Drought Resistance Evaluation of Casuarina equisetifolia Half-Sib Families at the Seedling Stage and the Response of Five NAC Genes to Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
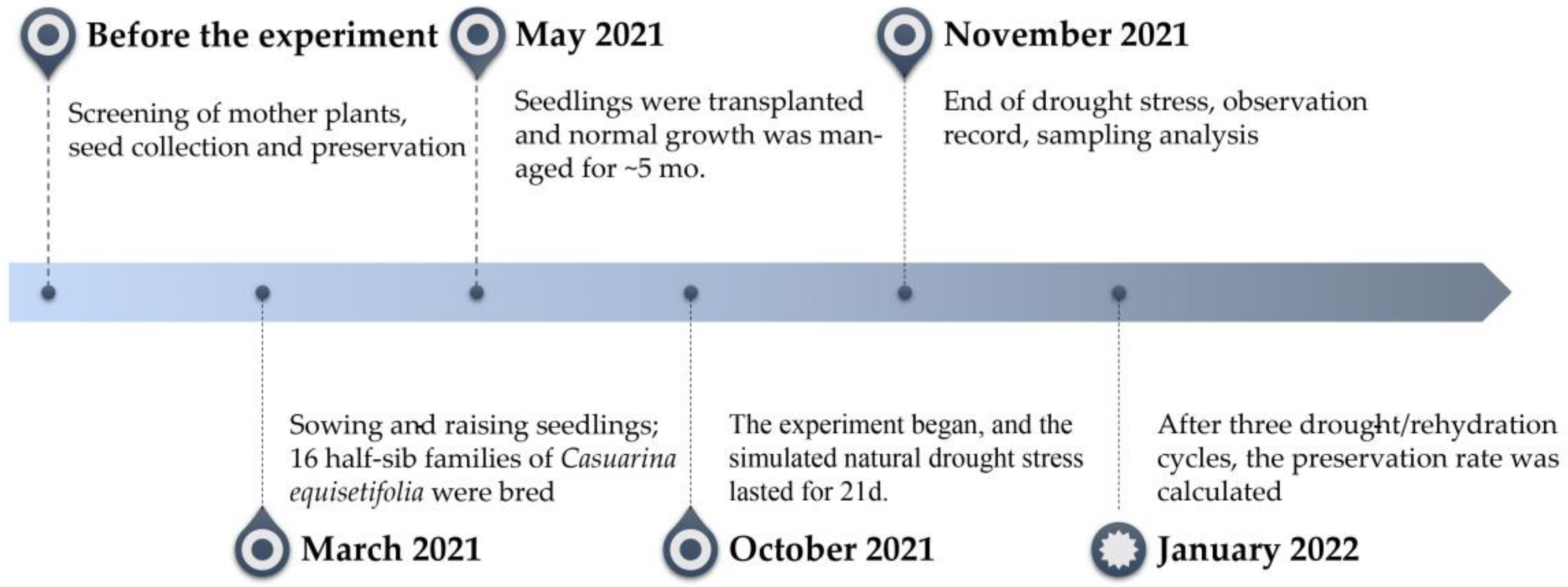
2.1. Test Materials and Design
2.1.1. Mother Plant Screening
2.1.2. Sowing and Raising Seedlings
2.1.3. Stress Experiment
2.1.4. Root Sampling
2.1.5. Observation and Statistics
2.2. Index Measurements
2.2.1. Determination of Substrate Field Water Holding Capacity (WHC), pH, and Nutrient Content
2.2.2. Determination of Growth and Morphological Indices
2.2.3. Determination of Physiological and Biochemical Indices
2.2.4. Determination of Relative NAC Gene Expression Levels
2.3. Data Processing and Analysis
3. Results
3.1. Measurements of Indices under Drought Stress and Normal Water Supply
3.2. Principal Component Analysis (PCA)
3.3. Comprehensive Evaluation of Drought Resistance
3.4. Distribution Characteristics and Differential Analysis of Preservation Rate
3.5. DC of Relative NAC Gene Expression
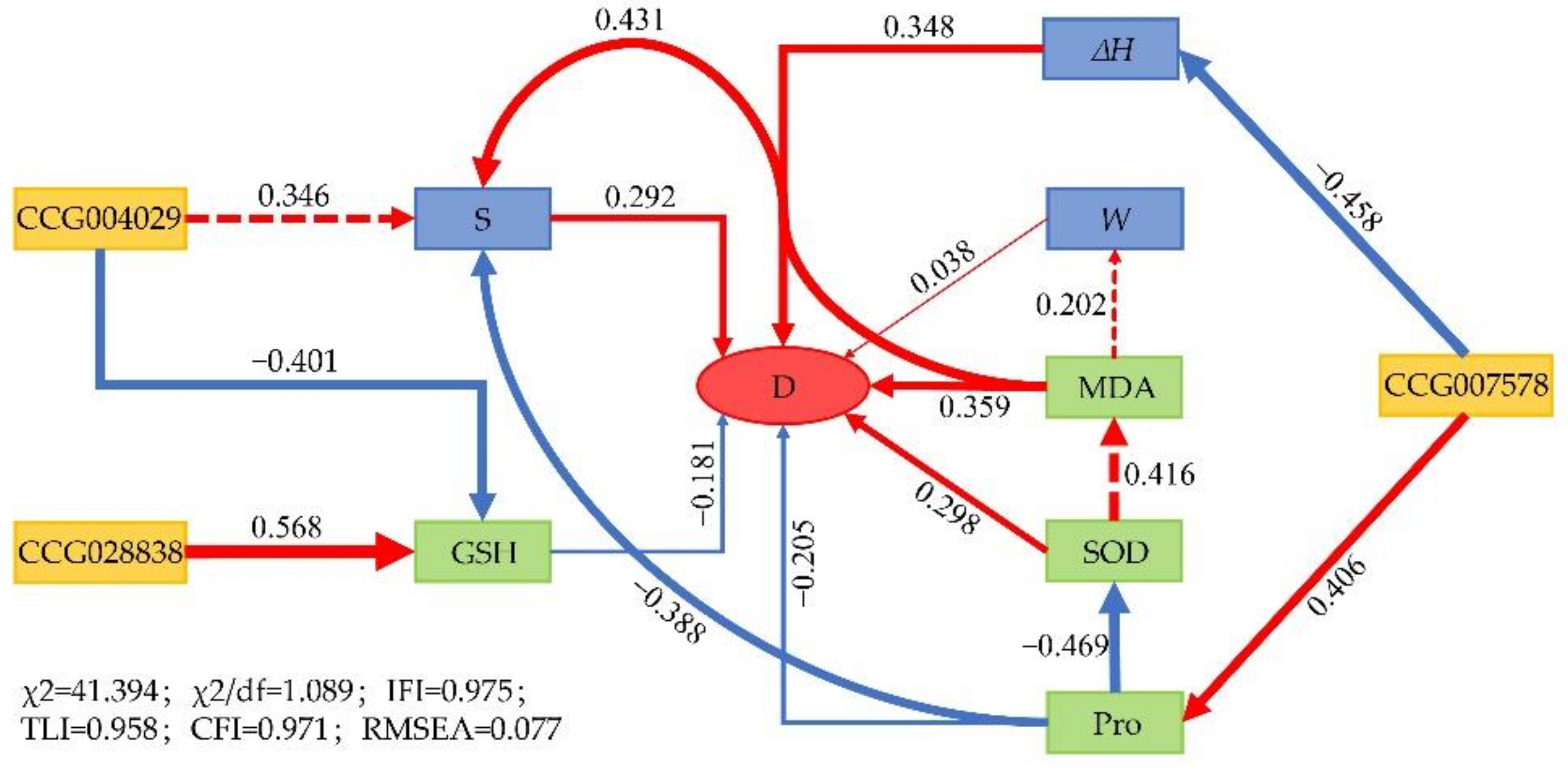
3.6. Correlation and Structural Equation Model Analyses
4. Discussion
4.1. Evaluation of Drought Resistance in C. equisetifolia
4.2. Response Mechanism of C. equisetifolia to Drought Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, K.; Yang, D.W.; Yang, H.B.; Li, Z.; Qin, Y.; Shen, Y. Spatio-temporal variation of drought in China during 1961–2012: A climatic perspective. J. Hydrol. 2015, 526, 253–264. [Google Scholar] [CrossRef]
- Han, L.Y.; Zhang, Q.; Jia, J.Y.; Wang, Y.H.; Huang, T. Drought severity, frequency, duration and regional differences in China. J. Desert Res. 2019, 39, 1–10. [Google Scholar]
- Cai, X.Z.; Zhang, R.Y. A study on drought and flood phases in the southeast coast area during the past 524 years. Trop. Geogr. 1994, 14, 250–259. [Google Scholar]
- Zhuang, Y.; Bao, R.J.; Zhang, R.Y.; He, J.H.; Wang, L. MCI-based climatic characteristics of drought in Fujian province. J. Meteor. Environ. 2021, 37, 93–99. [Google Scholar]
- Fang, Z.G.; Xie, J.R. Calculation and analysis of the aridity and the wind erosion climatic erosivity in the coastal area of Fujian. J. Fujian Nor. Univ (Nat. Sci. Ed.) 1997, 3, 99–106. [Google Scholar]
- Zhang, W.K. Study on the relationship between the landform and the development of dry climate in the coastal region in Fujian province. J. Fujian Nor. Univ (Nat. Sci. Ed.) 2001, 1, 94–98. [Google Scholar]
