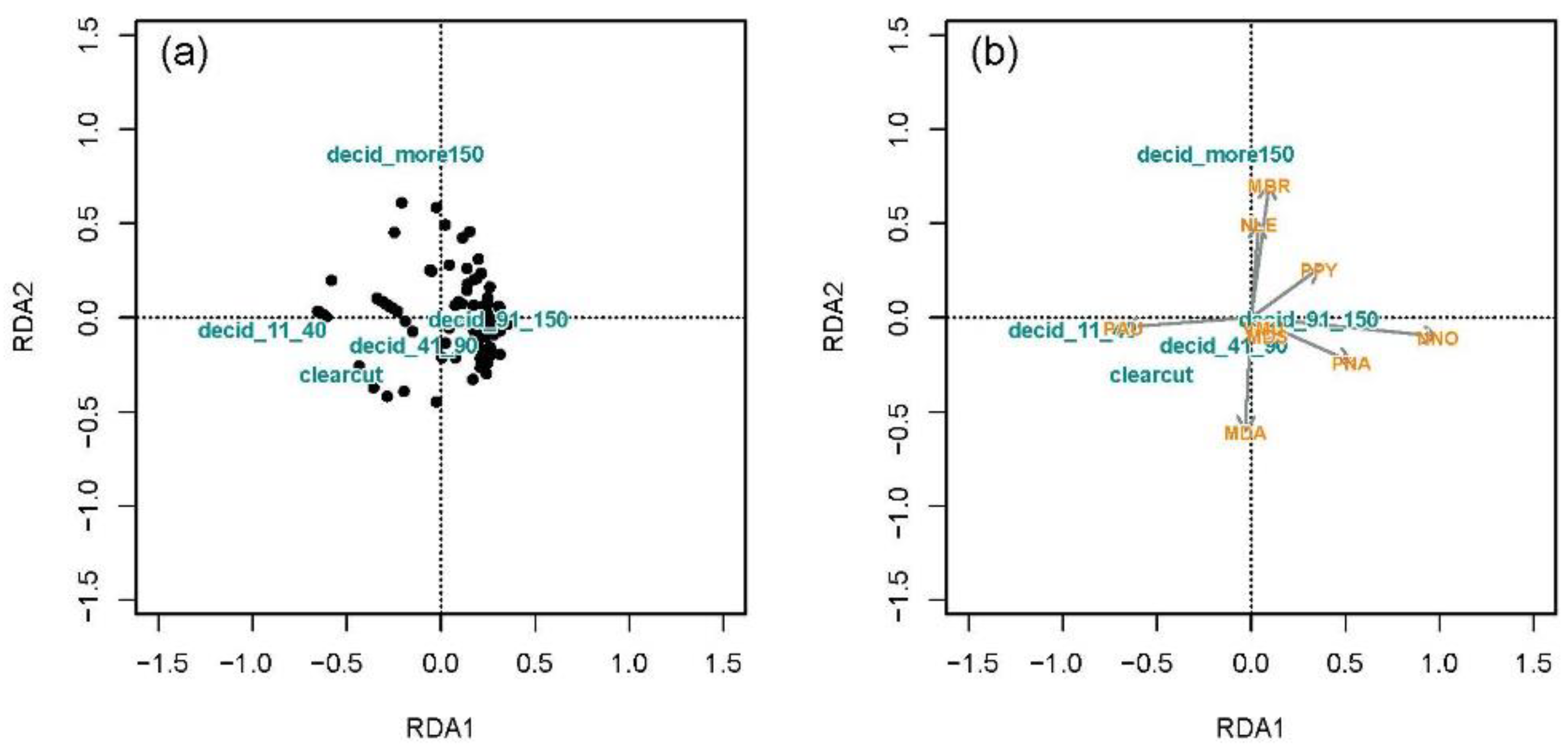
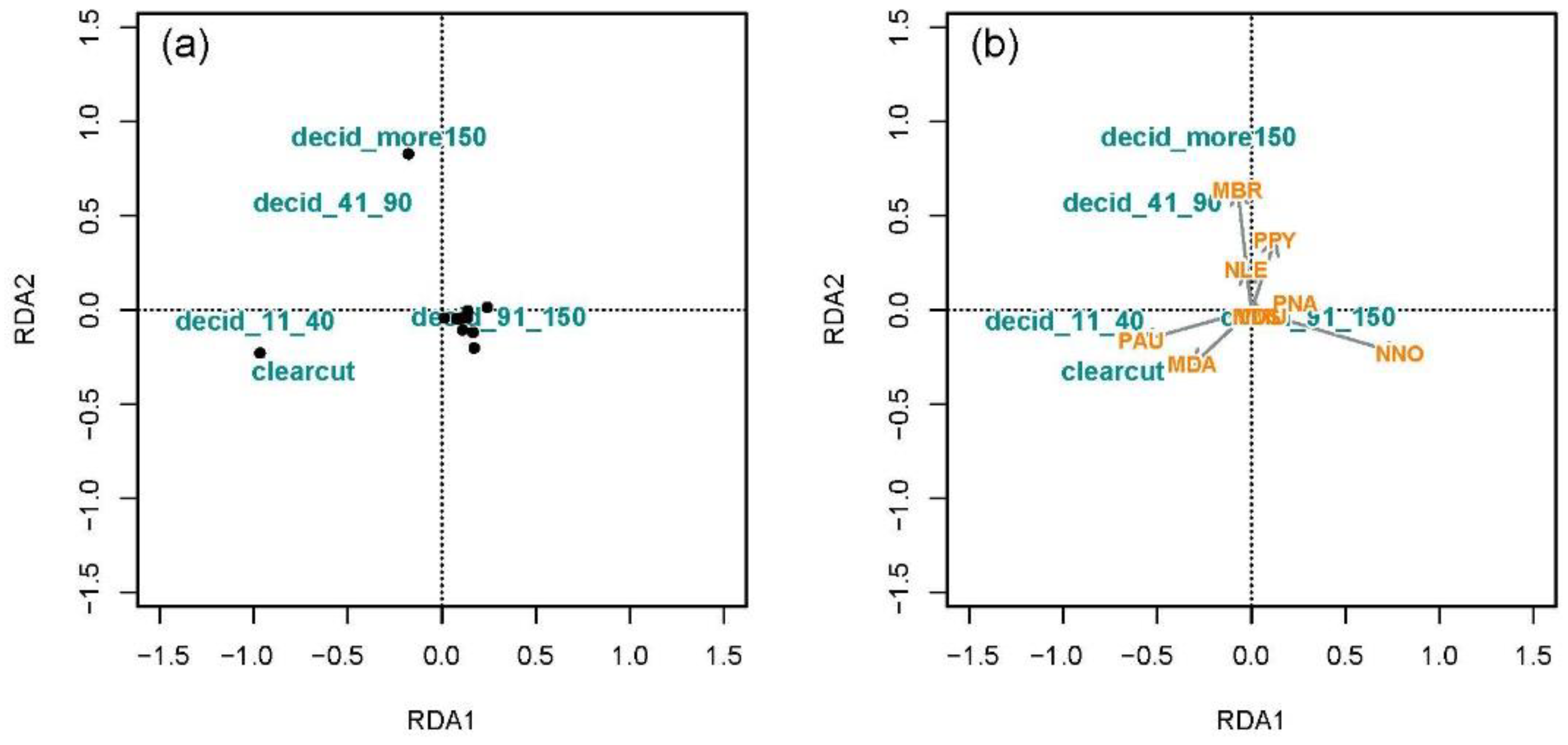
3.3. Land Cover Types, Forest Age and Bat Assemblage Structure
3.3.1. Land Cover Types and Forest Age of Study Plots
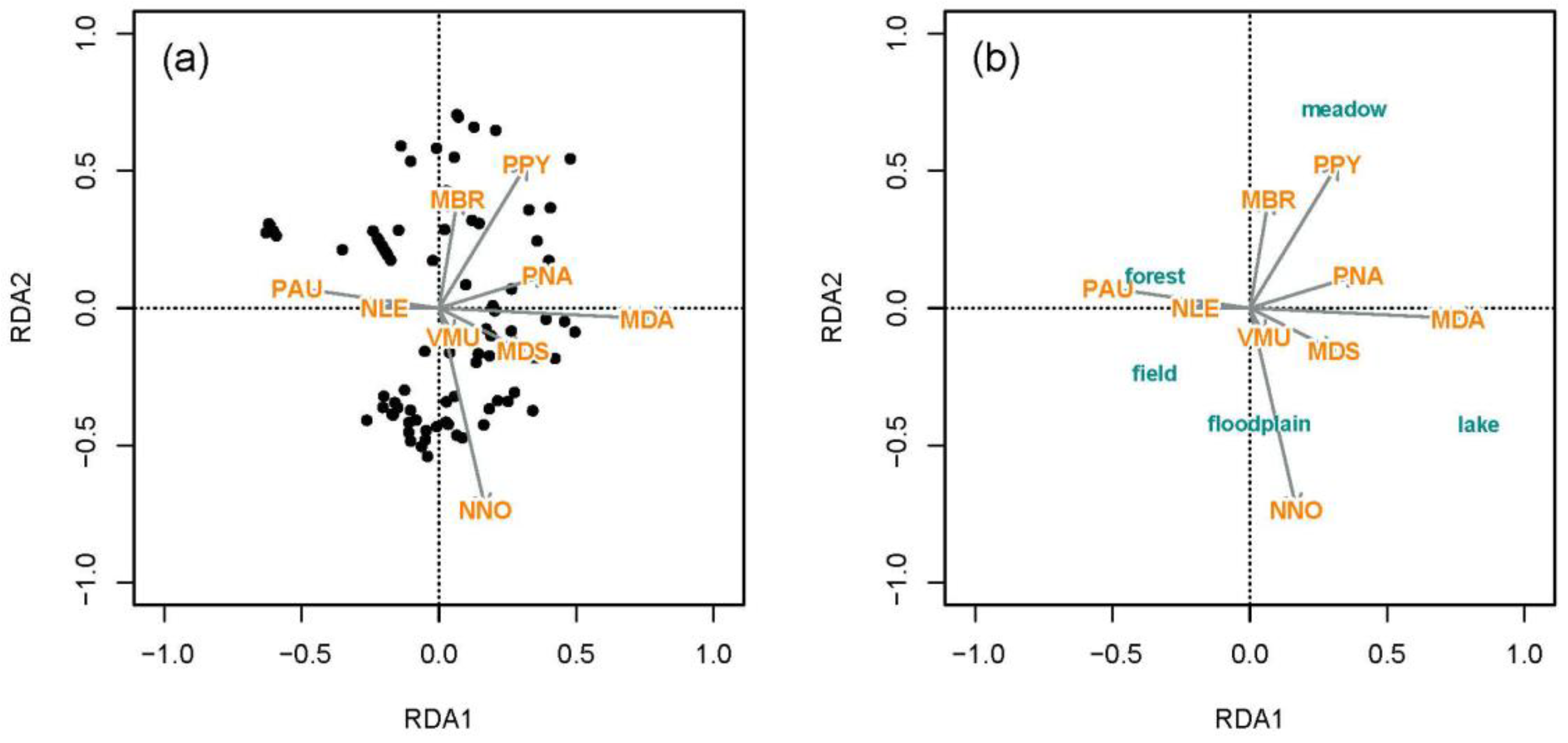
3.3.2. Associations of Bat Species with Land Cover Types and Forest Age
Association of Bat Species with Land Cover Types
Association of Bat Assemblages with Forest Age Structure
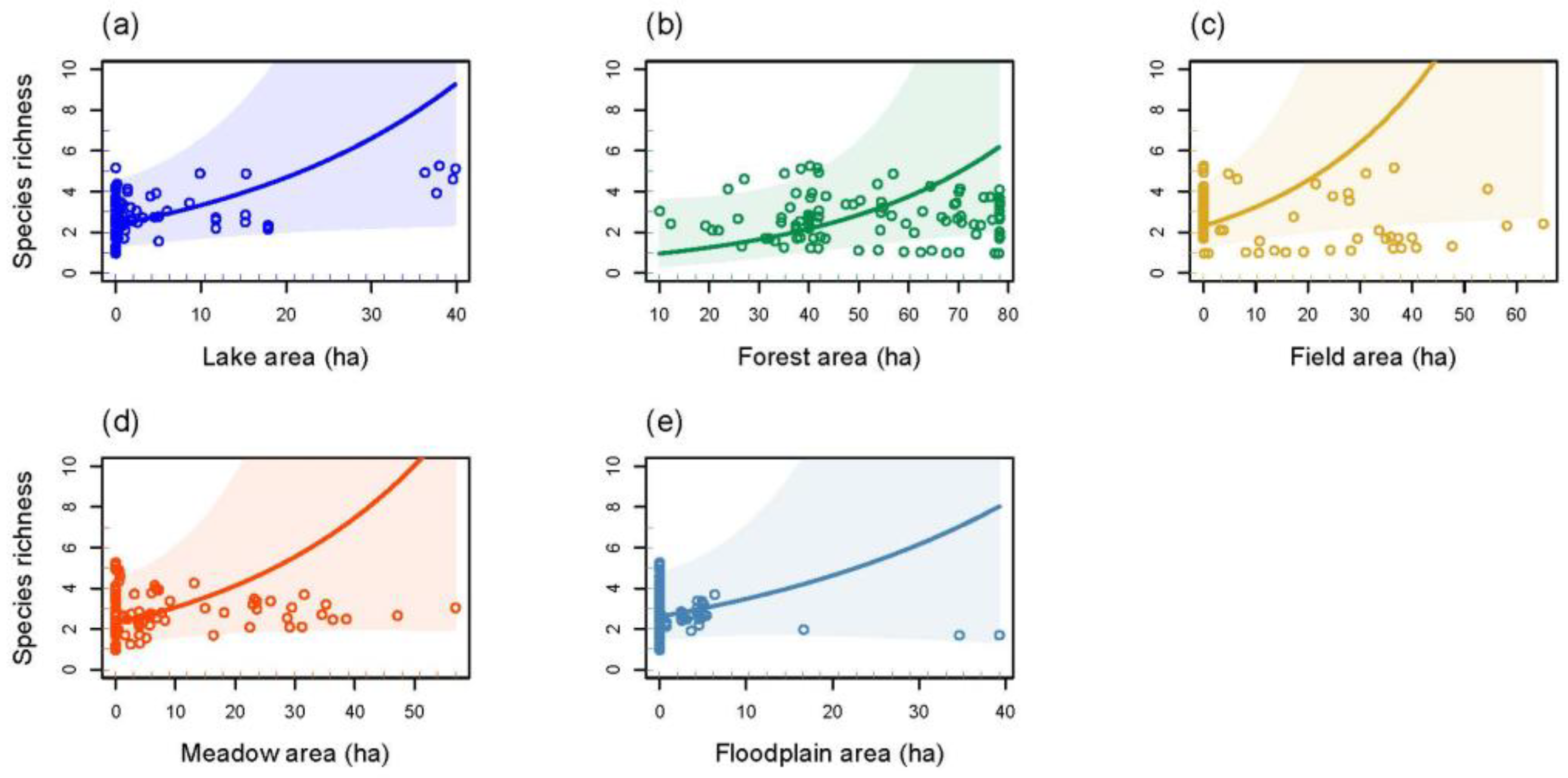
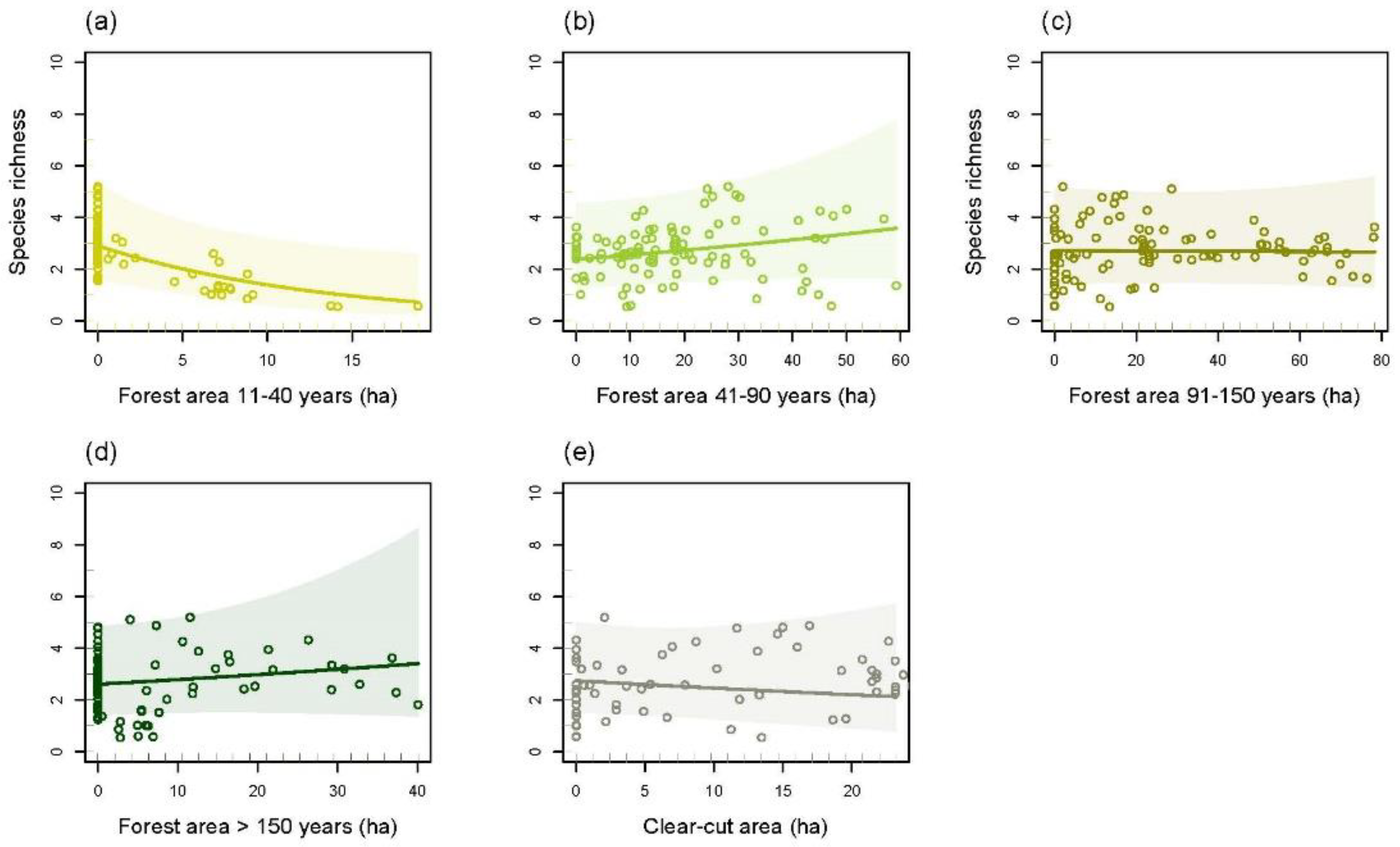
3.4. Species Richness and Total Bat Abundance in Relation to Land Cover Types and Forest Age Structure
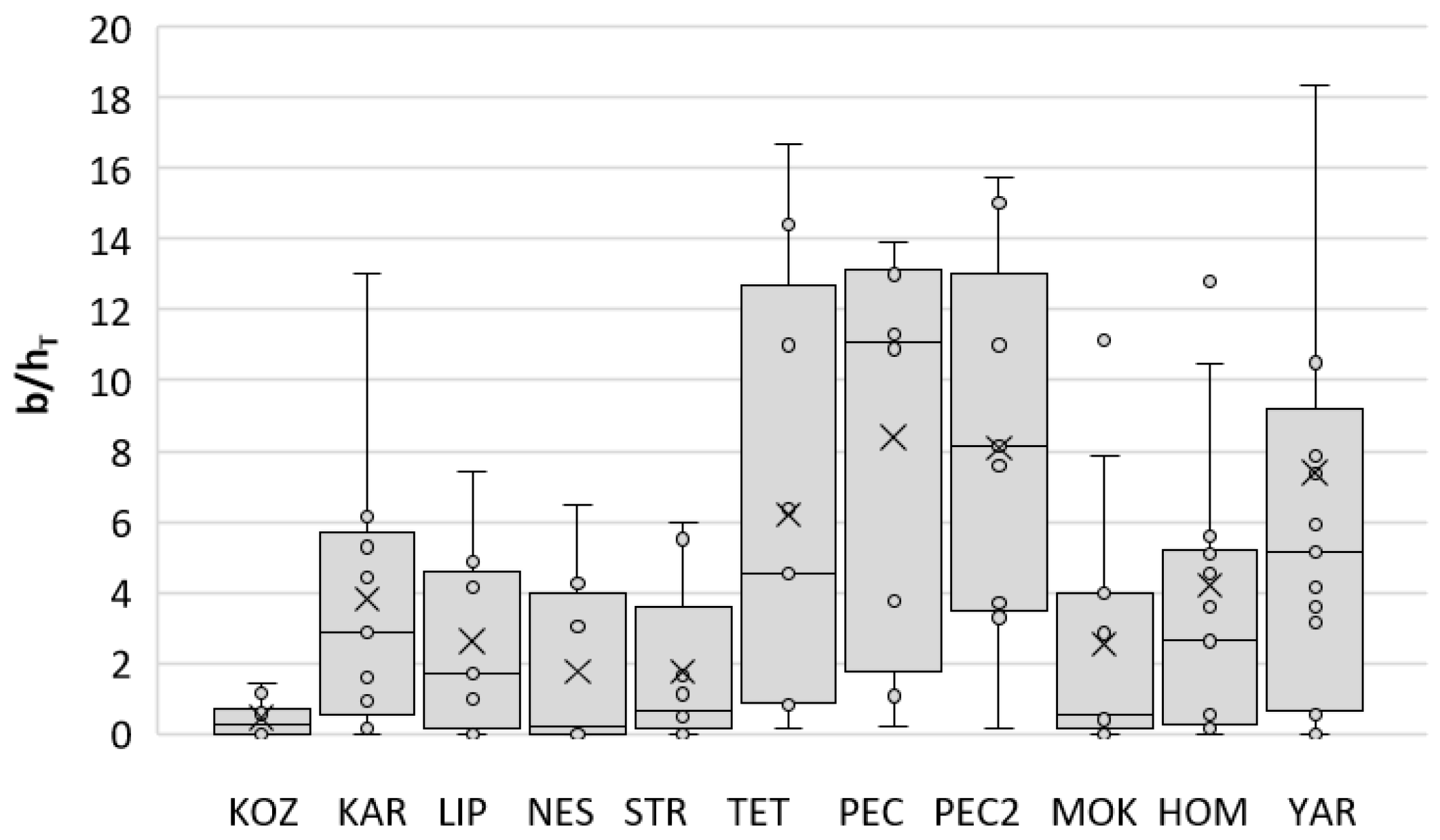
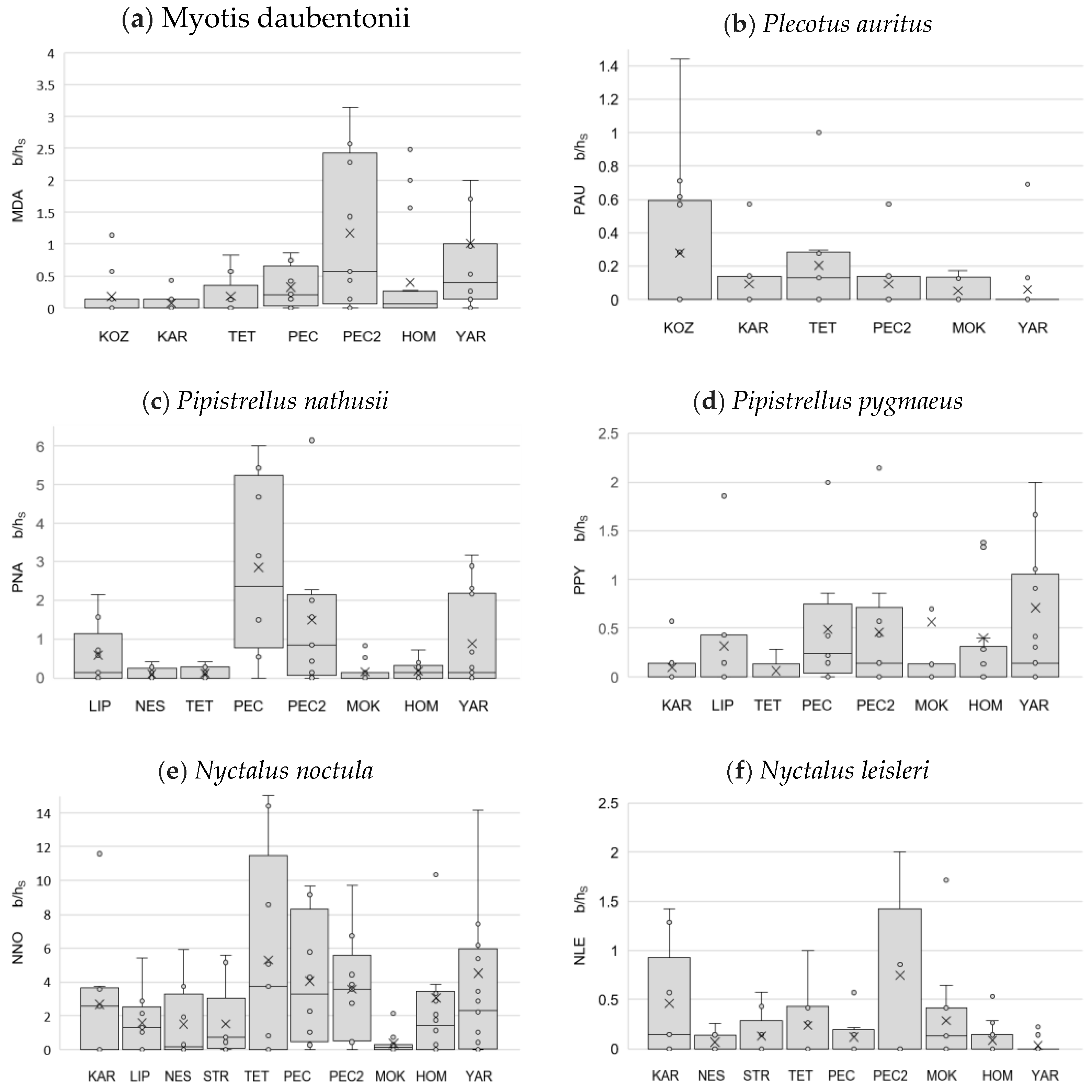
3.5. Indices of Bat Abundance B/h
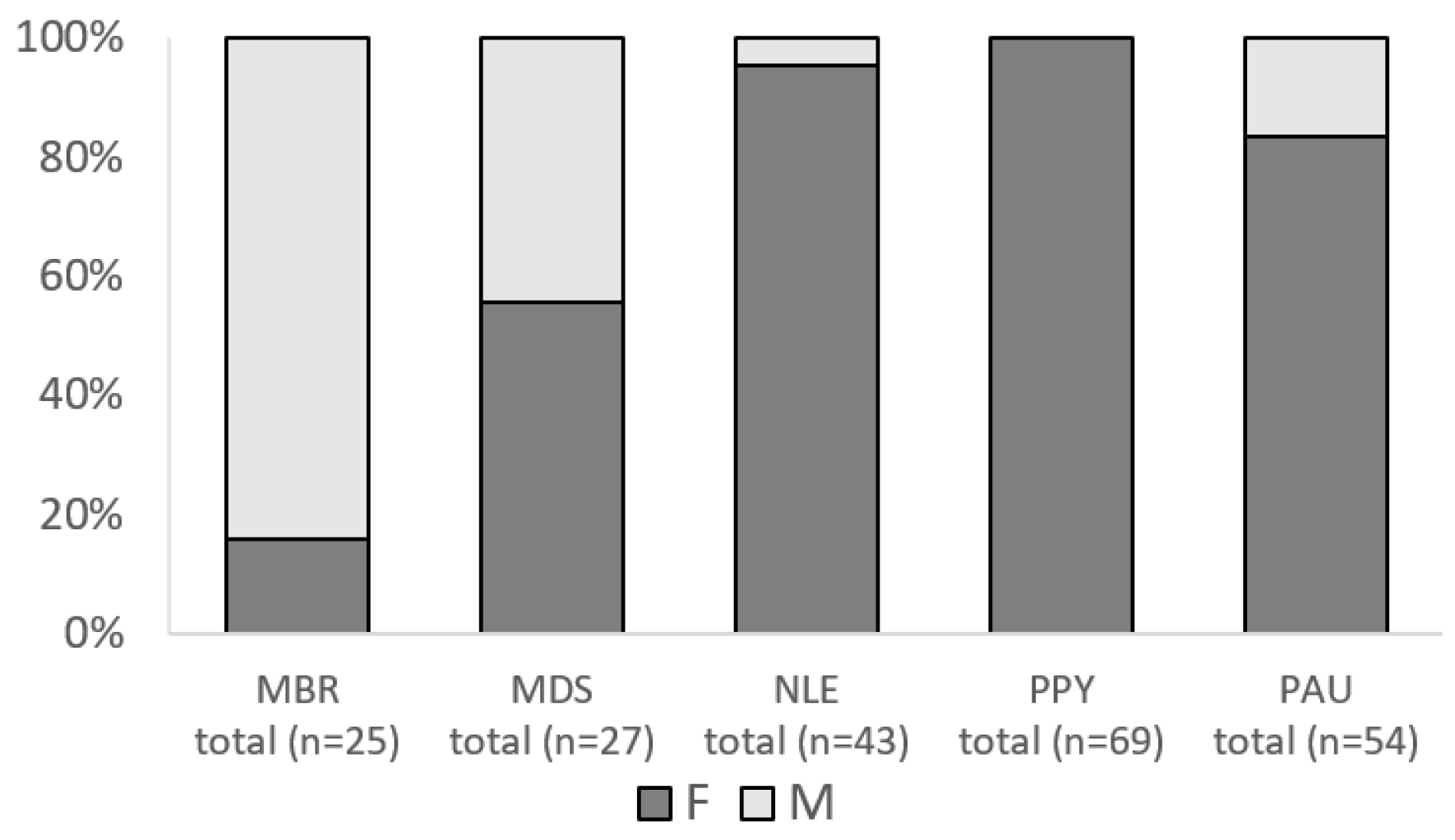
3.6. Sex Ratio and Breeding Activity of Bats
4. Discussion
4.1. Methodology
4.2. Structure of Bat Assemblages across Landscapes
4.3. Ecological Groups of Bats and Their Habitat Requirements
4.4. Impact of Forest Management on Bat Assemblages
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. The State of the World’s Forests 2020: Forests, Biodiversity and People; FAO and UNEP: Rome, Italy, 2020. [Google Scholar]
- Forest Europe. State of Europe’s Forests 2020; Forest Europe: Oslo, Norway, 2020; p. 392. [Google Scholar]
- Keenan, R.J.; Reams, G.A.; Achard, F.; de Freitas, J.V.; Grainger, V.A.; Lindquist, E. Dynamics of global forest area: Results from the FAO Global Forest Resources Assessment 2015. For. Ecol. Manag. 2015, 352, 9–20. [Google Scholar] [CrossRef]
- Song, X.P.; Hansen, M.C.; Stehman, S.V.; Potapov, P.V.; Tyukavina, A.; Vermote, E.F.; Townshend, J.R. Global land change from 1982 to 2016. Nature 2018, 560, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, F.; Bluhm, H.; Kun, Z.; Aksenov, D.; Atauri, J.; Kuemmerle, T. European primary forest database v2.0. Sci. Data 2021, 8, 220. [Google Scholar] [CrossRef]
- Paillet, Y.; Bergés, L.; Hjältén, J.; Ódor, P.; Avon, C.; Bernhardt-Römermann, M.; Bijlsma, R.-J.; de Bruyn, L.; Fuhr, M.; Grandin, U.; et al. Biodiversity differences between managed and unmanaged forests: Meta-analysis of species richness in Europe. Conserv. Biol. 2010, 24, 101–112. [Google Scholar] [CrossRef]
