Effect of Type of Forest Growth Conditions and Climate Elements on the Dynamics of Radial Growth in English Oak (Quercus robur L.) of Early and Late Phenological Forms
Abstract
:1. Introduction
2. Materials and Methods
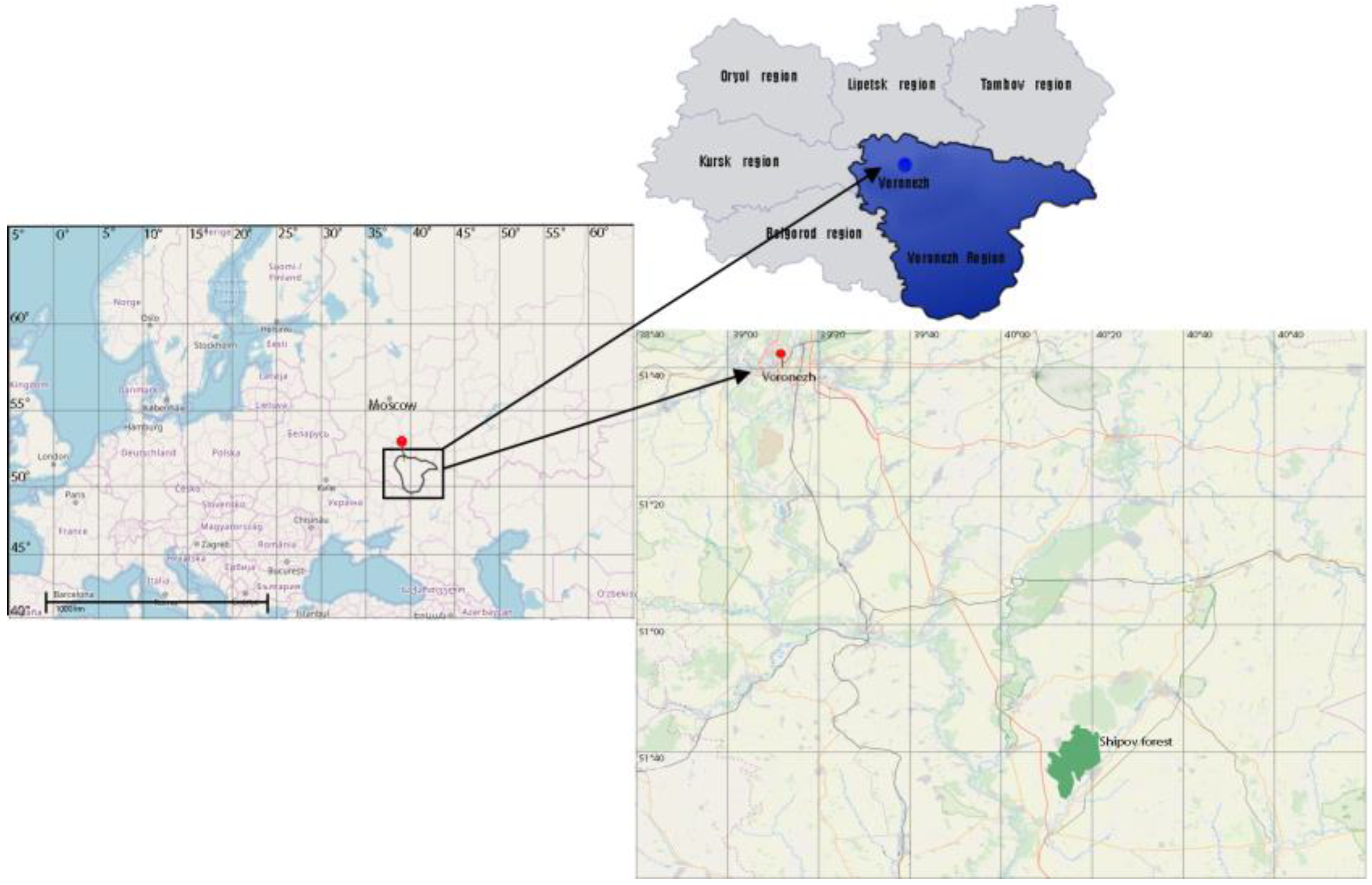
2.1. Edaphic and Orographic Conditions in Shipov Forest
2.2. Climatic Profile of the Study Area
2.3. Sampling Method
2.4. Preparation for Macroscopic Measurements
2.5. Statistical Analysis