- Zhang, Y.; Zhong, C.L.; Chen, Y.; Chen, Z.; Jiang, Q.B.; Wu, C.; Pinyopusarerk, K. Improving drought tolerance of Casuarina equisetifolia seedlings by arbuscular mycorrhizas under glasshouse conditions. New For. 2010, 40, 261–271. [Google Scholar] [CrossRef]
- Zhong, C.L.; Zhang, Y.; Chen, Y.; Jiang, Q.B.; Chen, Z.; Liang, J.F.; Pinyopusarerk, K.; Franche, C.; Bogusz, D. Casuarina research and applications in China. Symbiosis 2010, 50, 107–114. [Google Scholar] [CrossRef]
- Xie, Y.J. Evaluation of Ecosystem Service Value and Ecological Compensation Mechanism of Coastal Casuarina equisetifolia Forest in Fujian. Master’s Thesis, Fujian Normal University, Fuzhou, China, 2020. [Google Scholar]
- Xu, H.C.; Nie, S.; Yu, J.L.; You, L.H.; Ye, G.F.; Zhang, H.X.; Cheng, F.S.; Su, Q.G. Genetic variation and selection of Casuarina equisetifolia half-sibling families at seedling stage. Chin. J. Trop. Crops 2022. accepted. [Google Scholar]
- Wang, Q.M.; Zhang, Z.H.; Cui, J.G. The physiological age of asexual plants—Thingking arise from telomere length. For. Res. 2017, 30, 866–870. [Google Scholar]
- Li, M.J. Provenance characteristics and early selection of Casuarina equisetifolia. J. Trop. Subtrop. Bot. 2018, 26, 597–603. [Google Scholar]
- Wu, X.L.; Ye, G.F. Soil moisture dynamics and availability of Casuarina equisetifolia plantation in coastal sandy land. Straits Sci. 2008, 22, 46–50. [Google Scholar]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 2021, 242, 126626. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.X.; Ye, G.F.; Zheng, T.H.; Lai, W.S.; Xu, J.S.; Yang, X.M.; Tan, F.L.; Pan, H.Z. Study on drought resistance characteristics and application of different Casuarina species. Protect. For. Sci. Tech. 2000, 1, 173–176+193. [Google Scholar]
- Shi, C.G.; Ye, G.F.; Lai, W.S.; Lin, D.G.; Xu, J.S.; Lin, W.X.; Tan, F.L.; Pan, H.Z. Study on the growth and morphological characteristics of Casuarina equisetifolia seedlings under water stress. Protect. For. Sci. Tech. 2000, 1, 151–154. [Google Scholar]
- Ye, G.F.; Zhang, H.X.; Chen, B.H.; Nie, S.; Liu, H.; Gao, W.; Wang, H.Y.; Gao, Y.B.; Gu, L.F. De novo genome assembly of the stress tolerant forest species Casuarina equisetifolia provides insight into secondary growth. Plant J. 2019, 97, 779–794. [Google Scholar] [CrossRef] [Green Version]