- Pawson, S.M.; Brockerhoff, E.G.; Norton, D.A.; Didham, R.K. Clear-fell harvest impacts on biodiversity: Past research and the search for harvest size thresholds. Can. J. For. Res. 2006, 36, 1035–1046. [Google Scholar] [CrossRef]
- Fuller, R.J.; Robles, H. Conservation Strategies and Habitat Management for European Forest Birds. In Ecology and Conservation of Forest Birds; Mikusiński, G., Roberge, J.-M., Fuller, R.J., Eds.; Cambridge University Press: Cambridge, UK, 2018; pp. 455–507. [Google Scholar]
- Russo, D.; Billington, G.; Bontadina, F.; Dekker, J.; Dietz, M.; Gazaryan, S.; Jones, G.; Meschede, A.; Rebelo, H.; Reiter, G.; et al. Identifying Key Research Objectives to Make European Forests Greener for Bats. Front. Ecol. Evol. 2016, 4, 87. [Google Scholar] [CrossRef] [Green Version]
- Law, B.; Park, K.J.; Lacki, M.J. Insectivorous bats and silviculture: Balancing timber production and bat conservation. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Voigt, C.C., Kingston, T., Eds.; SpringerOpen: Cham, Switzerland; Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2016; pp. 105–150. [Google Scholar]
- Jones, G.; Jacobs, D.S.; Kunz, T.H.; Willig, M.R.; Racey, P.A. Carpe noctem: The importance of bats as bioindicators. Endang. Species Res. 2009, 8, 93–115. [Google Scholar] [CrossRef] [Green Version]
- Russo, D.; Jones, G. Bats as bioindicators. Mamm. Biol. 2015, 80, 157–246. [Google Scholar] [CrossRef] [Green Version]
- Russo, D.; Cistrone, L.; Garonna, A.P.; Jones, G. Reconsidering the importance of harvested forests for the conservation of tree-dwelling bats. Biodivers. Conserv. 2010, 9, 2501–2515. [Google Scholar] [CrossRef]
- Ruczyński, I.; Nicholls, B.; MacLeod, C.D.; Racey, P.A. Selection of roosting habitats by Nyctalus noctula and Nyctalus leisleri in Białowieża Forest—An adaptive response to forest management? For. Ecol. Manag. 2010, 259, 1633–1641. [Google Scholar] [CrossRef]
- Vlaschenko, A. Roost sites of Noctule bat (Nyctalus noctula Schreber, 1774) on the territory of Gomolsha forest. J. V. N. Karazin Kharkiv. Natl. Univ. Ser. Biol. 2005, 1, 122–133, (In Russian with English summary). [Google Scholar]