ai = 1/n ∑ xi × γ
j = 1
A11i = ∑ ai/11
i + 5
3. Results
3.1. Annual Ring Width (ARW)
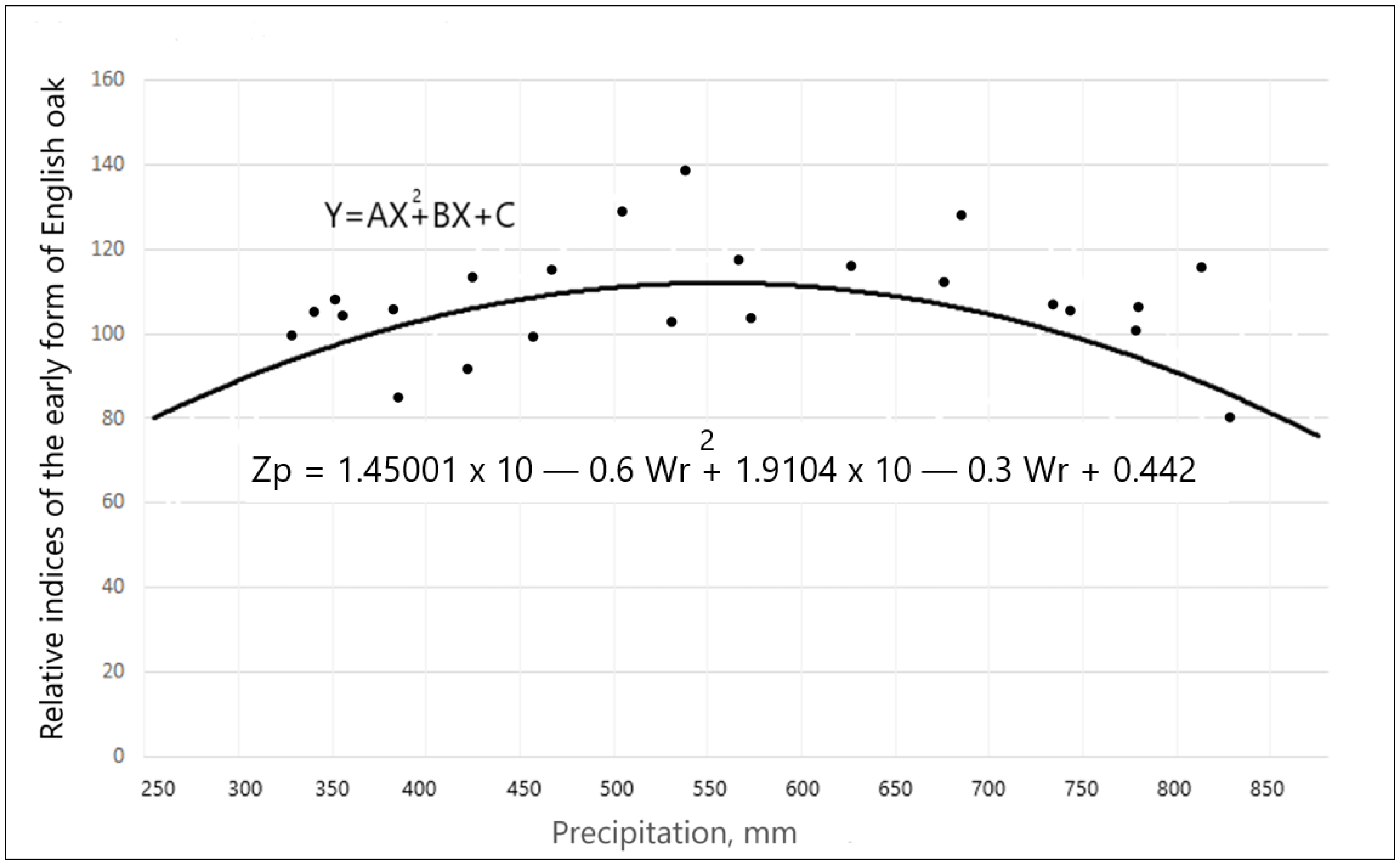
3.2. The Influence of Climatic Factors on ARW and Late Wood Width in Different Local Growth Conditions
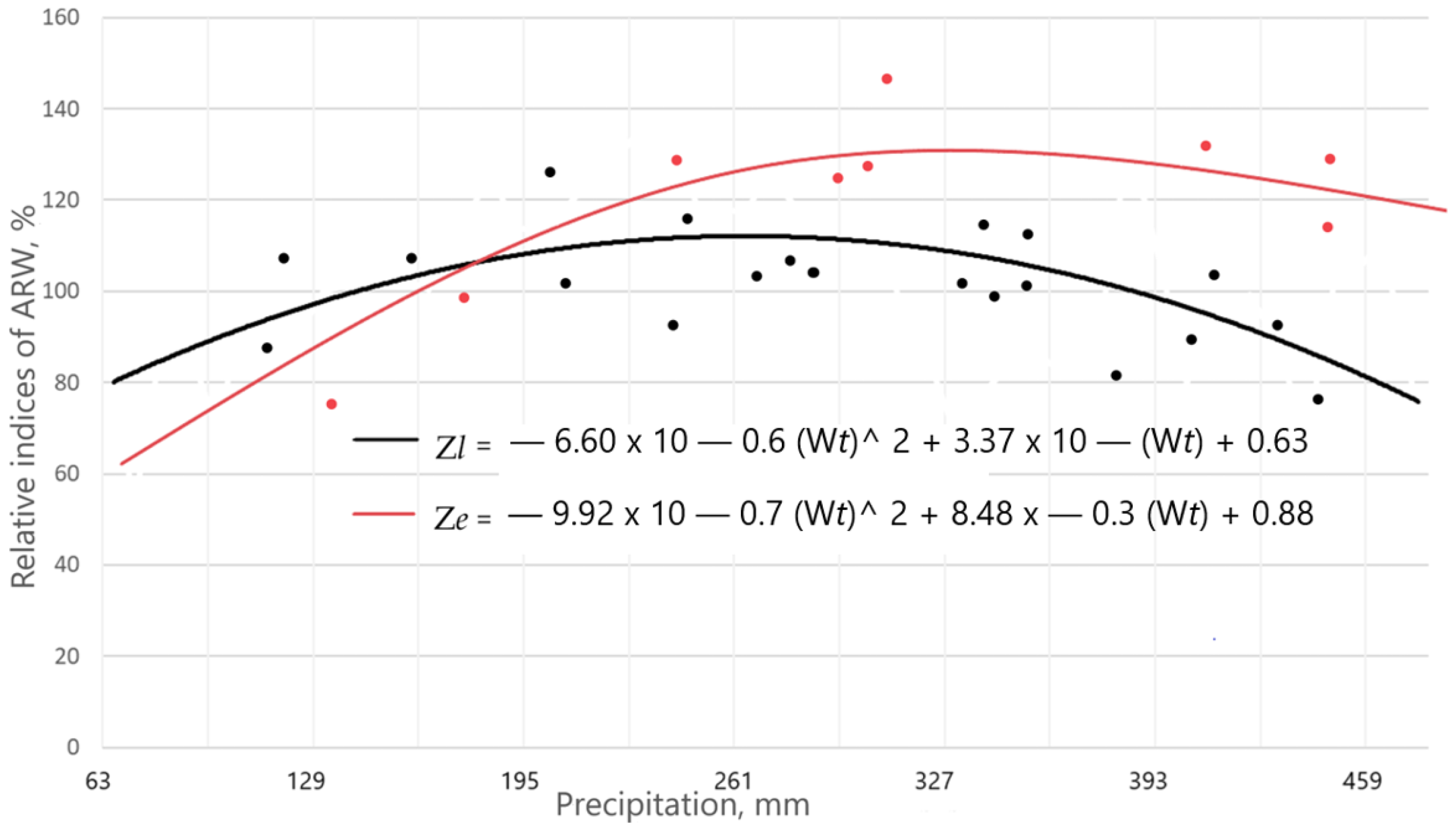
3.3. Effects of Climate Factors on ARW
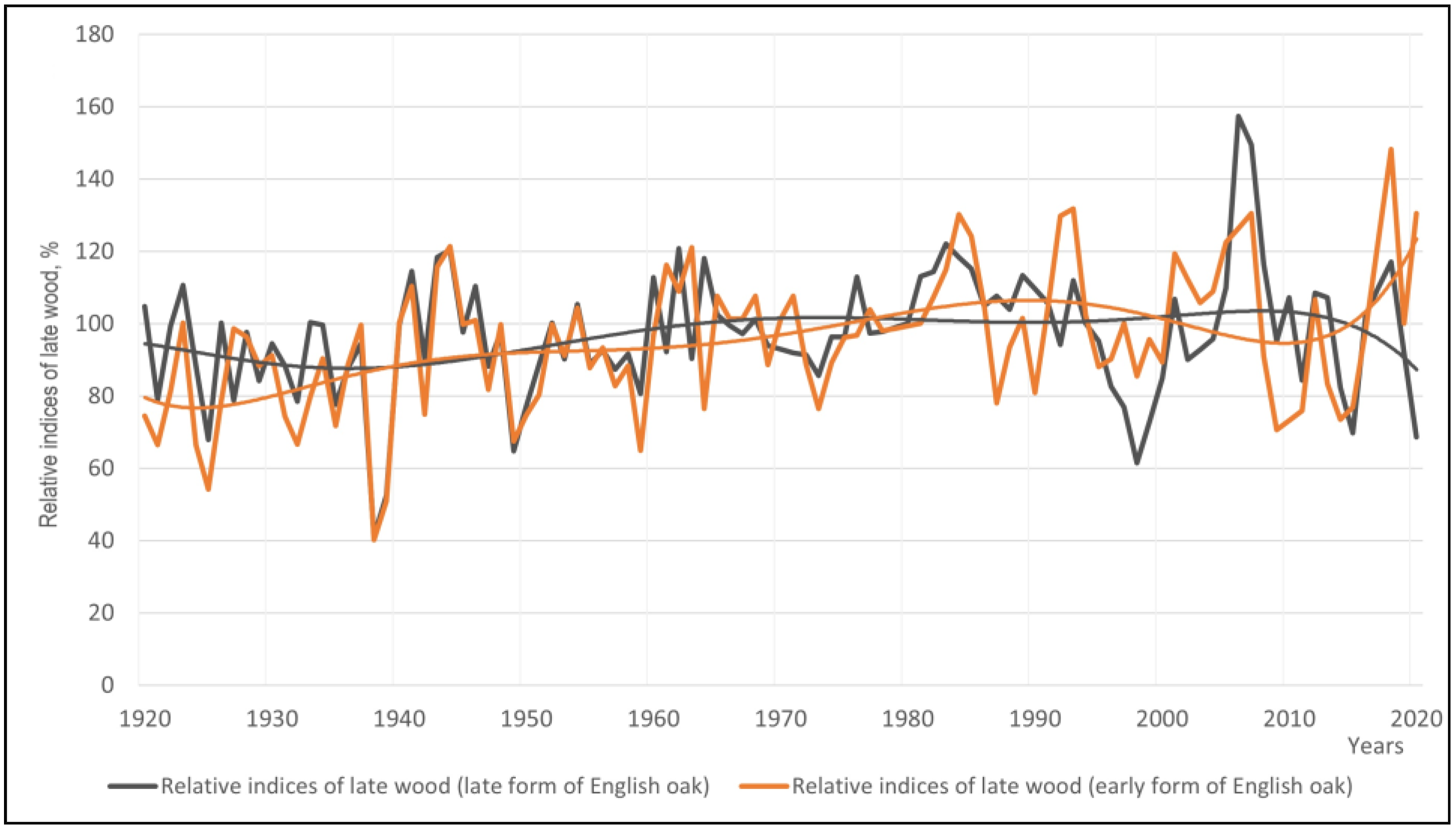
3.4. Differences between the Phenological Forms of English Oak Grown in Different Local Growth Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silchenko, I.I. Phenological forms of pedunculate oak (Quercus robur L.) in various types of landscapes of the Bryansk region. Bull. Bryansk State Univ. 2012, 4, 158–161. Available online: https://cyberleninka.ru/article/n/fenologicheskie-formy-duba-chereshchatogo-quercus-robur-l-v-razlichnyh-tipah-landshaftov-bryanskoy-oblasti (accessed on 29 May 2021). (In Russian).
