Physiological Responses of Chionanthus retusus Seedlings to Drought and Waterlogging Stresses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Drought and Flood Treatments
2.3. Measurements of Physiological Indexes
2.3.1. Determination of Morphological Indexes
- Grade 0: no damage symptoms.
- Grade I: mild water damage; less than 10% of the leaves show wilting, drooping, or wrinkling.
- Grade II: moderate water damage; 10%–20% of the leaves show wilting, drooping, or wrinkling.
- Grade III: severe water damage; 30%–59% of the leaves show wilting, drooping, or wrinkling.
- Grade IV: extremely severe water damage; more than 60% of the leaves show wilting, drooping, or shrinking.
series × total number of leaves) × 100%
2.3.2. Photosynthetic Parameters
2.3.3. Determination of Chlorophyll Fluorescence Index
2.3.4. Measurement of Antioxidase Activities, MDA, Thiobarbituric Acid and Pro Content
2.4. Statistical Analysis
3. Results
3.1. Effects of Water Stress on Growth Morphology of C. retusus
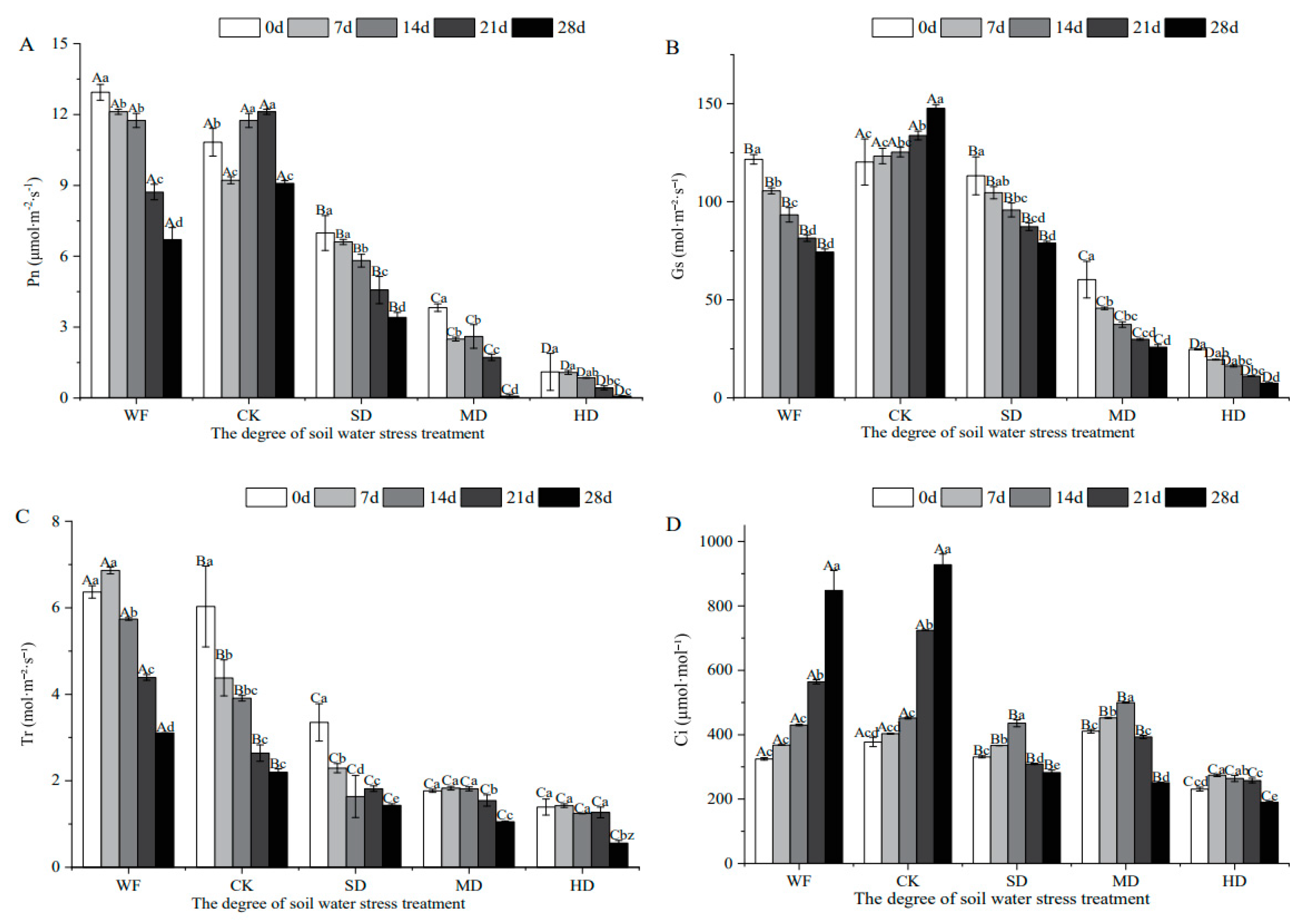
3.2. Effects of Water Stress on Gas Exchange Parameters of C. retusus
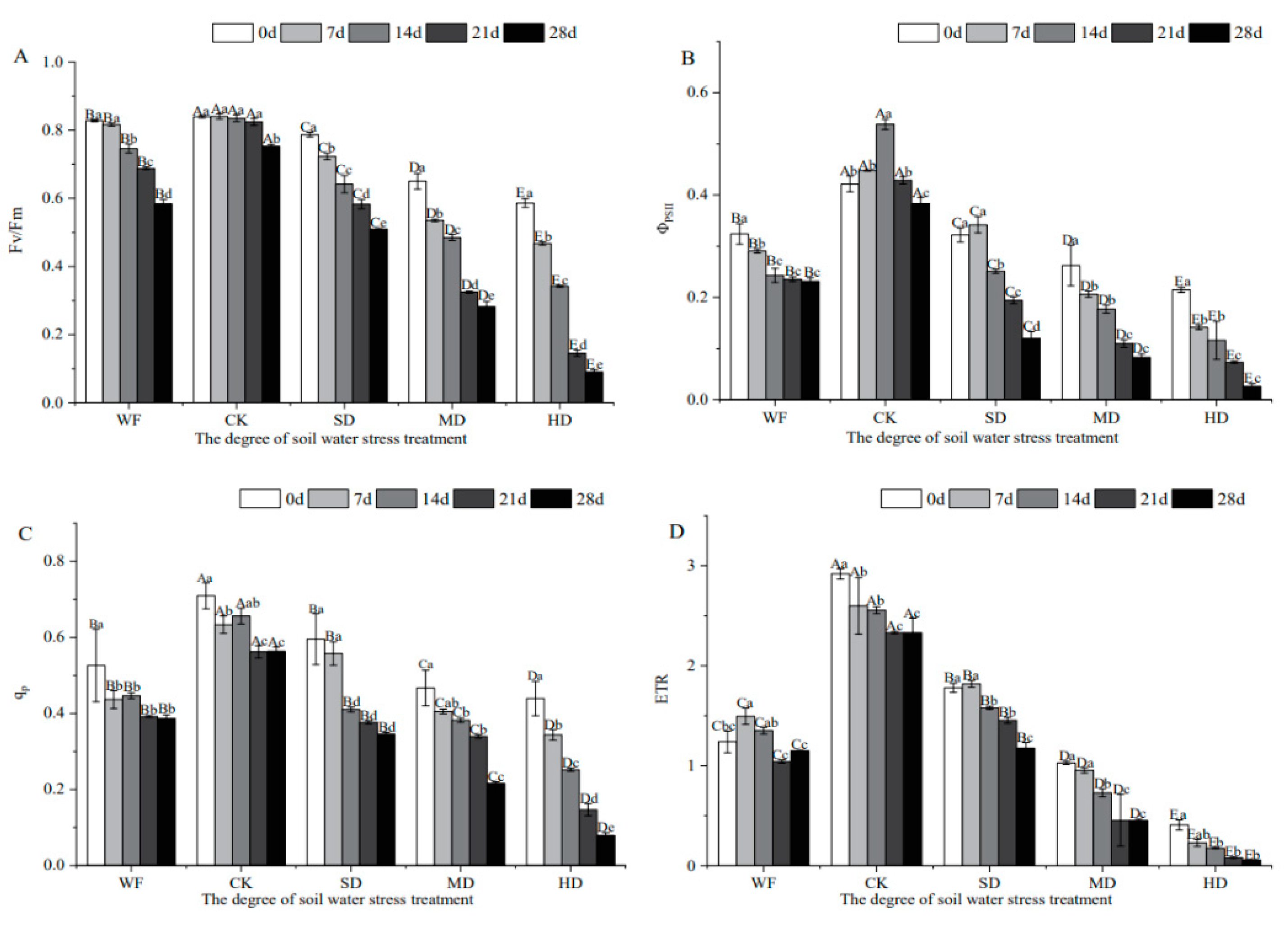
3.3. Effects of Water Stress on Chlorophyll Fluorescence Characteristics of C. retusus
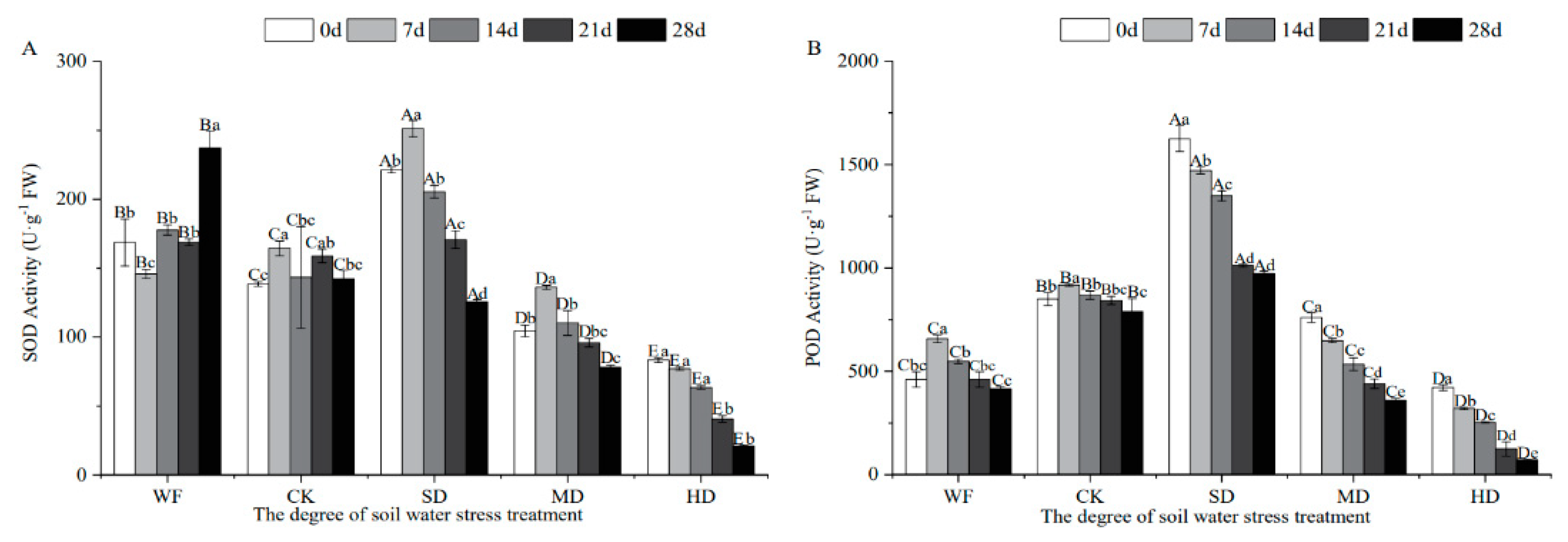
3.4. Effects of Water Stress on Protective Enzyme Activities in C. retusus
3.5. Effects of Water Stress on Malondialdehyde and Proline Contents in C. retusus
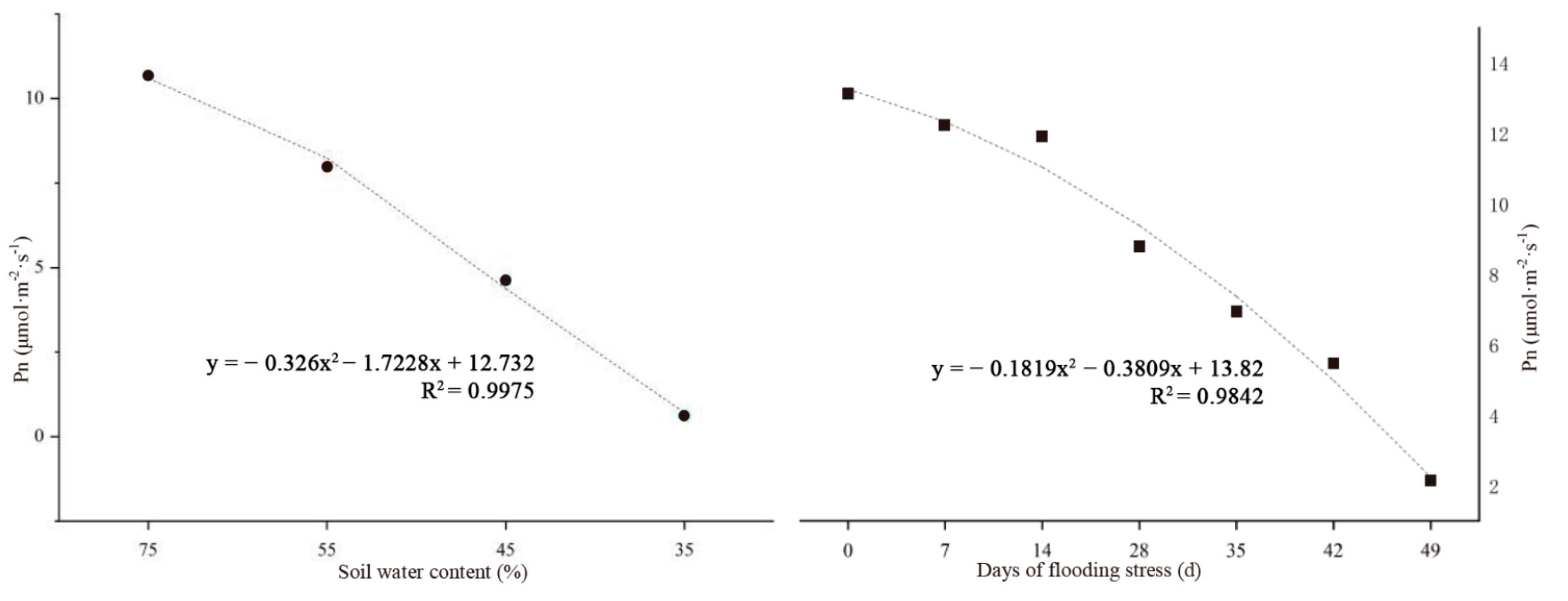
3.6. Drought Tolerance and Flood Tolerance Threshold Analysis of C. retusus
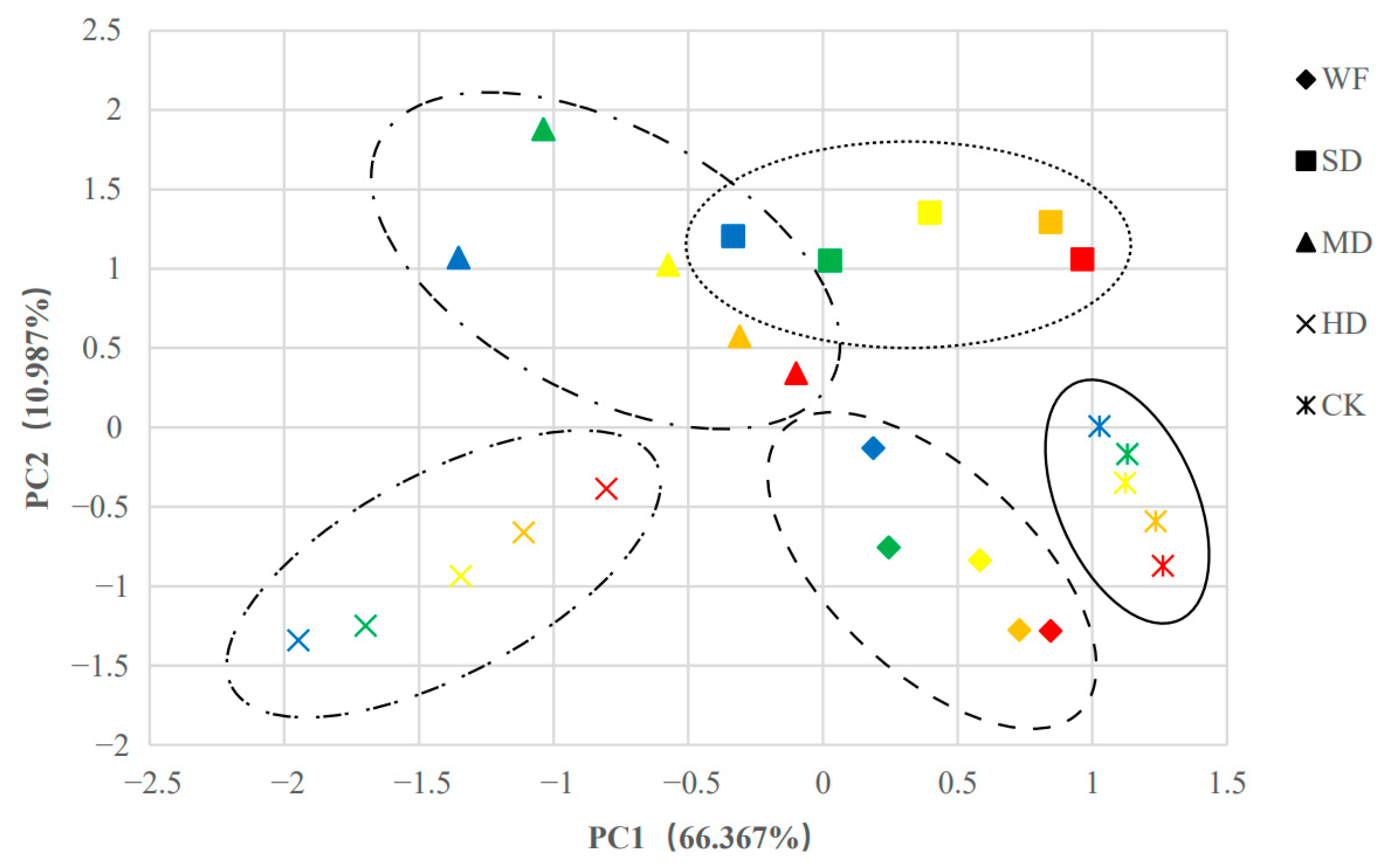
