Optimal Management Strategies to Maximize Carbon Capture in Forest Plantations: A Case Study with Pinus radiata D. Don
Abstract
1. Introduction
2. Materials and Methods
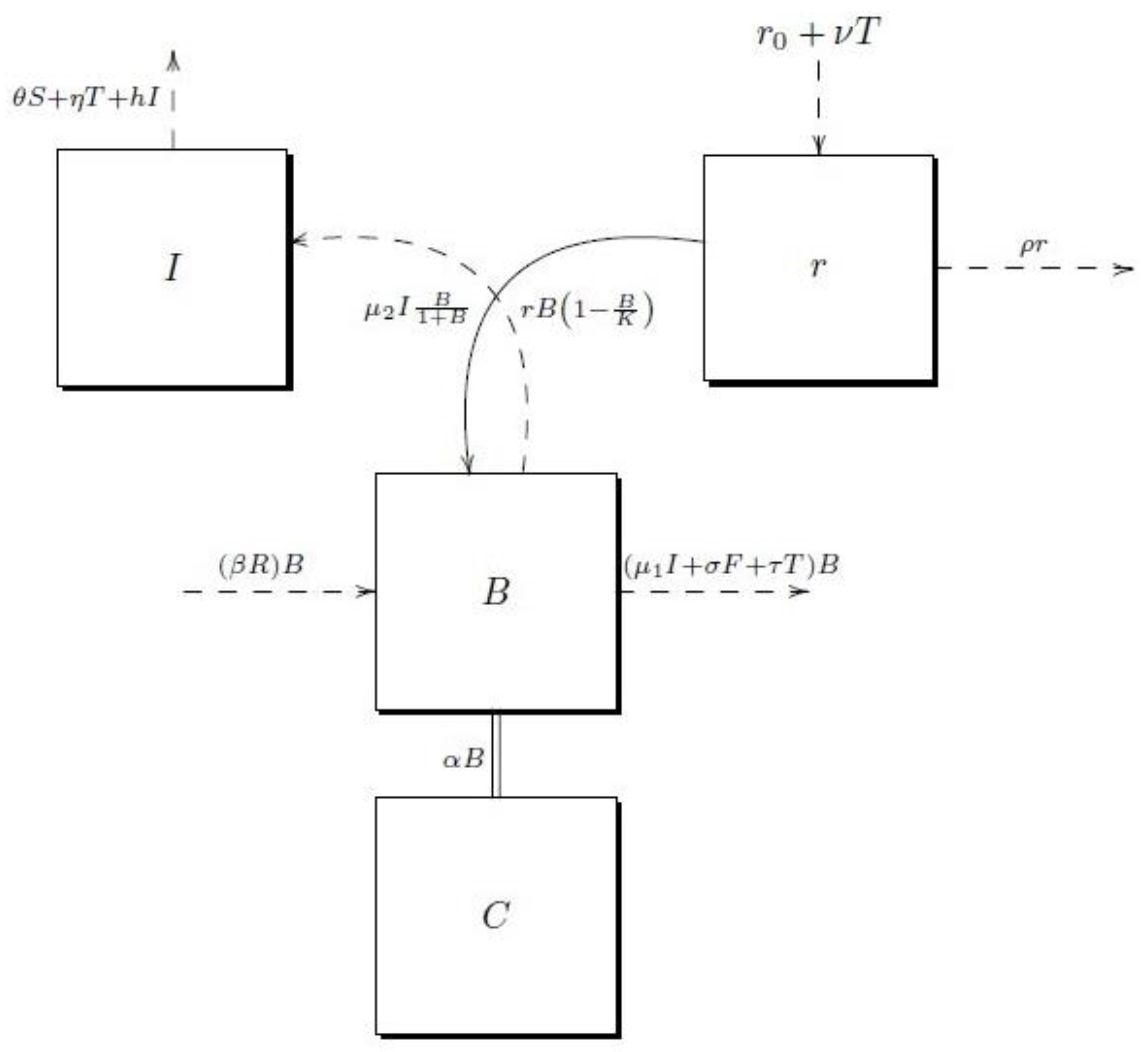
2.1. The Mathematical Model
- The model is formulated for fast-growing managed forest plantations;
- There are many plantations with different ages, thus biomass never goes to zero;
- The ambient humidity is considered constant for simplicity;
- No soil fertilization in each cycle of the forest regeneration is considered;
- The area burned per year is considered, but human intentionality is not taken into account;
- There are no incentives for reforestation or carbon capture;
- The harvesting method corresponds to clear-cutting;
- In the thinning, the thinner, lower quality and less commercially valuable trees will be removed. Two types of thinning effects are considered, which are explained below;
- The presence of artificial irrigation is neglected in the model;
- The budget for fire prevention is limited;
- Intensive management of forestry is not included in our model;
- Trees burned by fire are replaced by new plants and natural regeneration is not used;
- The mortality rate of extreme events is neglected in our model.