- Efimov, Y.P. On the question of the territorial distribution of the phenological forms of the petiolate oak. Genet. Village Fam. Introd. For. Species 1975, 2, 37–45. (In Russian) [Google Scholar]
- En’kova, E.I. Tellermanovskii Les i ego Vosstanovlenie (Tellerman Forest and Its Recovery); Voronezh Gos. Univ.: Voronezh, Russia, 1976; 214p. (In Russian) [Google Scholar]
- Puchałka, R.; Koprowski, M.; Przybylak, R. Fenologia liści i ksylogeneza w zróżnicowanej wiekowo populacji dębu szypułkowego. Klimatyczne uwarunkowania życia lasu. In Proceedings of the Ogólnopolska Konferencja Naukowa, Streszczenia Referatów, Rogów, Poland, 16–17 June 2015; pp. 16–17. [Google Scholar]
- Slepykh, O.O. Rhythm of phenology and distribution phenological forms of Pedunculate oak (Quercus robur L.) in Donetsk region. Biol. Syst. 2016, 8, 272–279. [Google Scholar]
- Wesołowski, T.; Rowiński, P. Late leaf development in pedunculate oak (Quercus robur): An antiherbivore defence? Scand. J. For. Res. 2008, 23, 386–394. [Google Scholar] [CrossRef]
- Orlović, S.; Šimunovački, D.; Djorđević, Z.; Pilipović, A.; Radosavljević, N. Očuvanje Genofonda I Proizvodnja Semena Hrasta Lužnjaka (Quercus robur L.). In 250 Godina Ravnog Srema; Vojvodinašume: Petrovaradin, Serbia, 2008; 378p, ISBN 978-86-906665-1-5. [Google Scholar]
- Batos, B.; Miljković, D.; Ninić-Todorović, J. Length of vegetation period as parameter of common oak (Quercus robur L.) phenological variability. Genetika 2012, 44, 139–152. [Google Scholar] [CrossRef]
- Utkina, I.; Rubtsov, V. Studies of Phenological Forms of Pedunculate Oak. Contemp. Probl. Ecol. 2017, 10, 804–811. [Google Scholar] [CrossRef]
- Pirko, Y.V.; Netsvetov, M.; Kalafat, L.O.; Pirko, N.M.; Rabokon, A.M.; Privalikhin, S.M.; Demkovich, A.Y.; Bilonozhko, Y.O.; Blume, Y.B. Genetic features of the phenological forms of Quercus robur (Fagaceae) according to the analysis of the introns polymorphism of β-tubulin genes and microsatellite loci. Ukr. Bot. J. 2018, 75, 489–500. [Google Scholar] [CrossRef] [Green Version]
- Ueno, S.; Klopp, C.; Noirot, C.; Léger, V.; Prince, E.; Kremer, A.; Plomion, C.; Le Provost, G. Detection of genes involved in bud phenology in sessile oak (Quercus petraea Matt. Liebl) combining digital expression analysis and Q-PCR. BMC Proc. 2011, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Chokheli, V.; Kozlovsky, B.; Sereda, M.; Lysenko, V.; Fesenko, I.; Varduny, T.; Kapralova, O.; Bondarenko, E. Preliminary comparative analysis of phenological varieties of Quercus robur by ISSR-markers. J. Bot. 2016, 2016, 7910451. [Google Scholar] [CrossRef] [Green Version]
- Puchałka, R.; Koprowski, M.; Przybylak, J.; Przybylak, R.; Dąbrowski, H.P. Did the late spring frost in 2007 and 2011 affect tree-ring width and earlywood vessel size in Pedunculate oak (Quercus robur) in northern Poland? Int. J. Biometeorol. 2016, 60, 1143–1150. [Google Scholar] [CrossRef] [Green Version]
- Puchałka, R.; Koprowski, M.; Gričar, J.; Przybylak, R. Does tree-ring formation follow leaf phenology in Pedunculate oak (Quercus robur L.)? Eur. J. For. Res. 2017, 136, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Kitin, P. Dynamics of cambial activity in the stem of early- and late-flushing forms of oak (Quercus robur vars. praecox and tardiflora) in the Park of Freedom, Sofia. Nauk za Gorata 1992, 27, 3–13. [Google Scholar]
- Kostic, S.; Orlovic, S.; Karaklic, V.; Kesić, L.; Zorić, M.; Stojanović, D.B. Allometry and Post-Drought Growth Resilience of Pedunculate Oak (Quercus robur L.) Varieties. Forests 2021, 12, 930. [Google Scholar] [CrossRef]
- Koval, I.M.; Kostyashkin, D.C. The influence of climate and recreation on formation of layers of annual wood of early and late forms Quercus robur L. in Kharkiv. Greenbelt. Sci. Bull. UNFU 2015, 25, 52–58. [Google Scholar] [CrossRef]