- Ruczynski, I.; Bogdanowicz, W. Roost cavity selection by Nyctalus noctula and N. leisleri (Vespertilionidae, Chiroptera) in BiaŁowieża Primeval Forest, eastern Poland. J. Mammal. 2005, 86, 921–930. [Google Scholar] [CrossRef]
- Boye, P.; Dietz, M. Development of good practical guidelines for woodland management for bats. eNRR 2005, 661, 1–89. [Google Scholar]
- Salvarina, I. Bats and aquatic habitats: A review of habitat use and anthropogenic impacts. Mammal Rev. 2016, 46, 131–143. [Google Scholar] [CrossRef]
- Blakey, R.V.; Law, B.S.; Straka, T.M.; Kingsford, R.T.; Milne, D.J. Importance of wetlands to bats on a dry continent: A review and meta-analysis. Hystrix Ital. J. Mammal. 2018, 29, 41–52. [Google Scholar]
- Meyer, C.F.J.; Aguiar, L.M.S.; Aguirre, L.F.; Baumgarten, J.; Clarke, F.M.; Cosson, J.F.; Villegas, S.E.; Fahr, J.; Faria, D.; Furey, N.; et al. Long-term monitoring of tropical bats for anthropogenic impact assessment: Gauging the statistical power to detect population change. Biol. Conserv. 2010, 143, 2797–2807. [Google Scholar] [CrossRef]
- Freudmann, A.; Mollik, P.; Tschapka, M.; Schulze, C.H. Impacts of oil palm agriculture on phyllostomid bat assemblages. Biodivers. Conserv. 2015, 24, 3583–3599. [Google Scholar] [CrossRef]
- Loureiro, L.O.; Gregorin, R. Structure of a bat assemblage from a fragmented landscape in the state of Minas Gerais, southeastern Brazil. Mastozool. Neotrop. 2015, 22, 35–42. [Google Scholar]
- Meyer, C.F.J.; Struebig, J.M.; Willig, M.R. Responses of Tropical Bats to Habitat Fragmentation, Logging, and Deforestation. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Voigt, C.C., Kingston, T., Eds.; SpringerOpen: Cham, Switzerland; Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2016; pp. 63–103. [Google Scholar]
- Wordley, C.F.R.; Sankaran, M.; Mudappa, D.; Altringham, J.D. Heard but not seen: Comparing bat assemblages and study methods in a mosaic landscape in the Western Ghats of India. Ecol. Evol. 2018, 8, 3883–3894. [Google Scholar] [CrossRef]
- Strelkov, P.P. Breeding area and its position in range of migratory bats species in East Europe and adjacent territories Communication 1. Zool. Zhurnal 1997, 76, 1073–1082. [Google Scholar]