| Notation | Definition | Unit |
|---|---|---|
| Rate of increase in biomass due to the effects of reforestation | ha−1 | |
| Relative humidity threshold to reduce fire | year−1 | |
| Rate at which biomass decreases due to fire effects | m−2 | |
| Fire parameter | year−1 | |
| Rate at which biomass decreases due to felling effects | ha−1 | |
| Rate at which biomass decreases due to thinning effects | ha−1 | |
| Maximum growth rate | year−2 | |
| Natural mortality rate | year−1 | |
| Rate of increase in thinning over individual growth | ha−1 | |
| Fire prevention rate | ha US$−1year−1 | |
| Thinning rate | m2 ha−1 year−1 | |
| Carbon capture rate | Tn C m−3 year−1 |
2.2. The Optimal Control Problem
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Existence of Solutions to the Optimal Control Problem
- The set of admissible controls and state variables of the problem is non-empty;
- The admissible control class is convex and bounded;
- The right-hand side of the system Equation (4) is bounded by a nonlinear function that depends on the state and control variables;
- The integral of the objective function is concave;
- There exist positive constants and satisfying the integrating of the objective functional, such that
- The solutions of the system (4) are considered to be bounded in a finite time interval and making use of a result from [65], the existence of a solution for the controlled system can be assured;
- From Equation (A1) the set of admissible controls is known to be topologically closed and convex by definition;
- For this point, let us represent the system (4) as follows:in its matrix formThe system (A3) is a nonlinear system with a bounded coefficient. So, now the system (A3) is defined as follows:From the second term of Equation (A4), we apply the inequality of Hölder we obtainwhere , and In addition, by the constraints of the solutions of Equation (4) and [33]. Then, the constant Z is positive, taking which is independent of the state variables, we have that
- To show the concavity of the integrand of the objective functional, let us denote as followsfor this, we must prove thatlet be two control vectors and . Applying Equation (A2), the definition of convex set, we obtaintherefore if one obtains from Equation (A6)then the integrating of the objective function is concave.
- Finally, considering that and it follows thatwhere depends on while for and the required is obtained.It is satisfied that there exists an optimal control quadruple such that which was given in Equation (5) is maximized. □
Appendix B. Description of Parameters
References
- Pedersen, J.S.T.; Santos, F.D.; van Vuuren, D.; Gupta, J.; Coelho, R.E.; Aparício, B.A.; Swart, R. An assessment of the performance of scenarios against historical global emissions for IPCC reports. Glob. Environ. Chang. 2021, 66, 102199. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Current status and challenges on microalgae-based carbon capture. Int. J. Greenh. Gas Control 2012, 10, 456–469. [Google Scholar] [CrossRef]
- Ambardekar, A.A.; Siebenmorgen, T.J.; Counce, P.A.; Lanning, S.B.; Mauromoustakos, A. Impact of field-scale nighttime air temperatures during kernel development on rice milling quality. Field Crops Res. 2011, 122, 179–185. [Google Scholar] [CrossRef]
- UNFCCC. Adoption of the Paris Agreement FCCC/CP/2015/L.9/Rev.1. United Nations Framework Convention on Climate Change. In Proceedings of the Parties Twenty-First Session, Paris, France, 30 November–11 December 2015. [Google Scholar]
- Kim, Y.; Tanaka, K.; Matsuoka, S. Environmental and economic effectiveness of the Kyoto Protocol. PLoS ONE 2020, 15, e0236299. [Google Scholar] [CrossRef] [PubMed]
- Karousakis, K. Incentives to reduce GHG emissions from deforestation: Lessons learned from Costa Rica and Mexico. OECD Pap. 2007, 7, 1–50. [Google Scholar] [CrossRef]
- Dixon, R.K.; Solomon, A.; Brown, S.; Houghton, R.; Trexier, M.; Wisniewski, J. Carbon pools and flux of global forest ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef] [PubMed]
- FAO yPNUMA. El Estado de Los Bosques del Mundo 2020: Los Bosques, la Biodiversidad y las Personas, Roma. Available online: https://www.fao.org/documents/card/en/c/ca8642es (accessed on 8 August 2022).