- Souer, E.; Van, H.A.; Kloos, D.; Mol, J.; Koes, R. The no apical meristem gene of Petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries. Cell 1996, 85, 159–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riechmann, J.L.; Heard, J.; Martin, G.; Reuber, L.; Jiang, C.Z.; Keddie, J.; Adam, L.; Pineda, O.; Ratcliffe, O.; Samaha, R. Arabidopsis transcription factors: Genome-wide comparative analysis among eukaryotes. Science 2000, 290, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Thirumaaikumar, V.P.; Devkar, V.; Mehterov, N.; Ali, S.; Ozgur, R.; Turkan, I.; Mueller, R.B.; Balazadeh, S. NAC transcription factor JUNGBRUNNEN1 enhances drought tolerance in tomato. Plant Biotechnol. J. 2018, 16, 354–366. [Google Scholar] [CrossRef] [Green Version]
- Tran, L.S.P.; Nakashima, K.; Sakuma, Y.; Simpson, S.D.; Fujita, Y.; Maruyama, K.; Fujita, M.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell 2004, 16, 2481–2498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, C.L.; Zhang, Y.; Wei, Y.C.; Meng, J.X.; Chen, Y.; Jiang, Q.S.; Chen, Z.; Li, Z.; Yu, W. Technical Regulations on Cultivation of Casuarina Seedling and Trees: LY/T 3092-2019; NFBA: Beijing, China, 2019. [Google Scholar]
- Tian, Y.; Sun, X.Y.; Li, S.Y.; Wang, H.Y.; Wang, L.Z.; Cao, J.X.; Zhang, L. Biochar made from green waste as peat substitute in growth media for Calathea rotundifola cv. Fasciata. Sci. Hortic. 2012, 143, 15–18. [Google Scholar] [CrossRef]
- Xu, C.; Wu, X.Q.; Zhang, H.Y. Impact of D-arg on drought resistance and endogenous polyamines in mycorrhizal Pinusma ssoniana. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2009, 33, 19–23. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protocols 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Kowarik, A.; Meindl, B.; Vojvodic, M.; Bostock, M.; Lebeau, F. Voronoi Treemap: Voronoi Treemaps with Added Interactivity by Shiny. R Package Version 0.2.0. 2019. Available online: https://CRAN.R-project.Org/package=VoronoiTreemap (accessed on 16 September 2022).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Peterson, B.G.; Carl, P. Performance Analytics: Econometric Tools for Performance and Risk Analysis. R Package Version 2.0.4. 2020. Available online: https://CRAN.R-project.org/package=PerformanceAnalytics (accessed on 16 September 2022).