- Vlaschenko, A.; Kravchenko, K.; Prylutska, A.; Ivancheva, E.; Sitnikova, E.; Mishin, A. Structure of summer bat assemblages in forests in European Russia. Turk. J. Zool. 2016, 40, 876–893. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’Amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial ecoregions of the world: A new map of life on earth. BioScience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Golikov, A.P.; Kazakova, N.A.; Shuba, M.B. Kharkov Region: Regional Development, Current and Perspectives; V.N. Karazin Kharkiv National University Press: Kharkiv, Ukraine, 2011. (In Russian) [Google Scholar]
- Kuemmerle, T.; Kaplan, J.O.; Prishchepov, A.V.; Rylsky, I.; Chaskovskyy, O.; Tikunov, V.S.; Müller, D. Forest transitions in Eastern Europe and their effects on carbon budgets. Glob. Change Biol. 2015, 21, 3049–3061. [Google Scholar] [CrossRef] [PubMed]
- Global Forest Watch. Available online: www.globalforestwatch.org (accessed on 18 December 2021).
- Vlaschenko, A.S. Research history and list of records of bats (Chiroptera) in the Kharkov Region in the XIX and XX centuries. Plecotus 2011, 14, 26–54, (In Russian with English summary). [Google Scholar]
- Kovalov, V.; Hukov, V.; Rodenko, O. New record of Nyctalus lasiopterus (Schreber, 1780) in Ukraine with a new confirmation of carnivory. North-Weast. J. Zool. 2019, 15, 91–95. [Google Scholar]
- Vlaschenko, A.S.; Naglov, A.V. A Marl Open Pit as a Unique Place of Bats (Chiroptera) Inhabiting. Vest Zool. 2005, 39, 94. [Google Scholar]
- Vlaschenko, A.; Yatsiuk, Y.; Hukov, V.; Prylutska, A.; Straka, T.; Kravchenko, K. Urban forest preserves local bat species diversity, but not forest-dweller specialists—Renewed study 65 years later (Kharkiv city, Ukraine). Mammal Res. 2021, 66, 615–626. [Google Scholar] [CrossRef]
- Vlaschenko, A. Biogeocenological Role of Bats (Mammalia, Chiroptera) on the South Part of Forest-Steppe Zone. Ph.D. Thesis, V.N. Karazin Kharkiv National University, Kharkiv, Ukraine, 2006. (In Russian with English summary). [Google Scholar]
- Strelkov, P.; Il’in, V. Bats (Chiroptera, Vespertilionidae) of the Southern Central and Lower Volga Region. Tr. Zool. Inst. Akad Nauk SSSR 1990, 225, 42–167. (In Russian) [Google Scholar]