3.7. PCA of 12 Indicators of C. retusus under Different Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teng, H.F.; Hu, Y.; Zhou, L.Q.; Zhou, S. Modelling and mapping soil erosion potential in China. J. Integr. Agric. 2019, 18, 251–264. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.G.; Xue, M.; Li, B.; Chen, J.; Tao, Z. Spatial characteristics of extreme rainfall over China with hourly through 24-hour accumulation periods based on national-level hourly rain gauge data. Adv. Atmos. Sci. 2016, 33, 1218–1232. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Fischer, T.; Lu, X.X. Larger asian rivers: Changes in hydro-climate and water environments. Quat. Int. 2013, 304, 1–4. [Google Scholar] [CrossRef]
- Sun, H.; Wang, G.; Li, X.; Chen, J.; Su, B.; Jiang, T. Regional frequency analysis of observed sub-daily rainfall maxima over eastern China. Adv. Atmos. Sci. 2017, 34, 209–225. [Google Scholar] [CrossRef]
- Jiang, Z.; Shen, Y.; Ma, T.; Zhai, P.; Fang, S. Changes of precipitation intensity spectra in different regions of mainland China during 1961–2006. J. Meteorol. Res. 2014, 28, 1085–1098. [Google Scholar] [CrossRef]
- Wang, G.; Fang, Q.; Wu, B.; Yang, H.; Xu, Z. Relationship between soil erodibility and modeled infiltration rate in different soils. J. Hydrol. 2015, 528, 408–418. [Google Scholar] [CrossRef]
- Dhital, Y.P.; Kayastha, R.B.; Shi, J. Soil bioengineering application and practices in Nepal. Environ. Manag. 2013, 51, 354–364. [Google Scholar] [CrossRef]
- Lee, D.H.; Chen, P.Y.; Wu, J.H.; Chen, H.L.; Yang, Y.E. Method of mitigating the surface erosion of a high-gradient mudstone slope in southwest Taiwan. Bull. Eng. Geol. Environ. 2013, 72, 533–545. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, B.; Liu, Y.; Zhao, W.; Wang, S. Vulnerability assessment of the global water erosion tendency: Vegetation greening can partly offset increasing rainfall stress. Land Degrad Dev. 2019, 30, 1061–1069. [Google Scholar] [CrossRef]