- Lewis, S.L.; Wheeler, C.E.; Mitchard, E.T.; Koch, A. Restoring natural forests is the best way to remove atmospheric carbon. Nature 2019, 568, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, N. Optimal rotation age for carbon sequestration and biodiversity conservation in Vietnam. For. Policy Econ. 2014, 38, 56–64. [Google Scholar] [CrossRef]
- Kaipainen, T.; Liski, J.; Pussinen, A.; Karjalainen, T. Managing carbon sinks by changing rotation length in European forests. Environ. Sci. Policy 2004, 7, 205–219. [Google Scholar] [CrossRef]
- Fragoso-López, P.I.; Rodríguez-Laguna, R.; Otazo-Sánchez, E.M.; González-Ramírez, C.A.; Valdéz-Lazalde, J.R.; Cortés-Blobaum, H.J.; Razo-Zárate, R. Carbon sequestration in protected areas: A case study of an Abies religiosa (HBK) Schlecht. et Cham Forest. Forests 2017, 8, 429. [Google Scholar] [CrossRef]
- Jörgensen, K.; Granath, G.; Lindahl, B.D.; Strengbom, J. Forest management to increase carbon sequestration in boreal Pinus sylvestris forests. Plant Soil 2021, 466, 165–178. [Google Scholar] [CrossRef]
- Pérez-Cruzado, C.; Mansilla-Salinero, P.; Rodríguez-Soalleiro, R.; Merino, A. Influence of tree species on carbon sequestration in afforested pastures in a humid temperate region. Plant Soil 2012, 353, 333–353. [Google Scholar] [CrossRef]
- Olmedo, G.F.; Guevara, M.; Gilabert, H.; Montes, C.R.; Arellano, E.C.; Barría-Knopf, B.; Gárate, F.; Mena-Quijada, P.; Acuña, E.; Bown, H.E.; et al. Baseline of carbon stocks in Pinus radiata and Eucalyptus spp. plantations of Chile. Forests 2020, 11, 1063. [Google Scholar] [CrossRef]
- Derwisch, S.; Schwendenmann, L.; Olschewski, R.; Hölscher, D. Estimation and economic evaluation of aboveground carbon storage of Tectona grandis plantations in Western Panama. New For. 2009, 37, 227–240. [Google Scholar] [CrossRef]
- Wakker, E.; Watch, S.; Rozario, J.D. Greasy Palms: The Social and Ecological Impacts of Large-Scale Oil Palm Plantation Development in Southeast Asia; AIDEnvironment: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Nasir, N.; Abd Aziz, M.I.; Banitalebi, A. Carbon absorption control model of oil palm plantation. Sains Malays. 2019, 48, 921–925. [Google Scholar] [CrossRef]
- Abd Aziz, M.I.; Nasir, N.; Banitalebi, A. The Optimal Felling Rate in the Palm Oil Plantation System. MATEMATIKA Malays. J. Ind. Appl. Math. 2019, 35, 95–104. [Google Scholar] [CrossRef]
- Reed, W.J. The effects of the risk of fire on the optimal rotation of a forest. J. Environ. Econ. Manag. 1984, 11, 180–190. [Google Scholar] [CrossRef]
- Sohngen, B.; Mendelsohn, R. An optimal control model of forest carbon sequestration. Am. J. Agric. Econ. 2003, 85, 448–457. [Google Scholar] [CrossRef]
- Brown, T.J.; Hall, B.L.; Westerling, A.L. The impact of twenty-first century climate change on wildland fire danger in the western United States: An applications perspective. Clim. Chang. 2004, 62, 365–388. [Google Scholar] [CrossRef]
- Ning, Z.; Sun, C. Forest management with wildfire risk, prescribed burning and diverse carbon policies. For. Policy Econ. 2017, 75, 95–102. [Google Scholar] [CrossRef]