- Dietz, C.; Kiefer, A. Die Fledermäuse Europas: Kennen, Bestimmen, Schützen; Kosmos VerlagKosmos: Stuttgart, Germany, 2014. [Google Scholar]
- Gukasova, A.; Vlaschenko, A. Effectiveness of mist-netting of bats (Chiroptera, Mammalia) during the non-hibernation period in oak forests of Eastern Ukraine. Acta Zool. Crac. Verterbrata 2011, 54, 77–93. [Google Scholar] [CrossRef]
- Vlaschenko, A.S.; Gukasova, A.S. Bat (Chiroptera) fauna and structure of assemblage of projecting protected area “Yaremovskoe” (Kharkov region). Nat. Reserves Ukr. 2010, 16, 44–50, (In Russian with English summary). [Google Scholar]
- Vlaschenko, A.; Hukov, V.; Naglov, A.; Prylutska, A.; Kravchenko, K.; Rodenko, O. Contribution to ecology of Brandt’s Bat, Myotis brandtii (Chiroptera, Vespertilionidae), in the North-eastern Ukraine: Comparison of local summer and winter bat assemblages. Vest. Zool. 2016, 50, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Gukasova, A.; Vlaschenko, A.; Kosenkov, G.; Kravchenko, K. Fauna and structure of bat (Chiroptera) assemblage of the National Park ‘Smolensk Lakeland’, western Russia. Acta Zool. Litu. 2011, 21, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Kunz, T.H.; Hodgkison, R.; Weise, C.D. Methods of capturing and handling bats. In Ecological and Behavioral Methods for the Study of Bats, 2nd ed.; Kunz, T., Parsons, S., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2009; pp. 3–35. [Google Scholar]
- Kravchenko, K.; Vlaschenko, A.; Prylutska, A.; Rodenko, O.; Hukov, V.; Shuvaev, V. Year-round monitoring of bat records in an urban area: Kharkiv (NE Ukraine), 2013, as a case study. Turk. J. Zool. 2017, 41, 530–548. [Google Scholar] [CrossRef]
- Sikes, R.S. The Animal Care and Use Committee of the American Society of Mammalogists. Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J. Mammal. 2016, 97, 663–688. [Google Scholar] [CrossRef] [PubMed]
- Quantum GIS Development Team. QGIS Geographic Information System; QGIS 2.18.11 2.18.11 Version; Open Source Geospatial Foundation Project: Beaverton, OR, USA, 2016; Available online: https://qgis.osgeo.org (accessed on 1 January 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 1 May 2022).