- Zou, J.; Hu, W.; Li, Y.X.; He, J.Q.; Zhu, H.H.; Zhou, Z.G. Screening of drought resistance indices and evaluation of drought resistance in cotton (Gossypium hirsutum L.). J. Integr. Agric. 2020, 19, 495–508. [Google Scholar] [CrossRef]
- Meng, Y.; Tian, W.Z.; Wen, P.F.; Ding, Z.Q.; Zhang, X.P.; He, L.; Duan, J.Z.; Liu, W.D.; Guo, T.C.; Feng, W. Comprehensive evaluation of drought resistance of wheat varieties based on synergy of different developmental stages. Acta Agron. Sin. 2022. accepted. [Google Scholar]
- Biju, S.; Fuentes, S.; Gupta, D. The use of infrared thermal imaging as a non-destructive screening tool for identifying drought-tolerant lentil genotypes. Plant Physiol. Biochem. 2018, 127, 11–24. [Google Scholar] [CrossRef]
- Tian, C.Y.; Bian, X.; Yu, H.X.; Lang, R.B.; Dong, L.H.; Tao, L.N.; Zhou, Q.M.; An, R.D.; Sun, Y.F.; Yang, L.H.; et al. Comprehensive evaluation of innovative Yunrui Sugarcane parents for drought resistance. J. Plant Genet. Resour. 2019, 20, 610–623. [Google Scholar]
- Qi, X.S.; Wang, X.R.; Xu, J.; Zhang, J.P.; Mi, J. Drought-resistance evaluation of flax germplasm at adult plant stage. Sci. Agric. Sin. 2010, 43, 3076–3087. [Google Scholar]
- Anjum, S.K.; Xie, X.Y.; Wang, L.C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Ludlow, M.M.; Bjorkman, O. Paraheliotropic leaf movement in Siratro as a proactive mechanism against drought induced damage to primary photosynthetic reactions: Damage by excessive light and heat. Planta 1984, 161, 505–518. [Google Scholar] [CrossRef]
- Smimoff, N. The role of active oxygen in response of paints to water deficit and desiccation. New Phytol. 1993, 125, 27–58. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Z.; Zheng, C.S.; Wang, X.F. Advances on drought tolerant mechanism in woody plant. Acta Bot. Boreal.-Occid. Sin. 2007, 27, 629–634. [Google Scholar]
- Tang, Z.C. The Accumulation of free proline and its roles in water-stressed sorghum seedlings. Acta Phytophysiol. Sin. 1989, l5, 105–110. [Google Scholar]
- Chen, H. Studies on the part physiological and chemical characters of drought tolerance in Casuarina equisetifolia. Fujian J. Agric. Sci. 2000, 1, 48–54. [Google Scholar]
- Singh, T.N.; Aspinall, D.; Paleg, L.G. Proline accumulation and varietal adaptability to drought in Barley: A potential metabolic measure of drought resistance. Nat. New Biol. 1972, 236, 188–190. [Google Scholar] [CrossRef]
- Hanson, A.D.; Nelsen, C.E.; Everson, E.H. Evaluation of free proline accumulation as an index of drought resistance using two contrasting Barley cultivars. Crop Sci. 1977, 17, 720–726. [Google Scholar] [CrossRef]