- Silva, P.H.; Campoe, O.C.; De Paula, R.C.; Lee, D.J. Seedling growth and physiological responses of sixteen eucalypt taxa under controlled water regime. Forests 2016, 7, 110. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Wang, J.; Hui, W.; Zhao, F.; Wang, P.; Su, C.; Gong, W. Physiology of Plant Responses to Water Stress and Related Genes: A Review. Forests 2022, 13, 324. [Google Scholar] [CrossRef]
- Hao, S.; Cao, H.; Wang, H.; Pan, X. The physiological responses of tomato to water stress and re-water in different growth periods. Sci. Hortic. 2019, 249, 143–154. [Google Scholar] [CrossRef]
- Chen, H.; Zamorano, M.F.; Ivanoff, D. Effect of flooding depth on growth, biomass, photosynthesis, and chlorophyll fluorescence of Typha domingensis. Wetlands 2010, 30, 957–965. [Google Scholar] [CrossRef]
- Myung, J.K.; Seong, H.L.; Su, Y.W. Growth and anatomical characteristics of different water and light intensities on cork oak (Quercus suber L.) seedlings. Afr. J. Biotechnol. 2011, 10, 10964–10979. [Google Scholar] [CrossRef] [Green Version]
- Campelo, D.D.H.; Lacerda, C.F.D.; Sousa, J.A.D.; Bezerra, A.M.E.; Araújo, J.D.M.; Neves, A.L.R.; Carvalho, C.H. Morphophysiological leaf characteristics and nutritional status of six woody species as a function of the soil water availability. Cienc. Florest. 2018, 28, 924–936. [Google Scholar] [CrossRef] [Green Version]
- Conesa, M.R.; De La Rosa, J.M.; Domingo, R.; Banon, S.; Pérez-Pastor, A. Changes induced by water stress on water relations, stomatal behaviour and morphology of table grapes (cv. Crimson Seedless) grown in pots. Sci. Hortic. 2016; 202, 9–16. [Google Scholar] [CrossRef]