- Hsiech, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012; 990p. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Gavin, L.; Solymos, S.P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 May 2022).
- Rousset, F.; Ferdy, J.-B. Testing environmental and genetic effects in the presence of spatial autocorrelation. Ecography J. 2014, 37, 781–790. [Google Scholar] [CrossRef]
- Vlaschenko, A.S.; Gukasova, A.S. Development of method for the inventory of species composition and population structure of Chiroptera. Nat. Reserves Ukr. 2009, 15, 49–57. (In Russian) [Google Scholar]
- Prylutska, A.S. Summer Population of Bats in Forests of Central Part of East European Plain: Inventory and Monitoring Using mist Nets. Ph.D. Thesis, Schmalhausen Institute of Zoology, National Academy of Sciences of Ukraine, Kyiv, Ukraine, 2014. (In Russian with English summary). [Google Scholar]
- Gashchak, S.P.; Vlaschenko, A.S.; Naglov, A.V.; Kravchenko, K.O.; Gukasova, A.S. Bat fauna of the Exclusion Zone in concern of assessment of environmental value of its areas. Prob. Chernobyl. Exclusion Zone 2013, 11, 56–78, (In Russian with English summary). [Google Scholar]
- Zeppelini, C.G.; Rego, K.M.C.; Lopez, L.C.S. Bats in settlements from an atlantic forest area in northeastern Brazil. Papéis Avulsos De Zool. 2017, 57, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Rocha, R.; Ferreira, D.F.; López-Baucells, A.; Farneda, F.Z.; Carreiras, J.M.B.; Palmeirim, J.M.; Meyer, C.F.J. Does sex matter? Gender-specific responses to forest fragmentation in Neotropical bats. Biotropica 2017, 47, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Rocha, R.; Ovaskainen, O.; López-Baucells, A.; Farneda, F.Z.; Sampaio, F.M.; Bobrowiec, P.E.D.; Cabeza, M.; Jorge, M.; Palmeirim, J.M.; Meyer, C.F.J. Secondary forest regeneration benefits old-growth specialist bats in a fragmented tropical landscape. Sci. Rep. 2018, 8, 3819. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.; Bordignon, M. Bat (Chiroptera) assemblages in three Cerrado fragments of Mato Grosso do Sul, southwestern Brazil. Check List 2014, 10, 1380–1386. [Google Scholar] [CrossRef]
- Dietz, M.; Pir, J.D. Distribution and habitat selection of Myotis bechsteinii in Luxembourg: Implications for forest management and conservation. Fol. Zool. 2009, 58, 327–340. [Google Scholar]
- Panutin, K.K. Bats. In Results of Mammals Banding; Kucheruk, V.V., Ed.; Nauka; USSR: Moscow, Russia, 1980; pp. 23–46, (In Russian with English summary). [Google Scholar]
- Vlaschenko, A.; Hukov, V.; Timofieieva, O.; Moiseienko, M.; Domanska, A.; Zinenko, O.; Prylutska, A. Leaping on urban islands: Further summer and winter range expansion of European bat species. Ukrainian Bat Rehabilitation Center, Kharkiv, Ukraine. Mammalia 2022. submitted. [Google Scholar]
- Vlaschenko, A.; Gashchak, S.; Gukasova, A.; Naglov, A. New record and current status of Nyctalus lasiopterus (Schreber, 1780) (Chiroptera: Vespertilionidae) in Ukraine. Lynx N. Praha 2010, 41, 209–216. [Google Scholar]
- Dombrovski, V.; Fenchuk, V.; Fenchuk, V.; Zhurauliou, D. The bat fauna (Mammalia, Chiroptera) of Stary Zhaden Zakaznik, Southern Belarus. Proc. Theriol. Sch. 2017, 15, 3–97. [Google Scholar] [CrossRef]
- Salvarina, I.; Gravier, D.; Rothhaupt, K.O. Seasonal bat activity related to insect emergence at three temperate lakes. Ecol. Evol. 2018, 8, 3738–3750. [Google Scholar] [CrossRef] [Green Version]
- Starik, N.; Göttert, T.; Zeller, U. Spatial Behavior and Habitat Use of Two Sympatric Bat Species. Animals 2021, 11, 3460. [Google Scholar] [CrossRef]
- Figueiredo, L.; Krauss, J.; Steffan-Dewenter, I.; Cabral, J.S. Understanding extinction debts: Spatio–temporal scales, mechanisms and a roadmap for future research. Ecography 2019, 42, 1973–1990. [Google Scholar] [CrossRef] [Green Version]
- Vlaschenko, A. Current status of Leisler’s bat (Chiroptera) on the territory of Kharkov region. Visnyk Lviv. Univ. Biol. Ser. 2009, 51, 145–156, (In Ukrainian with English summary). [Google Scholar]
- Dombrovski, V. Maternity colonies of Myotis brandtii in the Polesie State Radiation-Ecological Reserve. Theriol. Ukr. 2018, 16, 91–94. [Google Scholar] [CrossRef] [Green Version]
- Boston, E.S.M.; Dechmann, D.K.N.; Ruczynski, I. Leisler’s Noctule Nyctalus leisleri (Kuhl, 1817). In Handbook of the Mammals of Europe; Hackländer, K., Zachos, F.E., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 1–15. [Google Scholar]
- Alcaldé, J.; Juste, J.; Paunović, M. Nyctalus lasiopterus. The IUCN Red List of Threatened Species. 2016. e.T14918A22015318. Available online: https://www.iucnredlist.org/species/14918/22015318 (accessed on 30 May 2022).