- Li, J.; Phan, T.T.; Li, Y.R.; Xing, Y.X.; Yang, L.T. Isolation, transformation and overexpression of sugarcane SoP5CS gene for drought tolerance improvement. Sugar. Tech 2018, 20, 464–473. [Google Scholar] [CrossRef]
- Li, X.J.; Luo, Z.Q.; Lu, C.Q.; Tang, J.C.; Zhou, Z.; Cao, Q. Evaluation of drought resistance on twenty sugarcane varieties (strains). Chin. J. Trop. Crops 2020, 41, 2482–2491. [Google Scholar]
- Movahedi, A.; Zhang, J.X.; Gao, P.H.; Yang, Y.; Wang, L.; Yin, T.M.; Kadkhodaei, S.; Ebrahimi, M.; Zhu, G.Q. Expression of the chickpea CarNAC3 gene enhances salinity and drought tolerance in transgenic poplars. Plant Cell Tissue Organ. Cult. 2014, 120, 141–154. [Google Scholar] [CrossRef]
- Movahedi, A.; Zhang, J.X.; Yin, T.M.; Zhu, G.Q. Functional analysis of two orthologous NAC genes, CarNAC3, and CarNAC6 from Cicer arietinum, involved in abiotic stresses in Poplar. Plant Mol. Biol. Rep. 2015, 33, 1539–1551. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]




| Cycling Step | Temperature (°C) | Time | No. Cycles |
|---|---|---|---|
| Predenaturation | 95 | 5 min | 1 |
| Deformation | 95 | 10 s | 1 |
| Annealing | 60 | 30 s | 40 |
| Gene | Maker Type | Primer Sequence (5′-3′) |
|---|---|---|
| CCG003077 | NAC | F: AAACAACTTGAGGCTTGACGA |
| R: GTACCGATTCCGACGTGTCCA | ||
| CCG004029 | NAC | F: ACGGAAACTAGAGTCACGAA |
| R: TCTCTTGGACGATCTACTGCT | ||
| CCG007578 | NAC | F: ATCCCCAAAACTGCAAACTCA |
| R: CCCGTTTCTACCAATAGAGTGT | ||
| CCG007885 | NAC | F: CGTGACCTGCTTCTCCGAT |
| R: AATTCTGCTTTTGCGTTCCTC | ||
| CCG028838 | NAC | F: CACCTGCTGTGCGACCTCC |
| R: GGAACTCCGGCCCAAACCC |
| Family | H /cm | W /% | S /% | MDA /nmol·g−1 | SOD /U·g−1 | Pro /ug·g−1 | GSH /umol·g−1 | AsA /mg·g−1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DT | CK | DT | CK | DT | CK | DT | CK | DT | CK | DT | CK | DT | CK | DT | CK | |
| 1-195 | 2.437 | 2.972 | 17.857 | 0.000 | 100.000 | 100.000 | 80.645 | 10.164 | 531.338 | 216.704 | 65.240 | 26.421 | 0.506 | 0.362 | 0.219 | 0.045 |
| 3-224 | 4.487 | 5.973 | 11.528 | 0.000 | 100.000 | 100.000 | 93.548 | 10.540 | 301.262 | 109.648 | 304.310 | 28.882 | 0.330 | 0.847 | 0.210 | 0.062 |
| 3-265 | 2.722 | 2.904 | 44.531 | 0.000 | 81.250 | 100.000 | 96.774 | 9.831 | 480.000 | 149.693 | 146.059 | 57.190 | 0.585 | 0.424 | 0.259 | 0.067 |