- Couture, S.; Reynaud, A. Forest management under fire risk when forest carbon sequestration has value. Ecol. Econ. 2011, 70, 2002–2011. [Google Scholar] [CrossRef]
- Holsten, A.; Dominic, A.R.; Costa, L.; Kropp, J.P. Evaluation of the performance of meteorological forest fire indices for German federal states. For. Ecol. Manag. 2013, 287, 123–131. [Google Scholar] [CrossRef]
- Misra, A.; Verma, M.; Venturino, E. Modeling the control of atmospheric carbon dioxide through reforestation: Effect of time delay. Model. Earth Syst. Environ. 2015, 1, 24. [Google Scholar] [CrossRef]
- Verma, M.; Misra, A. Optimal control of anthropogenic carbon dioxide emissions through technological options: A modeling study. Comput. Appl. Math. 2018, 37, 605–626. [Google Scholar] [CrossRef]
- Verma, M.; Verma, A.K. Effect of plantation of genetically modified trees on the control of atmospheric carbon dioxide: A modeling study. Nat. Resour. Model. 2021, 34, e12300. [Google Scholar] [CrossRef]
- Caetano, M.A.L.; Gherardi, D.F.M.; Yoneyama, T. Optimal resource management control for CO2 emission and reduction of the greenhouse effect. Ecol. Model. 2008, 213, 119–126. [Google Scholar] [CrossRef]
- Kerdan, I.G.; Giarola, S.; Hawkes, A. A novel energy systems model to explore the role of land use and reforestation in achieving carbon mitigation targets: A Brazil case study. J. Clean. Prod. 2019, 232, 796–821. [Google Scholar] [CrossRef]
- Hudiburg, T.W.; Law, B.E.; Wirth, C.; Luyssaert, S. Regional carbon dioxide implications of forest bioenergy production. Nat. Clim. Chang. 2011, 1, 419–423. [Google Scholar] [CrossRef]
- Jin, X.; Pukkala, T.; Li, F.; Dong, L. Optimal management of Korean pine plantations in multifunctional forestry. J. For. Res. 2017, 28, 1027–1037. [Google Scholar] [CrossRef]
- Altamirano-Fernández, A.; Rojas-Palma, A.; Espinoza-Meza, S. A mathematical model to study the dynamics of carbon capture in forest plantations. J.Phys. Conf. Ser. 2022, 1259, 012001. [Google Scholar] [CrossRef]
- Gaoue, O.G.; Jiang, J.; Ding, W.; Agusto, F.B.; Lenhart, S. Optimal harvesting strategies for timber and non-timber forest products in tropical ecosystems. Theor. Ecol. 2016, 9, 287–297. [Google Scholar] [CrossRef]
- Tahvonen, O. Optimal choice between even-and uneven-aged forestry. Nat. Resour. Model. 2009, 22, 289–321. [Google Scholar] [CrossRef]
- Du, E.; Tang, Y. Distinct Climate Effects on Dahurian Larch Growth at an Asian Temperate-Boreal Forest Ecotone and Nearby Boreal Sites. Forests 2021, 13, 27. [Google Scholar] [CrossRef]
- Favero, A.; Daigneault, A.; Sohngen, B. Forests: Carbon sequestration, biomass energy, or both? Sci. Adv. 2020, 6, eaay6792. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, S.; Workman, J.T. Optimal Control Applied to Biological Models; Chapman and Hall/CRC: Boca Raton, FL, USA, 2007. [Google Scholar]
- Fleming, W.H.; Rishel, R.W. Deterministic and Stochastic Optimal Control; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 1. [Google Scholar]
- Huong, V.T. Solution existence theorems for finite horizon optimal economic growth problems. Optimization 2021, 71, 4243–4263. [Google Scholar] [CrossRef]