- Eksteen, A.B.; Grzeskowiak, V.; Jones, N.B.; Pammenter, N.W. Stomatal characteristics of Eucalyptus grandis clonal hybrids in response to water stress. J. For. Res. 2013, 75, 105–111. [Google Scholar] [CrossRef]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef]
- Nicolas, B.; Kyaw, T.P.; Matthew, E.G. A dynamic model of RuBP-regeneration limited photosynthesis accounting for photoinhibition, heat and water stress. Agric. For. Meteorol. 2020, 285, 107911. [Google Scholar] [CrossRef]
- He, Y.; Yang, J.; Zhu, B.; Zhu, Z.J. Low root zone temperature exacerbates the ion imbalance and photosynthesis inhibition and induces antioxidant responses in tomato plants under salinity. J. Integr. Agric. 2014, 13, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Aghaie, P.; Tafreshi, S.A.H.; Ebrahimi, M.A.; Haerinasab, M. Tolerance evaluation and clustering of fourteen tomato cultivars grown under mild and severe drought conditions. Sci. Hortic. 2018, 232, 1–12. [Google Scholar] [CrossRef]
- Sancho-Knapik, D.; Sanz, M.Á.; Peguero-Pina, J.J.; Niinemets, Ü.; Gil-Pelegrín, E. Changes of secondary metabolites in Pinus sylvestris L. needles under increasing soil water deficit. Ann. For. Sci. 2017, 74, 24. [Google Scholar] [CrossRef]
- Li, J.H. Varieties Appreciation of Chinese Fringe Tree, 1st ed.; China Forestry Publishing House: Beijing, China, 2022; pp. 2–6. [Google Scholar]
- Wang, I.C.; Chen, L.C.; Chang, T.H.; Chen, C.L.; Sung, P.J.; Lin, R.A.; Cheng, M.J.; Hsiao, J.W.; Chen, J.J. New Coumarin and Bioactive Constituents of Chionanthus retusus. Chem. Nat. Compd. 2021, 57, 835–839. [Google Scholar] [CrossRef]