- Estók, P. Present status of a rare bat species, Nyctalus lasiopterus (Schreber, 1780), in Hungary. Hystrix Ital. J. Mammal. 2011, 22, 99–104. [Google Scholar]
- Dombrovski, V.; Fenchuk, V.; Zhurauliou, D. New occurrence and the first breeding record of Nyctalus lasiopterus in Belarus. Vespertilio 2016, 18, 55–59. [Google Scholar]
- Vasenkov, D.A.; Golovina, G.A.; Sidorchuk, N.V. The first registration of Greater noctule (Nyctalus lasiopterus) in Vladimir region. Plecotus 2016, 19, 32–36, (In Russian with English summary). [Google Scholar]
- Naďo, L.; Lőbbová, D.; Hapl, E.; Ceľuch, M.; Uhrin, M.; Šara, M.; Kaňuch, P. Highly selective roosting of the Giant Noctule Bat and its astonishing foraging activity by GPS tracking in a mountain environment. Mammal Res. 2019, 64, 587–594. [Google Scholar] [CrossRef]
- Popa-Lisseanu, A.G.; Bontadina, F.; Ibáñez, C. Giant noctule bats face conflicting constraints between roosting and foraging in a fragmented and heterogeneous landscape. J. Zool. 2009, 278, 126–133. [Google Scholar] [CrossRef]
- Krüger, F.; Clare, E.L.; Greif, S.; Siemers, B.M.; Symondson, W.O.; Sommer, R.S. An integrative approach to detect subtle trophic niche differentiation in the sympatric trawling bat species Myotis dasycneme and Myotis daubentonii. Mol. Ecol. 2014, 23, 3657–3671. [Google Scholar] [CrossRef]
- Encarnacao, J.A.; Backer, N.I. Daubenton’s Bat Myotis daubentonii (Kuhl, 1817). In Handbook of the Mammals of Europe; Hackländer, K., Zachos, F.E., Eds.; Springer Nature: Switzerland, Switzerland, 2020; pp. 1–31. [Google Scholar]
- Ciechanowski, M.; Zapart, A.; Kokurewicz, T.; Rusiński, M.; Lazarus, M. Habitat selection of the pond bat Myotis dasycneme during pregnancy and lactation in Northern Poland. J. Mammal. 2017, 98, 232–245. [Google Scholar] [CrossRef] [Green Version]
- Dietz, M.; Encarnacao, J.A.; Kalko, E. Small scale distribution patterns of female and male Daubenton’s bats (Myotis daubentonii). Acta Chir. 2006, 8, 403–415. [Google Scholar] [CrossRef]
- Abbott, I.M.; Sleeman, D.P.; Harrison, S. Bat activity affected by sewage effluent in Irish rivers. Biol. Con. 2009, 142, 2904–2914. [Google Scholar] [CrossRef]
- Langton, S.D.; Briggs, P.A.; Haysom, K.A. Daubenton’s bat distribution along rivers—Developing and testing a predictive model. Aquatic Conserv. Mar. Freshw. Ecosyst. 2010, 20, 45–54. [Google Scholar] [CrossRef]
- Bogdarina, S.V.; Strelkov, P.P. Distribution of Chiroptera in northern European Russia. Plecotus 2003, 6, 7–28, (In Russian with English summary). [Google Scholar]
- Blomberg, A.S.; Vasko, V.; Salonen, S.; Pētersons, G.; Lilley, T.M. First record of a Nathusius’ pipistrelle (Pipistrellus nathusii) overwintering at a latitude above 60° N. Mammalia 2020, 85, 74–78. [Google Scholar] [CrossRef]
- Tidenberg, E.M.; Liukko, U.M.; Stjernberg, T. Atlas of Finnish bats. Ann. Zool. Fenn. 2019, 56, 207–250. [Google Scholar] [CrossRef] [Green Version]
- Flaquer, C.; Puig-Montserrat, X.; Goiti, U.; Vidal, F.; Curco, A.; Russo, D. Habitat selection in Nathusius’ pipistrelle (Pipistrellus nathusii): The importance of wetlands. Acta Chir. 2009, 11, 149–155. [Google Scholar] [CrossRef]
- Ancillotto, L.; Tomassini, A.; Russo, D. The fancy city life: Kuhl’s pipistrelle, Pipistrellus kuhlii, benefits from urbanisation. Wildl. Res. 2016, 42, 598–606. [Google Scholar] [CrossRef]
- Maxinová, E.; Kipson, M.; Naďo, L.; Hradická, P.; Uhrin, M. Foraging Strategy of Kuhl’s Pipistrelle at the Northern Edge of the Species Distribution. Acta Chir. 2016, 18, 215–222. [Google Scholar] [CrossRef]
- Bilushenko, A.A. The Current Status of Kuhl’s Pipistrelle, Pipistrellus kuhlii (Chiroptera, Vespertilionidae), in the Central Forest-Steppe of Ukraine. Vest. Zool. 2013, 47, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Hukov, V.; Timofieieva, O.; Prylutska, A.; Rodenko, O.; Moiseienko, M.; Bohodist, V.; Domanska, A.; Vlaschenko, A. Wintering of an urban bat (Pipistrellus kuhlii lepidus) in recently occupied areas. Eur. J. Ecol. 2020, 6, 102–120. [Google Scholar] [CrossRef]
- Kruskop, S.V. On distribution of pipistrelles from Pipistrellus pipistrellus/pygmaeus complex (Chiroptera, Vespertilionidae) in Russia. Plecotus 2007, 10, 36–46, (In Russian with English summary). [Google Scholar]
- Godlevska, L.V.; Shpak, A.V. Bats in the collection of the Zoological museum of Belarusian State University. Proc. Natl. Acad. Sci. Belarus. Biol. Ser. 2020, 65, 412–420, (In Russian with English abstract). [Google Scholar] [CrossRef]
- Godlevska, L.; Rebrov, S. Bats of the Left-Bank Dnipro Region in the northern part of Ukraine. Theriol. Ukr. 2018, 16, 25–50, (In Ukrainian with English abstract). [Google Scholar] [CrossRef] [Green Version]
- Jones, G.; Froidevaux, J.S.P. Soprano Pipistrelle Pipistrellus pygmaeus (Leach, 1825). In Handbook of the Mammals of Europe; Hackländer, K., Zachos, F.E., Eds.; Springer Nature: Switzerland, Switzerland, 2020; pp. 1–25. [Google Scholar]
- Petrov, O.V. Mammals of the “Forest on Vorskla” forestry and its environs. Uch. App. Leningr. State Univ. Ser. Biol. Sciences. Integr. Res. For.-Steppe Oak For. For. Vorskla 1971, 52, 127–132. (In Russian) [Google Scholar]
- Vlaschenko, A.S.; Godlevska, L.V.; Kravchenko, K.O.; Tyshchenko, V.M.; Gukasova, A.S.; Sudakova, M.V. Contribution to bat fauna of Holosiyivsky National Nature Park. Nat. Reserves Ukraine 2012, 18, 51–58, (In Russian with English abstract). [Google Scholar]
- Dietz, M.; Brombacher, M.; Erasmy, M.; Fenchuk, V.; Simon, O. Bat community and roost site selection of tree-dwelling bats in a well-preserved European lowland forest. Acta Chir. 2018, 20, 117–127. [Google Scholar] [CrossRef]
- Asbeck, T.; Pyttel, P.; Bauhus, F.L. Predicting abundance and diversity of tree-related microhabitats in Central European montane forests from common forest attributes. For. Ecol. Manag. 2019, 432, 400–408. [Google Scholar] [CrossRef]
- Crampton, H.L.; Barclay, R. Selection of Roosting and Foraging Habitat by Bats in Different-Aged Aspen Mixedwood Stands. Conserv. Biol. 1998, 12, 1347–1358. [Google Scholar] [CrossRef]