| 3-52 | 4.446 | 5.934 | 51.563 | 0.000 | 83.333 | 100.000 | 96.774 | 11.507 | 592.208 | 158.865 | 50.652 | 25.600 | 0.544 | 0.300 | 0.186 | 0.072 |
| 3-80 | 2.857 | 3.322 | 25.558 | 0.000 | 93.750 | 100.000 | 93.548 | 11.454 | 576.923 | 104.969 | 74.715 | 21.497 | 0.523 | 0.444 | 0.214 | 0.085 |
| 4-128 | 4.892 | 6.325 | 43.452 | 0.000 | 78.571 | 100.000 | 67.742 | 9.265 | 361.976 | 225.370 | 842.648 | 30.523 | 0.951 | 0.362 | 0.302 | 0.137 |
| 4-213 | 5.529 | 6.863 | 52.083 | 0.000 | 83.333 | 100.000 | 83.871 | 10.160 | 897.902 | 343.450 | 1106.898 | 40.369 | 0.579 | 0.331 | 0.337 | 0.029 |
| 4-383 | 2.071 | 3.168 | 32.292 | 0.000 | 100.000 | 100.000 | 109.677 | 9.534 | 1035.115 | 205.086 | 55.351 | 29.170 | 0.723 | 0.550 | 0.169 | 0.027 |
| 4-389 | 1.434 | 2.335 | 64.063 | 0.000 | 90.000 | 100.000 | 103.226 | 9.781 | 471.000 | 341.891 | 662.450 | 39.138 | 0.611 | 0.837 | 0.431 | 0.036 |
| 5-218 | 3.550 | 9.012 | 43.750 | 0.000 | 75.000 | 100.000 | 83.871 | 10.008 | 727.686 | 283.728 | 979.586 | 46.113 | 0.917 | 0.517 | 0.347 | 0.036 |
| 5-398 | 3.202 | 7.321 | 21.786 | 0.000 | 89.722 | 100.000 | 80.645 | 10.150 | 617.045 | 234.214 | 410.101 | 32.164 | 0.584 | 0.496 | 0.210 | 0.027 |
| 5-335 | 3.407 | 9.535 | 41.250 | 0.000 | 73.750 | 100.000 | 77.419 | 11.294 | 174.217 | 290.179 | 365.936 | 26.831 | 0.435 | 0.475 | 0.203 | 0.036 |
| 5-80 | 2.273 | 2.428 | 33.125 | 0.000 | 82.500 | 100.000 | 70.968 | 11.064 | 485.473 | 248.393 | 174.012 | 37.497 | 0.556 | 0.372 | 0.340 | 0.030 |
| 6-207 | 3.670 | 4.024 | 33.125 | 0.000 | 87.500 | 100.000 | 83.871 | 11.315 | 513.692 | 262.012 | 298.880 | 48.574 | 0.523 | 0.382 | 0.343 | 0.040 |
| 6-394 | 4.642 | 7.493 | 42.361 | 0.000 | 71.667 | 100.000 | 90.323 | 12.093 | 605.629 | 196.989 | 707.848 | 39.549 | 0.454 | 0.413 | 0.336 | 0.025 |
| 6-445 | 2.273 | 2.824 | 41.146 | 0.000 | 83.333 | 100.000 | 103.226 | 10.696 | 621.673 | 257.854 | 803.944 | 37.087 | 0.540 | 0.641 | 0.336 | 0.027 |
| Average | 3.368 | 5.152 | 37.467 | 0.000 | 85.857 | 100.000 | 88.508 | 10.554 | 562.071 | 226.815 | 440.539 | 35.413 | 0.585 | 0.485 | 0.278 | 0.049 |
| StDev | 1.133 | 2.359 | 13.244 | 0.000 | 8.901 | 0.000 | 11.517 | 0.794 | 202.524 | 69.565 | 347.040 | 9.305 | 0.156 | 0.160 | 0.075 | 0.029 |
| CV/% | 0.336 | 0.458 | 0.353 | 0.000 | 0.104 | 0.000 | 0.130 | 0.075 | 0.360 | 0.307 | 0.788 | 0.263 | 0.266 | 0.329 | 0.271 | 0.594 |