- Lee, Y.G.; Lee, H.; Jung, J.W.; Seo, K.H.; Lee, D.Y.; Kim, H.G.; Ko, J.H.; Lee, D.S.; Baek, N.I. Flavonoids from Chionanthus retusus (Oleaceae) flowers and their protective effects against glutamate-induced cell toxicity in HT22 cells. Int. J. Mol. Sci. 2019, 20, 3517. [Google Scholar] [CrossRef] [Green Version]
- Atchudan, R.; Edison, T.N.J.I.; Chakradhar, D.; Perumal, S.; Shim, J.J.; Lee, Y.R. Facile green synthesis of nitrogen-doped carbon dots using Chionanthus retusus fruit extract and investigation of their suitability for metal ion sensing and biological applications. Sens. Actuators B 2017, 246, 497–509. [Google Scholar] [CrossRef]
- Gao, J.Y.; Yin, W.P. Flower Essential Oil Composition of Chionanthus retusus. Chem. Nat. Compd. 2016, 52, 934–936. [Google Scholar] [CrossRef]
- Quilantang, N.G.; Ryu, S.H.; Park, S.H.; Byun, J.S.; Chun, J.S.; Lee, J.S.; Rodriguez, J.P.; Yun, Y.S.; Jacinto, S.D.; Lee, S. Inhibitory activity of methanol extracts from different colored flowers on aldose reductase and HPLC-UV analysis of quercetin. Hortic. Environ. Biotechnol. 2018, 59, 899–907. [Google Scholar] [CrossRef]
- Cipollini, D.; Rigsby, C.M. Incidence of infestation and larval success of emerald ash borer (Agrilus planipennis) on white fringetree (Chionanthus virginicus), Chinese fringetree (Chionanthus retusus), and devilwood (Osmanthus americanus). Environ. Entomol. 2015, 44, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.T. Genetic Diversity and Distribution within Cultivated Gene Pools of Chionanthus retusus (Oleaceae) in the United States. In Proceedings of the 2014 ASHS Annual Conference, Orlando, Florida, USA, 27 July–1 August 2014. [Google Scholar]
- Zhang, L.; Su, B.Y.; Xu, H.; Li, Y.G. Growth and photosynthetic responses of four landscape shrub species to elevated ozone. Photosynthetica 2012, 50, 67–76. [Google Scholar] [CrossRef]