- Pontryagin, L.S. Mathematical Theory of Optimal Processes; CRC Press: Boca Raton, FL, USA, 1987. [Google Scholar]
- Kirk, D.E. Optimal Control theory: An introduction; Courier Corporation. Dover Publications: New York, NY, USA, 2004. [Google Scholar]
- Grüne, L.; Pannek, J. Nonlinear model predictive control. In Nonlinear Model Predictive Control; Springer: Berlin/Heidelberg, Germany, 2017; pp. 45–69. [Google Scholar]
- Campos, C.; Silva, C.J.; Torres, D.F. Numerical optimal control of HIV transmission in Octave/MATLAB. Math. Comput. Appl. 2019, 25, 1. [Google Scholar] [CrossRef]
- Higham, D.; Higham, N. MATLAB Guide; SIAM: Philadelphia, PA, USA, 2016; Volume 150. [Google Scholar]
- Navarrete, E.; Bustos, J. Faustmann optimal pine stands stochastic rotation problem. For. Policy Econ. 2013, 30, 39–45. [Google Scholar] [CrossRef]
- Shukla, P.R.; Skeg, J.; Buendia, E.C.; Masson-Delmotte, V.; Pörtner, H.O.; Roberts, D.; Zhai, P.; Slade, R.; Connors, S.; Van Diemen, S.; et al. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2019; in press. [Google Scholar]
- Diaz-Hormazabal, I.; Gonzalez, M.E. Spatio-temporal analyses of wildfires in the region of Maule, Chile. Bosque 2016, 37, 147–158. [Google Scholar]
- Rodríguez, M.P.R.; Rodríguez, Y.C.; Sierra, C.A.M.; Batista, A.C.; Tetto, A.F. Relación entre variables meteorológicas e incendios forestales en la Provincia Pinar del Río, Cuba. Floresta 2017, 47. [Google Scholar] [CrossRef]
- Song, L.; Zhou, Y. The COVID-19 pandemic and its impact on the global economy: What does it take to turn crisis into opportunity? China World Econ. 2020, 28, 1–25. [Google Scholar] [CrossRef]
- Schneider, G.; Troeger, V.E. War and the world economy: Stock market reactions to international conflicts. J. Confl. Resolut. 2006, 50, 623–645. [Google Scholar] [CrossRef]
- Bekessy, S.A.; Wintle, B.A. Using carbon investment to grow the biodiversity bank. Conserv. Biol. 2008, 22, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Burgin, S. BioBanking: An environmental scientist’s view of the role of biodiversity banking offsets in conservation. Biodivers. Conserv. 2008, 17, 807–816. [Google Scholar] [CrossRef]
- Meleason, M.A.; Quinn, J.M. Influence of riparian buffer width on air temperature at Whangapoua Forest, Coromandel Peninsula, New Zealand. For. Ecol. Manag. 2004, 191, 365–371. [Google Scholar] [CrossRef]
- Georgi, N.J.; Zafiriadis, K. The impact of park trees on microclimate in urban areas. Urban Ecosyst. 2006, 9, 195–209. [Google Scholar] [CrossRef]
- Peng, G.; Li, J.; Chen, Y.; Norizan, A.P.; Tay, L. High-resolution surface relative humidity computation using MODIS image in Peninsular Malaysia. Chin. Geogr. Sci. 2006, 16, 260–264. [Google Scholar] [CrossRef]
- Kumar, M.; Sheikh, M.A.; Bhat, J.A.; Bussmann, R.W. Effect of fire on soil nutrients and under storey vegetation in Chir pine forest in Garhwal Himalaya, India. Acta Ecol. Sin. 2013, 33, 59–63. [Google Scholar] [CrossRef]