| t-value | −3.800 | 26.091 | 10.959 | −6.154 | 6.370 | 4.560 | 1.604 | 10.342 | ||||||||
| p-value | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.130 | 0.000 | ||||||||
| Family | H /cm | W /% | S /% | MDA /nmol·g−1 | SOD /U·g−1 | Pro /ug·g−1 | GSH /umol·g−1 | AsA /mg·g−1 |
|---|---|---|---|---|---|---|---|---|
| 1-195 | 0.620 | 0.424 | 0.717 | 7.469 | 3.074 | 17.898 | 1.098 | 13.488 |
| 3-224 | 0.751 | 0.115 | 1.000 | 8.876 | 2.748 | 10.536 | 0.389 | 3.400 |
| 3-265 | 0.805 | 0.411 | 0.833 | 9.651 | 2.411 | 21.677 | 0.842 | 12.343 |
| 3-52 | 0.749 | 0.516 | 0.833 | 8.410 | 3.728 | 1.979 | 1.816 | 2.566 |
| 3-80 | 0.860 | 0.256 | 0.938 | 8.167 | 5.496 | 3.476 | 1.176 | 2.525 |
| 4-128 | 0.937 | 0.445 | 0.813 | 9.844 | 3.207 | 2.554 | 1.381 | 3.886 |
| 4-213 | 0.806 | 0.521 | 0.833 | 8.255 | 2.614 | 27.419 | 1.750 | 11.707 |
| 4-383 | 0.654 | 0.323 | 1.000 | 11.504 | 5.047 | 1.898 | 1.314 | 6.329 |
| 4-389 | 0.614 | 0.641 | 0.900 | 10.554 | 1.378 | 16.926 | 0.730 | 12.134 |
| 5-218 | 0.394 | 0.438 | 0.750 | 8.380 | 2.565 | 21.243 | 1.775 | 9.714 |
| 5-398 | 0.437 | 0.218 | 0.897 | 7.946 | 2.635 | 12.750 | 1.178 | 7.753 |
| 5-335 | 0.357 | 0.413 | 0.738 | 6.855 | 0.600 | 13.639 | 0.915 | 5.687 |
| 5-80 | 0.936 | 0.331 | 0.825 | 6.414 | 1.954 | 4.641 | 1.494 | 11.242 |
| 6-207 | 0.912 | 0.331 | 0.875 | 7.412 | 1.961 | 6.153 | 1.368 | 8.669 |
| 6-394 | 0.820 | 0.179 | 1.000 | 7.934 | 2.452 | 2.469 | 1.398 | 4.840 |
| 6-445 | 0.773 | 0.435 | 0.786 | 7.312 | 1.606 | 27.607 | 2.628 | 2.211 |
| Average | 0.714 | 37.467 | 0.859 | 8.436 | 2.717 | 12.054 | 1.328 | 7.406 |
| StDev | 0.181 | 13.244 | 0.089 | 1.322 | 1.210 | 8.904 | 0.505 | 3.852 |
| CV/% | 0.253 | 0.353 | 0.104 | 0.157 | 0.445 | 0.739 | 0.380 | 0.520 |
| Index | Factor Pattern | |||
|---|---|---|---|---|
| P1 | P2 | P3 | P4 | |
| H/cm | 0.412 | −0.349 | 0.348 | 0.738 |
| W/% | −0.655 | 0.190 | 0.627 | 0.018 |
| S/% | 0.849 | 0.215 | −0.073 | 0.048 |
| MDA/nmol·g−1 | 0.371 | 0.684 | 0.538 | −0.173 |
| SOD/U·g−1 | 0.687 | 0.018 | 0.435 | −0.178 |
| Pro/ug·g−1 | −0.784 | 0.118 | 0.053 | −0.084 |
| GSH/umol·g−1 | −0.292 | −0.734 | 0.479 | −0.165 |
| AsA/mg·g−1 | −0.556 | 0.544 | −0.022 | 0.468 |
| Eigenvalue | 2.937 | 1.521 | 1.232 | 0.863 |
| Contribution rate/% | 36.712 | 19.011 | 15.399 | 10.785 |
| Cumulative contribution rate/% | 36.712 | 55.723 | 71.122 | 81.907 |
| Factor weight | 0.448 | 0.232 | 0.188 | 0.132 |