- Batos, B. Diversity of Pedunculate Oak (Quercus robur L.); Foundation Andrejević: Belgrade, Serbia, 2012; ISBN 1450-801X. [Google Scholar]
- Bobinac, M.; Batos, B.; Miljković, D.; Radulović, S. Polycyclism and phenological variability in the common oak (Quercus robur L.). Arch. Biol. Sci. 2012, 64, 97–105. [Google Scholar] [CrossRef]
- Levlev, V.V. Ecotypes and Forms of the Petiolate Oak in the Voronezh Nature Reserve: Abstract of the Dissertation of the Phd of Agricultural Sciences; Voronezh State Forestry Academy: Voronezh, Russia, 1970; 20p. (In Russian) [Google Scholar]
- Lukyanets, V.B. Intraspecific Variability of the Petiolate Oak in the Central Forest-Steppe; Voronezh Gos. Univ.: Voronezh, Russia, 1979; 215p. (In Russian) [Google Scholar]
- Shitov, V.P. The Form Diversity of Floodplain Oak Forests of Polesie and the Ways of Their Economic Use: Abstract of the Dissertation of the Phd of Agricultural Sciences; BGU: Bryansk, Russia, 1986; 25p. (In Russian) [Google Scholar]
- Shutyaev, A.M. Biodiversity of the Pedunculate oak (Quercus robur L.) and Its Use in Breeding and Afforestation: Abstract of the Dissertation of the Dr. Agricultural Sciences; Research Institute of Forest Genetics and Breeding: Bryansk, Russia, 1998; 43p. (In Russian) [Google Scholar]
- Kobranov, N.P. Oak Breeding; New Village: Voronezh, Russia, 1925; 25p, Available online: http://lestehjournal.ru/sites/default/files/journal_pdf/81-98.pdf (accessed on 27 September 2022). (In Russian)
- Pukacka, S. Wzrost i rozwój. In Dęby. Quercus robur L., Quercus petraea Liebl; Bugała, W., Ed.; Nasze Drzewa Leśne: Kórnik, Poland, 2006; Volume 11, pp. 165–303. [Google Scholar]
- Antin, C.; Pélissier, R.; Vincent, G.; Couteron, P. Crown allometries are less responsive than stem allometry to tree size and habitat variations in an Indian monsoon forest. Trees Struct. Funct. 2013, 27, 1485–1495. [Google Scholar] [CrossRef]
- Rubtsov, V.V.; Utkina, I.A. Adaptatsionnyye Reaktsii Duba na Defoliatsiyu; Institute of Forestry of the Russian Academy of Sciences: Moscow, Russia, 2008; 302p. (In Russian) [Google Scholar]
- Kozarac, J. Kasni (pozni) hrast (Quercus pedunculata var. tardissima Simonkai). Sumar List 1898, 22, 41–53. [Google Scholar]
- Vikhrov, V.E. Stroenie i Fiziko-Mekhanicheskie Svoistva Drevesiny duba; Izd-vo Akademii nauk SSSR: Moskva, Russia, 1954; 264p. (In Russian) [Google Scholar]
- Izdebski, K. Wstępne badania nad ekologią i rozmieszczeniem dębu szypułkowego (Quercus robur L.) w Polsce. Sylwan 1956, 11, 415–506. [Google Scholar]
- Savolainen, O.; Bokma, F.; García-Gil, R.; Komulainen, P.; Repo, T. Genetic variation in cessation of growth and frost hardiness and consequences for adaptation of Pinus sylvestris to climatic changes. For. Ecol. Manag. 2004, 197, 79–89. [Google Scholar] [CrossRef]
- Skrøppa, T.; Johnsen, Ø. Patterns of adaptive genetic variation in forest tree species; the reproductive enviroment as an evolutionary force in Picea abies. In Forest Genetics and Sustainability; Springer: Dordrecht, The Netherlands, 1999; pp. 49–58. [Google Scholar]
- Mátyás, C. Modelling climate change effects with provenance test data. Tree Physiol. 1994, 14, 797–804. [Google Scholar] [CrossRef] [Green Version]
- Mátyás, C. Migratory, genetic and phenetic response potential of forest tree populations facing climate change. Acta Silv. Lignaria Hung. 2006, 2, 33–46. [Google Scholar]
- Food and Agriculture Organization of the United Nations. FAO/UNESCO «Soil Map of the World». Available online: https://www.fao.org/soils-portal/data-hub/soil-maps-and-databases/faounesco-soil-map-of-the-world/en/ (accessed on 27 September 2022).