- Kwak, M.J.; Lee, J.K.; Park, S.; Lim, Y.J.; Kim, H.; Kim, K.N.; Je, S.M.; Park, C.R.; Woo, S.Y. Evaluation of the importance of some East Asian tree species for refinement of air quality by estimating air pollution tolerance index, anticipated performance index, and air pollutant uptake. Sustainability 2020, 12, 3067. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Cai, F.; Zhang, C.; Zhang, M.; Li, Y.; Duan, Y. Characterization of the complete chloroplast genome of the ornamental plant Osmanthus cooperi. Mitochondrial DNA Part B 2019, 4, 2314–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias, R.S.; Techen, N.; Rinehart, T.A.; Olsen, R.T.; Kirkbride, J.H.; Scheffler, B.E. Development of simple sequence repeat markers for Chionanthus retusus (Oleaceae) and effective discrimination of closely related taxa. HortScience 2011, 46, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.; Andrews, A.; Baas, P.; Bond, J.E.; Auad, M.; Dute, R. Pit membranes and their evolution in the Oleinae of the Oleaceae. IAWA J. 2017, 38, 201–219. [Google Scholar] [CrossRef]
- Alexander, L.W.; Thammina, C.S.; Kramer, M. Cross-transferability of SSR markers in Osmanthus. Genet. Resour. Crop Evol. 2018, 65, 125–136. [Google Scholar] [CrossRef]
- Ye, Y.; Tam, N.F.; Wong, Y.S.; Lu, C.Y. Growth and physiological responses of two mangrove species (Bruguiera gymnorrhiza and Kandelia candel) to waterlogging. Environ. Exp. Bot. 2003, 49, 209–221. [Google Scholar] [CrossRef]
- Keleş, Y.; Öncel, I. Response of antioxidative defence system to temperature and water stress combinations in wheat seedlings. Plant Sci. 2002, 163, 783–790. [Google Scholar] [CrossRef]