| Family | Subordinate Function Value | D Value | Rank | Category | Drought Resistance | |||
|---|---|---|---|---|---|---|---|---|
| 4-383 | 0.998 | 0.755 | 0.994 | 0.108 | 0.824 | 1 | Ⅰ | Drought-resistant |
| 3-80 | 1.000 | 0.347 | 0.683 | 0.353 | 0.703 | 2 | Ⅰ | Drought-resistant |
| 3-265 | 0.637 | 0.428 | 1.000 | 0.518 | 0.641 | 3 | Ⅰ | Drought-resistant |
| 3-224 | 0.926 | 0.628 | 0.061 | 0.412 | 0.626 | 4 | Ⅰ | Drought-resistant |
| 1-195 | 0.845 | 0.338 | 0.285 | 0.537 | 0.582 | 5 | Ⅰ | Drought-resistant |
| 4-389 | 0.180 | 1.000 | 0.809 | 0.520 | 0.534 | 6 | Ⅱ | Intermediate drought tolerance |
| 6-445 | 0.271 | 0.790 | 0.669 | 0.727 | 0.526 | 7 | Ⅱ | Intermediate drought tolerance |
| 3-52 | 0.553 | 0.269 | 0.993 | 0.209 | 0.524 | 8 | Ⅱ | Intermediate drought tolerance |
| 6-207 | 0.481 | 0.352 | 0.441 | 0.838 | 0.491 | 9 | Ⅱ | Intermediate drought tolerance |
| 5-80 | 0.359 | 0.273 | 0.381 | 1.000 | 0.428 | 10 | Ⅱ | Intermediate drought tolerance |
| 4-213 | 0.060 | 0.501 | 0.915 | 0.642 | 0.400 | 11 | Ⅱ | Intermediate drought tolerance |
| 5-398 | 0.468 | 0.554 | 0.136 | 0.102 | 0.377 | 12 | Ⅱ | Intermediate drought tolerance |
| 6-394 | 0.053 | 0.594 | 0.498 | 0.548 | 0.328 | 13 | Ⅲ | Drought-sensitive |
| 5-218 | 0.000 | 0.522 | 0.625 | 0.000 | 0.239 | 14 | Ⅲ | Drought-sensitive |
| 4-128 | 0.049 | 0.000 | 0.838 | 0.198 | 0.205 | 15 | Ⅲ | Drought-sensitive |
| 5-335 | 0.015 | 0.487 | 0.000 | 0.063 | 0.128 | 16 | Ⅲ | Drought-sensitive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Yu, J.; You, L.; Xiao, S.; Nie, S.; Li, T.; Ye, G.; Lin, D. Drought Resistance Evaluation of Casuarina equisetifolia Half-Sib Families at the Seedling Stage and the Response of Five NAC Genes to Drought Stress. Forests 2022, 13, 2037. https://doi.org/10.3390/f13122037
Xu H, Yu J, You L, Xiao S, Nie S, Li T, Ye G, Lin D. Drought Resistance Evaluation of Casuarina equisetifolia Half-Sib Families at the Seedling Stage and the Response of Five NAC Genes to Drought Stress. Forests. 2022; 13(12):2037. https://doi.org/10.3390/f13122037
Chicago/Turabian StyleXu, Huichang, Jinlin Yu, Longhui You, Shengwu Xiao, Sen Nie, Tuhe Li, Gongfu Ye, and Dichu Lin. 2022. "Drought Resistance Evaluation of Casuarina equisetifolia Half-Sib Families at the Seedling Stage and the Response of Five NAC Genes to Drought Stress" Forests 13, no. 12: 2037. https://doi.org/10.3390/f13122037