- Kalcounis-Rüppell, C.M.; Psyllakis, M.J.; Brigham, R.M. Tree Roost Selection by Bats: An Empirical Synthesis Using Meta-Analysis. Wildl. Soc. Bull. 2005, 33, 1123–1132. [Google Scholar] [CrossRef]
- Wesołowski, T.; Martin, K. Tree holes and hole nesting birds in European and North American forests. In Ecology and Conservation of Forest Birds; Mikusiński, G., Roberge, J.-M., Fuller, R.J., Eds.; Cambridge University Press: Cambridge, UK, 2018; pp. 79–133. [Google Scholar]
- Lesinski, G.; Kowalski, M.; Wojtowicz, B.; Gulatowska, J.; Lisowska, A. Bats on forest islands of different size in an agricultural landscape. Folia Zool. 2007, 56, 153–161. [Google Scholar]
- de Jong, J. Habitat use and species richness of bats in a patchy landscape. Acta Theriol. 1995, 40, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Quine, C.P. The Curious Case of the Even-aged Plantation: Wretched, Funereal or Misunderstood? In Europe’s Changing Woods and Forests: From Wildwood to Managed Landscapes; Kirby, K., Watkins, C., Eds.; CABI: Wallingford, UK, 2015; pp. 207–223. [Google Scholar]
- Paquette, A.; Messier, C. The role of plantations in managing the world’s forests in the Anthropocene. Front. Ecol. Environ. 2010, 8, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Himes, A.; Betts, M.; Messier, C.; Seymour, R. Perspectives: Thirty years of triad forestry, a critical clarification of theory and recommendations for implementation and testing. For. Ecol. Manag. 2022, 510, 120103. [Google Scholar] [CrossRef]

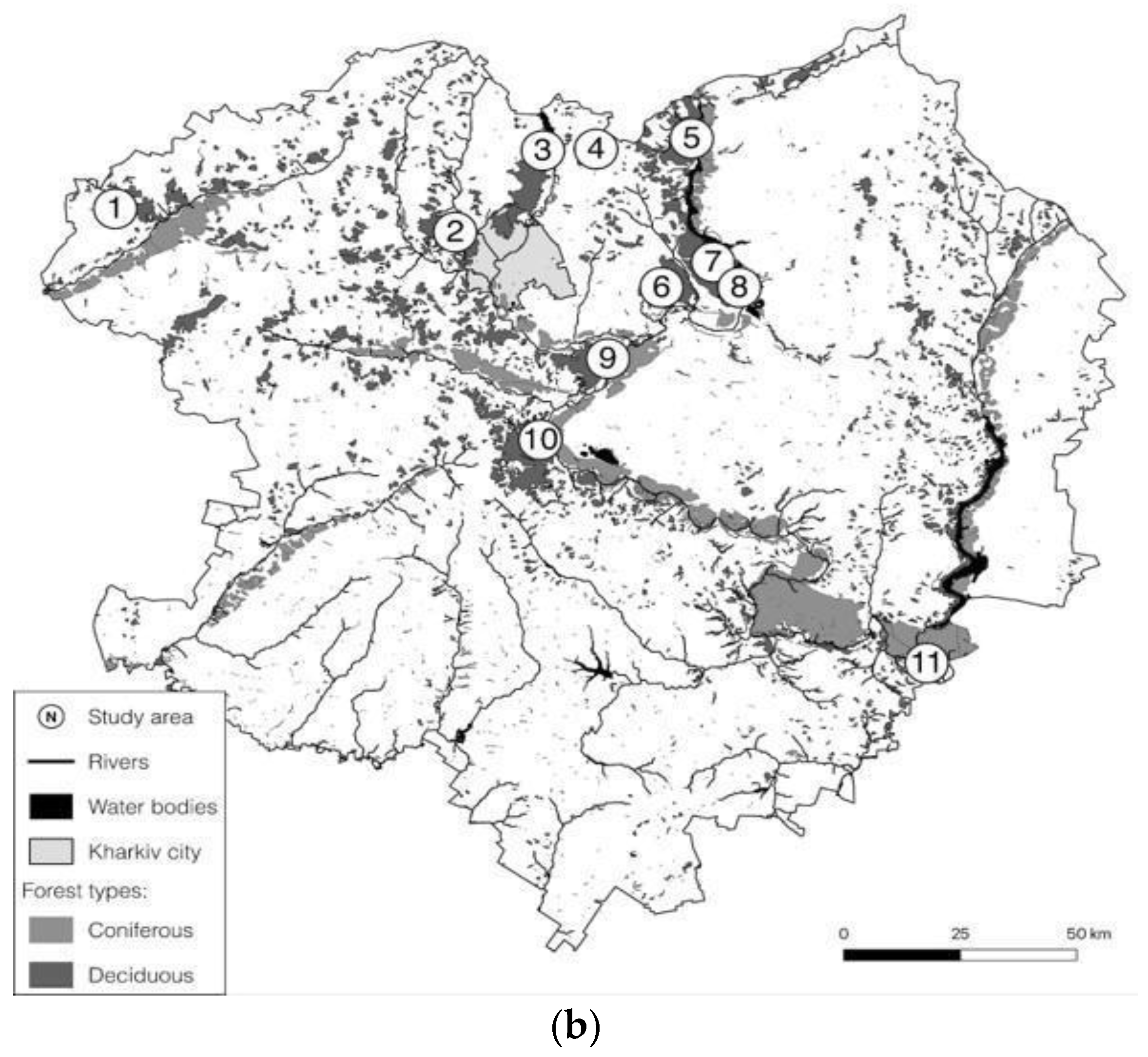


| Names, Acronyms and Total Area (ha) | Coordinates, Field Work Period and Plot Number According to Figure 1b | Land Cover Types | Research Effort | Management Type (According to IUCN Protected Area Categories) |
|---|---|---|---|---|
| Koziyivka KOZ 759.1 | 50°07′30″ N 35°05′04″ E 27 June–2 July 2012 1 | Deciduous upland forest far from big rivers and in the vicinity of water bodies | MN hours—88; MN sites—13; Bat species—3; Individuals—39. | Managed forest; the local Forest Authority held FSC FM certificate in 2008. Natural Monuments or Feature “Koziyivska 1” and “Koziyivska 2” (IUCN category III); total area 8.1 ha; restriction of clear cutting. |
| Karavan KAR 491.8 | 50°02′48″ N 36°06′16″ E 15–19 July 2018 2 | Deciduous upland forest on a bank of water reservoir | MN hours—63; MN sites—9; Bat species—7; Individuals—239. | Managed forest. |
| Liptsy LIP 221.2 | 50°12′40″ N 36°22′23″ E 8–13 July 2016 3 | Deciduous upland forest on a bank of water reservoir | MN hours—64.75; MN sites—9; Bat species—6; Individuals—168. | Managed forest. |
| Neskuchne NES 478.6 | 50°12′06″ N 36°32′19″ E 27 July–1 August 2012 4 | Two small, isolated patches of deciduous forest within an agricultural landscape | MN hours—58; MN sites—8; Bat species—6; Individuals—105. | Managed forest, only selection cuttings. |
| Starytsia STR 1067.8 | 50°13′19″ N 36°49′37″ E 25–29 July 2018 5 | Large deciduous upland forest at the right bank of the main river in the region | MN hours—62; MN sites—8; Bat species—6; Individuals—110. | Managed forest; “Kholodnoyarske” habitat management area (IUCN category IV); 10 ha; minor restrictions of forest management. |
| Tetleha TET 496.7 | 49°54′58″ N 36°43′02″ E 5–10 July 2012 6 | Large deciduous upland forest with big rivers and water bodies in the vicinity | MN hours—54.75; MN sites—9; Bat species—8; Individuals—328. | Managed forest. “Kochetotsky forest” habitat management area (IUCN category IV); 240 ha; minor restrictions of forest management. |
| Pechenihy PEC 681.6 | 49°57′01″ N 36°51′59″ E 10–15 July 2012 7 | Large deciduous upland forest at the right bank of the large artificial water reservoir | MN hours—39.75; MN sites—8; Bat species—7; Individuals—358. | Managed forest. “Pechenizky forest” habitat management area (IUCN category IV); 358 ha; minor restrictions of forest management. |
| Pechenihy2 PEC2 1031.9 | 49°54′25″ N 36°56′14″ E 11–15 July 2018 8 | Large deciduous upland forest at the right bank of the large artificial water reservoir | MN hours—64; MN sites—9; Bat species—9; Individuals—514. | Managed forest; “Pechenizky forest” habitat management area (IUCN category IV); 1031.9 ha; minor restrictions of forest management. |
| Mokhnach MOK 813.6 | 49°45′25″ N 36°32′32″ E 18–24 July 2012 9 | Large deciduous upland forest at the right bank of the main river in the region | MN hours—75.75; MN sites—11; Bat species—8; Individuals—186. | Managed forest; “Mokhnachansky” and “Serednyodonetsky” habitat management areas; (IUCN category IV); 701 ha; restriction of clear cutting. |
| Homil′sha HOM 575.8 | 49°37′10″ N 36°19′32″ E two cycles 2–10 & 11–20 July 2011 10 | Large deciduous upland forest at the right bank of the main river in the region | MN hours—130.75; MN sites—9 (double); Bat species—10; Individuals—545. | Protected area; National Nature Park (IUCN category II) since 2004; 567 ha; complete restriction of clear and selective cuttings since 2007. |
| Yaremivka YAR 550.9 | 49°06′38″ N 37°25′00″ E two cycles 4–11 & 12–19 July 2009 11 | Floodplain deciduous woodland at the left bank of the main river in the region | MN hours—101.5; MN sites—8 (double); Bat species—10; Individuals—660. | Managed forest, but under private protection; restriction of clear cuttings and limitation of selective cuttings. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlaschenko, A.; Kravchenko, K.; Yatsiuk, Y.; Hukov, V.; Kramer-Schadt, S.; Radchuk, V. Bat Assemblages Are Shaped by Land Cover Types and Forest Age: A Case Study from Eastern Ukraine. Forests 2022, 13, 1732. https://doi.org/10.3390/f13101732
Vlaschenko A, Kravchenko K, Yatsiuk Y, Hukov V, Kramer-Schadt S, Radchuk V. Bat Assemblages Are Shaped by Land Cover Types and Forest Age: A Case Study from Eastern Ukraine. Forests. 2022; 13(10):1732. https://doi.org/10.3390/f13101732
Chicago/Turabian StyleVlaschenko, Anton, Kseniia Kravchenko, Yehor Yatsiuk, Vitalii Hukov, Stephanie Kramer-Schadt, and Viktoriia Radchuk. 2022. "Bat Assemblages Are Shaped by Land Cover Types and Forest Age: A Case Study from Eastern Ukraine" Forests 13, no. 10: 1732. https://doi.org/10.3390/f13101732