- Du, E.; Terrer, C.; Pellegrini, A.F.; Ahlström, A.; van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.; Jackson, R.B. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- Du, E. Evidence of soil nutrient availability as the proximate constraint on growth of treeline trees in northwest A laska: Comment. Ecology 2016, 97, 801–803. [Google Scholar] [CrossRef]
- Toro, J.; Gessel, S. Radiata pine plantations in Chile. New For. 1999, 18, 33–44. [Google Scholar] [CrossRef]
- Castillo, E.; Rodriguez, F. Determining response times for the deployment of terrestrial resources for fighting forest fires: A case study: Mediterranean-Chile. Cienc. Investig. Agrar. Rev. Latinoam. Cienc. Agric. 2015, 42, 97–107. [Google Scholar] [CrossRef]
- Apud, E.; Meyer, F. Factors influencing the workload of forest fire-fighters in Chile. Work 2011, 38, 203–209. [Google Scholar] [CrossRef]
- Cartes-Rodríguez, E.; Rubilar-Pons, R.; Acuña-Carmona, E.; Cancino-Cancino, J.; Rodríguez-Toro, J.; Burgos-Tornería, Y. Potential of Pinus radiata plantations for use of harvest residues in characteristic soils of south-central Chile. Rev. Chapingo Ser. Cienc. For. Ambiente 2016, 22, 221–233. [Google Scholar] [CrossRef]
- CONAF. Situación Diaria de Incendios Forestales, Sistema de Información Digital para el Control de Operaciones. 2022. Available online: https://www.conaf.cl/situacion-nacional-de-incendios-forestales/ (accessed on 8 August 2022).
- Lukes, D.L. Differential Equations: Classical to Controlled; Mathematics in Science and Engineering; Academic Press: New York, NY, USA, 1982. [Google Scholar]
- Khan, M.; Ali, K.; Bonyah, E.; Okosun, K.; Islam, S.; Khan, A. Mathematical modeling and stability analysis of Pine Wilt Disease with optimal control. Sci. Rep. 2017, 7, 3115. [Google Scholar] [CrossRef] [PubMed]
- Logan, J.D. An Introduction to Nonlinear Partial Differential Equations; John Wiley & Sons: Hoboken, NJ, USA, 2008; Volume 89. [Google Scholar]
- Soto Aguirre, D. Anuario Forestal. 2021. Available online: https://bibliotecadigital.infor.cl/handle/20.500.12220/31292 (accessed on 1 March 2021).
- Akinbode, O.; Eludoyin, A.; Fashae, O. Temperature and relative humidity distributions in a medium-size administrative town in southwest Nigeria. J. Environ. Manag. 2008, 87, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Valdivieso, J.P.; Rivera, J.d.D. Effect of wind on smoldering combustion limits of moist pine needle beds. Fire Technol. 2014, 50, 1589–1605. [Google Scholar] [CrossRef]
- Ojeda, H.; Rubilar, R.A.; Montes, C.; Cancino, J.; Espinosa, M. Leaf area and growth of Chilean radiata pine plantations after thinning across a water stress gradient. N. Z. J. For. Sci. 2018, 48, 10. [Google Scholar] [CrossRef]
- Asenjo, S.B. Evolución de las plantaciones forestales en Chile. For. Reforestación. Cienc. Investig. For. 2018, 24, 89–115. [Google Scholar] [CrossRef]
- Gallardo Vera PD, L.; Morales Agoni, R.P.; Sáez, G. Manual de manejo silvícola para coníferas en Aysén. bibliotecadigital.infor.cl 2000, 24, 1–24. [Google Scholar] [CrossRef]
- Alzamora, M.R.; Apiolaza, L.; Ide, S. Physical and economic evaluation of volume losses due to Rhyacionia buoliana (Schiff.) damage in Pinus radiata (D. Don) plantations in Southern Chile. Bosque 2002, 23, 29–42. [Google Scholar] [CrossRef]