- Myasoedov, S.S. Shipov Forest: Forestry-Household. Characteristics and Overview of Scientific Works: (To the 60th Anniversary of the Experience. Affairs in the Array), the All-Union. Scientific-Research. Institute of Forestry and Mechanization of Forestry. Shipovskaya Forest Experience. Station; Publishing House of Voronezh University: Voronezh, Russia, 1969; 276p. [Google Scholar]
- Rivas-Martínez, S.; Sáenz, S.; Penas, A. Worldwide Bioclimatic Classification System. Glob. Geobot. 2011, 1, 1–638. [Google Scholar] [CrossRef]
- Resolution of The Government of the Russian Federation from December 9, 2020 N 2047 “On the Approval of the Rules of Sanitary Safety in Forests”. Available online: https://docs.cntd.ru/document/573053313 (accessed on 9 September 2022).
- Martin-StPaul, N.K.; Limousin, J.-M.; Vogt-Schilb, H.; Rodríguez-Calcerrada, J.; Rambal, S.; Longepierre, D.; Misson, L. The temporal response to drought in a Mediterranean evergreen tree: Comparing a regional precipitation gradient and a throughfall exclusion experiment. Glob. Chang. Biol. 2013, 19, 2413–2426. [Google Scholar] [CrossRef]
- Bolychev, V.G. Annual layers of oak wood as an indicator of manifestation extreme meteorological conditions in secular cycles of climate fluctuations. In Proceedings of the All-Union Meeting of the Scientific Conference on Dendrochronology and Dendroclimatology, Vilnius, Lithuania, 7–8 July 1968; pp. 45–48. [Google Scholar]
- Wilks, D.S. Statistical Methods in the Atmospheric Sciences, 2nd ed.; International Geophys Series Number 91; Academic Press: Burlington, VT, USA, 2006; 649p. [Google Scholar]
- Kharchenko, N.A.; Mikhno, V.B.; Kharchenko, N.N.; Tsaralunga, V.V.; Korchagin, O.M.; Matveev, S.M.; Melnikov, E.E.; Zapletin, V.Y. Degradation of Oak Forests of the Central Chernozem Region; Fed. agency for Education, FSB HPE “VGLTA”: Voronezh, Russia, 2010; 604p. (In Russian) [Google Scholar]
- Vlasenko, A.A. Growth, Condition, Durability and Renewal of the Oak Petiolate in the Conditions of the Dry Steppe. Abstract of the Dissertation of the Phd of Agricultural Sciences; All-Russian Scientific Research; Institute of Forestry and Mechanization of Forestry: Pushkino, Russia, 2012; 21p. (In Russian) [Google Scholar]
- Kalashnikova, I.V.; Migalina, S.V. Influence of habitat on growth and changes in productivity parameters of birch trees at the ash dump of power station. In Problems of Botany in Southern Siberia and Mongolia, Proceedings of the XVIII International Scientific and Practical Conference, Barnaul, Russia, 20–23 May 2019; Altai State University: Barnaul, Russia, 2019; pp. 503–507. (In Russian) [Google Scholar]
- Kostin, S.I. Solar activity and tree growth. In Proceedings of the All-Union Meeting of the Scientific Conference on Dendrochronology and Dendroclimatology, Vilnius, Lithuania, 7–8 July 1968; pp. 119–124. (In Russian). [Google Scholar]
- Martynenko, S.N. Dendrochronological Features of Carbon Deposition by Oak Plantations of the Central Forest-Steppe. Abstract of the Dissertation of the Phd of Agricultural Sciences; Voronezh State Forestry Academy: Voronezh, Russia, 2005; 18p. (In Russian) [Google Scholar]
- Melnikov, E.E. Temporal and Spatial Aspects of Successions in Upland Oak Forests of the Central Forest-Steppe. Abstract of the Dissertation for the Phd of Biological Sciences; Voronezh State University: Voronezh, Russia, 2009; 23p. (In Russian) [Google Scholar]
- Tarankov, V.I.; Matveev, S.M.; Van Kun, V.; Mamonov, D.N. Features of radial growth of coniferous species in the Irkutsk region. Izv. Vuzov. Lesn. Zh. 1994, 4, 54–57. (In Russian) [Google Scholar]
- Holtmaeir, F.K. Waldgrenzen und Klimaaschwandunden. Ohologische Aspekte eines vieldiskutirten Pehenomens. Geoocod Ynamic 1995, 1, 24. [Google Scholar]
- Batos, B.; Šešlija Jovanović, D.; Miljković, D. Spatial and temporal variability of flowering in the pedunculate oak (Quercus robur L.). Šumarski List 2014, 7–8, 371–379. [Google Scholar]
- Order from October 8, 2015 N 353 “The Establishment of Forest-Seed Zoning”; Ministry of Natural Resources and Ecology of the Russian Federation, Federal Forestry Agency: Moscow, Russia, 2015.