- Blanke, M.M.; Cooke, D.T. Effects of flooding and drought on stomatal activity, transpiration, photosynthesis, water potential and water channel activity in strawberry stolons and leaves. Plant Growth Regul. 2004, 42, 153–160. [Google Scholar] [CrossRef]
- Azizi, S.; Tabari, M.; Striker, G.G. Growth, physiology, and leaf ion concentration responses to long-term flooding with fresh or saline water of Populus euphratica. S. Afr. J. Bot. 2017, 108, 229–236. [Google Scholar] [CrossRef]
- Pociecha, E. Different physiological reactions at vegetative and generative stage of development of field bean plants exposed to flooding and undergoing recovery. J. Agron. Crop Sci. 2013, 199, 195–199. [Google Scholar] [CrossRef]
- Pei, B.; Zhang, G.G.; Zhang, S.Y.; Wu, Q.; Xu, Z.Q.; Xu, P. Effects of soil drought stress on photosynthesis and antioxidant enzyme activities in sea-buckthorn leaves. Acta Ecol. Sin. 2013, 33, 1386–1396. [Google Scholar]
- Zou, J.; Hu, W.; Li, Y.X.; He, J.Q.; Zhu, H.H.; Zhou, Z.G. Screening of drought resistance indices and evaluation of drought resistance in cotton (Gossypium hirsutum L.). J. Integr. Agric. 2020, 19, 495–508. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef]
- Javadi, T.; Rohollahi, D.; Ghaderi, N.; Nazari, F. Mitigating the adverse effects of drought stress on the morpho-physiological traits and anti-oxidative enzyme activities of Prunus avium through β-amino butyric acid drenching. Sci. Hortic. 2017, 218, 156–163. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, G.; Chao, W.; Xu, F.; Sun, X.; Chen, Z. Physiological responses and tolerance evaluation of five poplar varieties to waterlogging. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 658–667. [Google Scholar] [CrossRef] [Green Version]