- Crecente-Campo, F.; Pommerening, A.; Rodríguez-Soalleiro, R. Impacts of thinning on structure, growth and risk of crown fire in a Pinus sylvestris L. plantation in northern Spain. For. Ecol. Manag. 2009, 257, 1945–1954. [Google Scholar] [CrossRef]
- Fernández, M.P.; Basauri, J.; Madariaga, C.; Menéndez-Miguélez, M.; Olea, R.; Zubizarreta-Gerendiain, A. Effects of thinning and pruning on stem and crown characteristics of radiata pine (Pinus radiata D. Don). Iforest-Biogeosci. For. 2017, 10, 383. [Google Scholar] [CrossRef]
- White, D.A.; Silberstein, R.P.; Balocchi-Contreras, F.; Quiroga, J.J.; Meason, D.F.; Palma, J.H.; de Arellano, P.R. Growth, water use, and water use efficiency of Eucalyptus globulus and Pinus radiata plantations compared with natural stands of Roble-Hualo forest in the coastal mountains of central Chile. For. Ecol. Manag. 2021, 501, 119676. [Google Scholar] [CrossRef]
- CONAF. Incendios. 2022. Available online: https://www.conaf.cl/incendios-forestales/incendios-forestales-en-chile/estadistica-deocurrencia-diaria/ (accessed on 1 June 2022).
- Hidalgo, M.G. Disciplinamiento de las subjetividades como estrategia de prevención de incendios: El caso de las plantaciones forestales en el sur de Chile. Perspect. Rural. Nueva Época 2018, 16, 117–141. [Google Scholar]
- Raison, R.; Myers, B. The biology of forest growth experiment: Linking water and nitrogen availability to the growth of Pinus radiata. For. Ecol. Manag. 1992, 52, 279–308. [Google Scholar] [CrossRef]



| Notation | Definition | Unit |
|---|---|---|
| Volume of living biomass | m3 ha−1 | |
| Intrinsic growth of biomass | year−1 | |
| Burned area per year | m2 year−1 | |
| Carbon capture | Tn C ha−1year−1 | |
| Forest reforestation | ha year−1 | |
| Forest felling | ha year−1 | |
| Fire prevention | US$m2 ha−1year−1 | |
| Forest thinning | ha year−1 |
| 400 | 0.055 | 2 × 10−5 | 2 × 10−5 | 1 × 10−5 | 0.06 | 0.07275 | 0.159 | 0.4097 | 0.15 | 9.3 × 10−7 | 0.135 | 0.5 |
| 400 | 0.02 | 0.1 | 0.09 | 1 × 10−5 | 0.06 | 0.061 | 0.03 | 0.47 | 0.052 | 9.3 × 10−7 | 0.11 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altamirano-Fernández, A.; Rojas-Palma, A.; Espinoza-Meza, S. Optimal Management Strategies to Maximize Carbon Capture in Forest Plantations: A Case Study with Pinus radiata D. Don. Forests 2023, 14, 82. https://doi.org/10.3390/f14010082
Altamirano-Fernández A, Rojas-Palma A, Espinoza-Meza S. Optimal Management Strategies to Maximize Carbon Capture in Forest Plantations: A Case Study with Pinus radiata D. Don. Forests. 2023; 14(1):82. https://doi.org/10.3390/f14010082
Chicago/Turabian StyleAltamirano-Fernández, Alex, Alejandro Rojas-Palma, and Sergio Espinoza-Meza. 2023. "Optimal Management Strategies to Maximize Carbon Capture in Forest Plantations: A Case Study with Pinus radiata D. Don" Forests 14, no. 1: 82. https://doi.org/10.3390/f14010082
APA StyleAltamirano-Fernández, A., Rojas-Palma, A., & Espinoza-Meza, S. (2023). Optimal Management Strategies to Maximize Carbon Capture in Forest Plantations: A Case Study with Pinus radiata D. Don. Forests, 14(1), 82. https://doi.org/10.3390/f14010082