- Romanowsky, M.G.; Mamaev, V.V. Dinamika aktivnosti pogloshchayushchikh kornei duba (Oak absorbing roots activity dynamics). Vestn. Mosk. Gos. Univ. Lesa—Lesn. Vestn. 2012, 7, 78–82. [Google Scholar]
- Molchanov, A.G. Photosynthesis intensity of phenological forms of common oak in conditions of insufficient moisture. Lesovedenie 2012, 4, 31–38. [Google Scholar]
- Yurkevich, I.D.; Sidorovich, I.A. The phenomenforms and ecotypes of the pedunculate oak and their productivity in the floodplain of the Dnieper river. Lesovedenie 1969, 2, 24–32. (In Russian) [Google Scholar]
- Zvorykina, K.V.; Elagina, I.N. The association of early and late oak forms with relief elements. Izv. All-Union Geogr. Soc. 1965, 97, 287–290. [Google Scholar]
- Mironenko, A.Y. Distribution of early and late foliation forms of common oak related to soil-ground conditions, in Lesovedenie i lesnoe khozyastvo. For. Sci. For. Minsk. 1970, 3, 46–50. [Google Scholar]
- Buras, A.; Sass-Klaassen, U.; Verbeek, I.; Copini, P. Provenance selection and site conditions determine growth performance of pedunculate oak. Dendrochronologia 2020, 61, 125705. [Google Scholar] [CrossRef]
- Ljubojević, M.; Sebolt, A.; Ognjanov, V.; Iezzoni, A. Heritability of anatomical characteristics in cherry interspecific hybrids. J. Plant Growth Regul. 2021, 41(4), 1–18. [Google Scholar] [CrossRef]
- Pellizzari, E.; Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Carrer, M. Wood anatomy and carbon-isotope discrimination support long-term hydraulic deterioration as a major cause of drought-induced dieback. Glob. Chang. Biol. 2016, 22, 2125–2137. [Google Scholar] [CrossRef]
- Kostin, S.I. Frequency of appearance for dry and wet periods in the Central Forest-Steppe of Russian Plain. In Issues to Increase of Agricultural Productivity; Scientific Notes of Voronezh Forestry Institute; Voronezh State University Press: Voronezh, Russia, 1963; Volume XXIX, pp. 91–101. (In Russian) [Google Scholar]
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK; San Francisco, CA, USA; New York, NY, USA, 1976; 566p. [Google Scholar]
- Molchanov, A.A. Dendroclimatic Foundations of Weather Forecasts; Nauka: Moscow, Russia, 1976; 168p. (In Russian) [Google Scholar]
- Pretzsch, H.; Schütze, G.; Uhl, E. Resistance of European tree species to drought stress in mixed versus pure forests: Evidence of stress release by inter-specific facilitation. Plant Biol. 2013, 15, 483–495. [Google Scholar] [CrossRef]
- Bosela, M.; Štefančík, I.; Petráš, R.; Vacek, S. The effects of climate warming on the growth of European beech forests depend critically on thinning strategy and site productivity. Agric. For. Meteorol. 2016, 222, 21–31. [Google Scholar] [CrossRef]
- Prykhodko, N.F.; Parpan, T.V.; Prykhodko, M.M. Radial increment in European spruce (Picea abies) as indicator of sanitary condition of spruce forests in the Ukrainian Carpathians. Artic. Biosyst. Divers. 2020, 28, 131–138. [Google Scholar] [CrossRef]
- Zhirina, L.S. Dendroclimate Analysis of the Growth of Common Oak Stalked of Early and Late Phenoforms in the Bryansk Oblast, Abstract of the Dissertation of the Phd of Biological Sciences; Tartu State Univ.: Tartu, Estonia, 1985. [Google Scholar]



| Sample Plot No. | Stand Composition | Origin | Mean Age, yrs | Mean Height, m | Mean Stem Diameter, cm | Soil Type |
|---|---|---|---|---|---|---|
| 1 | 1st storey: 90% O 10% A; 2nd storey: 100% M + st P | Natural, by seed | 170 | 33.1 | 54.0 | Degraded chernozem |
| 2 | 1st storey: 80% O 20% A; 2nd storey: 10% M, st L | Natural, by seed | 170 | 31.4 | 49.0 | Degraded chernozem |
| 3 | 90% O 10% A + st M, P | Natural, by coppice | 75 | 21.1 | 24.3 | Light brown, forest, loamy |
| 4 | 50% O 30% A 10% M 10% L | Natural, by coppice | 75 | 22.1 | 26.6 | Light brown, forest, loamy |
| 5 | 100% O st A | Natural, by coppice | 95 | 17.0 | 23.2 | Light gray, solonetzic |