- Showler, A.T. Effects of water deficit stress, shade, weed competition, and kaolin particle film on selected foliar free amino acid accumulations in cotton, Gossypium hirsutum (L.). J. Chem. Ecol. 2002, 28, 631–651. [Google Scholar] [CrossRef]
- Qi, M.; Liu, X.; Li, Y.; Song, H.; Yin, Z.; Zhang, F.; He, Q.; Xu, Z.; Zhou, G. Photosynthetic resistance and resilience under drought, flooding and rewatering in maize plants. Photosynth. Res. 2021, 148, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mafakheri, A.; Siosemardeh, A.F.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar] [CrossRef]
- Nxele, X.; Klein, A.; Ndimba, B.K. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. S. Afr. J. Bot. 2017, 108, 261–266. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, J.L.; Hao, R.M.; Xu, W.G.; Pan, W.M.; Jiao, Z.Y. Changes of some physiological indexes of ten tree species under flooding stress and comparison of their waterlogging tolerance. J. Plant Resour. Environ. 2007, 1, 69–73. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, M.; Zhao, T.; Xu, D.; Liu, C.; Liu, Y.; Sun, M.; Xie, H.; Li, J. Physiological Responses of Chionanthus retusus Seedlings to Drought and Waterlogging Stresses. Forests 2023, 14, 429. https://doi.org/10.3390/f14020429
Niu M, Zhao T, Xu D, Liu C, Liu Y, Sun M, Xie H, Li J. Physiological Responses of Chionanthus retusus Seedlings to Drought and Waterlogging Stresses. Forests. 2023; 14(2):429. https://doi.org/10.3390/f14020429
Chicago/Turabian StyleNiu, Muge, Tianran Zhao, Dong Xu, Cuishuang Liu, Yuan Liu, Maotong Sun, Huicheng Xie, and Jihong Li. 2023. "Physiological Responses of Chionanthus retusus Seedlings to Drought and Waterlogging Stresses" Forests 14, no. 2: 429. https://doi.org/10.3390/f14020429