| 6 | 100% O | Natural, by coppice | 95 | 16.0 | 26.0 | Gray, forest, solonetzic |
| 7 | 80% O 10% A 10% L | Natural, by coppice | 78 | 21.8 | 24.8 | Humus-carbonate on cretacious deposits |
| Age, yrs | Annual Width | Statistics | Late Wood Percentage, % | ||||
|---|---|---|---|---|---|---|---|
| Xavg ± m, mm | ±σ, mm | CV,% | p | t-Test | |||
| 10 | ARW | 2.82 ± 0.097 | 0.44 | 15.5 | <0.001 | 3.65 | 75 |
| EWW | 0.77 ± 0015 | 0.07 | 0.9 | <0.05 | 2.20 | ||
| LWW | 2.05 ± 0.087 | 0.39 | 19.0 | <0.01 | 2.84 | ||
| 30 | ARW | 2.35 ± 0.092 | 0.41 | 17.5 | <0.05 | 2.41 | 66 |
| EWW | 0.79 ± 0019 | 0.09 | 13.5 | <0.01 | 3.13 | ||
| LWW | 1.56 ± 0.073 | 0.32 | 20.8 | <0.01 | 3.01 | ||
| 50 | ARW | 1.52 ± 0.036 | 0.16 | 10.6 | <0.001 | 3.24 | 59 |
| EWW | 0.62 ± 0025 | 0.11 | 21.1 | <0.001 | 3.94 | ||
| LWW | 0.90 ± 0.023 | 0.12 | 13.3 | <0.001 | 4.41 | ||
| 70 | ARW | 1.03 ± 0.023 | 0.11 | 10.4 | <0.001 | 4.12 | 56 |
| EWW | 0.45 ± 0023 | 0.10 | 19.2 | <0.001 | 3.78 | ||
| LWW | 0.58 ± 0.018 | 0.08 | 13.9 | <0.001 | 3.98 | ||
| 90 | ARW | 1.14 ± 0.058 | 0.26 | 22.6 | <0.01 | 2.87 | 57 |
| EWW | 0.49 ± 0027 | 0.12 | 26.3 | <0.001 | 3.38 | ||
| LWW | 0.65 ± 0.044 | 0.19 | 30.4 | <0.01 | 2.96 | ||
| 110 | ARW | 1.55 ± 0.070 | 0.31 | 27.5 | <0.05 | 2.47 | 60 |
| EWW | 0.62 ± 0041 | 0.18 | 34.3 | <0.01 | 2.69 | ||
| LWW | 0.93 ± 0.051 | 0.23 | 24.7 | <0.05 | 2.88 | ||
| 130 | ARW | 1.74 ± 0.059 | 0.26 | 15.2 | <0.001 | 3.45 | 59 |
| EWW | 0.70 ± 0036 | 0.16 | 29.1 | <0.001 | 3.72 | ||
| LWW | 1.04 ± 0.054 | 0.24 | 23.2 | <0.001 | 3.64 | ||
| 150 | ARW | 1.29 ± 0.065 | 0.29 | 22.7 | <0.001 | 3.57 | 57 |
| EWW | 0.55 ± 0029 | 0.11 | 17.1 | - | 1.78 | ||
| LWW | 0.74 ± 0.046 | 0.21 | 28.2 | <0.001 | 3.65 | ||
| 170 | ARW | 1.15 ± 0.072 | 0.31 | 27.3 | <0.01 | 2.75 | 53 |
| EWW | 0.54 ± 0033 | 0.15 | 25.3 | <0.05 | 2.23 | ||
| LWW | 0.61 ± 0.057 | 0.25 | 40.4 | <0.01 | 2.71 | ||
| Climate Factor | LGCs | Pheno- Form | Effect Size Index | Effect Size Index Error | F-Test | t-Test | ||
|---|---|---|---|---|---|---|---|---|
| p = 0.05 | p = 0.01 | |||||||
| Annual precipitation | D2 D1 D0 | late early late early late early | 0.18 0.06 0.21 0.08 0.13 0.06 | 0.048 0.050 0.052 0.060 0.050 0.054 | 3.54 1.20 4.04 1.33 2.60 1.11 | 2.36 2.40 2.36 | 3.3 | |
| Summer precipitation | D2 D1 D0 | late early late early late early | 0.24 0.10 0.30 0.38 0.09 0.29 | 0.046 0.050 0.030 0.029 0.050 0.040 | 5.11 2.00 10.0 13.1 1.82 7.25 | 2.34 2.40 2.34 | 3.3 | |
| Composite indicators | Selyaninov’s hydrothermal coefficient | D2 D1 D0 | late early late early late early | 0.67 0.71 0.24 0.20 0.34 0.17 | 0.019 0.016 0.050 0.052 0.038 0.048 | 35.26 44.37 4.80 3.80 8.95 3.54 | 2.36 2.40 2.36 | 3.3 |
| Lang’s rain factor | D2 D1 D0 | late early late early late early | 0.20 0.31 0.18 0.15 0.22 0.13 | 0.046 0.039 0.038 0.040 0.044 0.050 | 4.35 7.95 4.74 3.75 5.00 2.6 | 2.35 2.40 2.35 | 3.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milenin, A.I.; Popova, A.A.; Shestibratov, K.A. Effect of Type of Forest Growth Conditions and Climate Elements on the Dynamics of Radial Growth in English Oak (Quercus robur L.) of Early and Late Phenological Forms. Forests 2023, 14, 11. https://doi.org/10.3390/f14010011
Milenin AI, Popova AA, Shestibratov KA. Effect of Type of Forest Growth Conditions and Climate Elements on the Dynamics of Radial Growth in English Oak (Quercus robur L.) of Early and Late Phenological Forms. Forests. 2023; 14(1):11. https://doi.org/10.3390/f14010011
Chicago/Turabian StyleMilenin, Andrey I., Anna A. Popova, and Konstantin A. Shestibratov. 2023. "Effect of Type of Forest Growth Conditions and Climate Elements on the Dynamics of Radial Growth in English Oak (Quercus robur L.) of Early and Late Phenological Forms" Forests 14, no. 1: 11. https://doi.org/10.3390/f14010011






