Response of Common Garden Plant Leaf Traits to Air Pollution in Urban Parks of Suzhou City (China)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Collection and Measurement of Samples
2.3. Sample Collection and Measurement
2.3.1. Measurement of Leaf Structural Traits
2.3.2. Measurement of Leaf Physiological Traits
2.4. Data Processing and Statistics
3. Results
3.1. Air Pollution Gradient Analysis
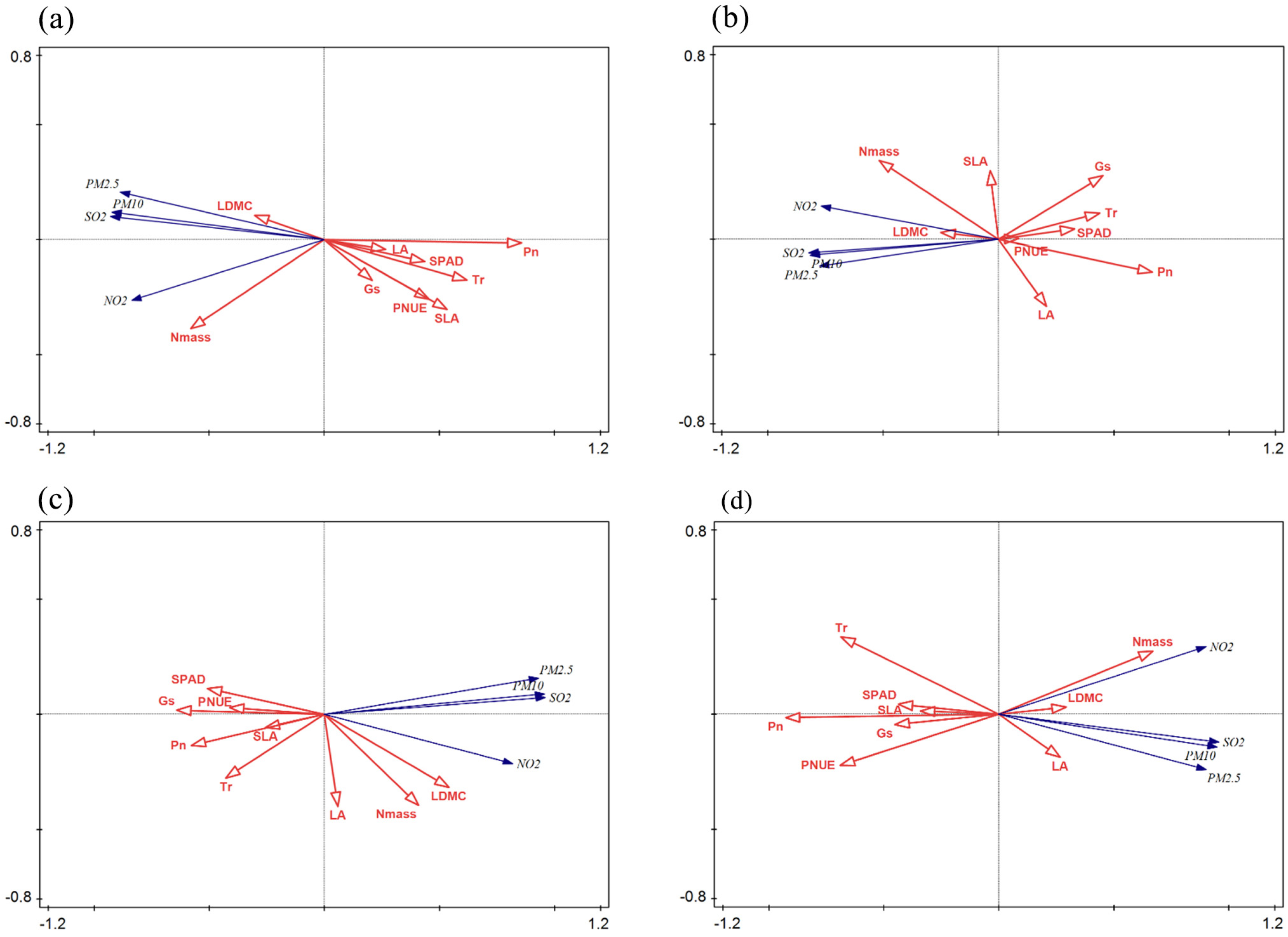
3.2. Relationships between Air Pollutants and Leaf Functional Traits
3.3. Leaf Functional Traits
3.4. Characteristics of Variation in Plant Leaf Traits in Different Gradient Zones
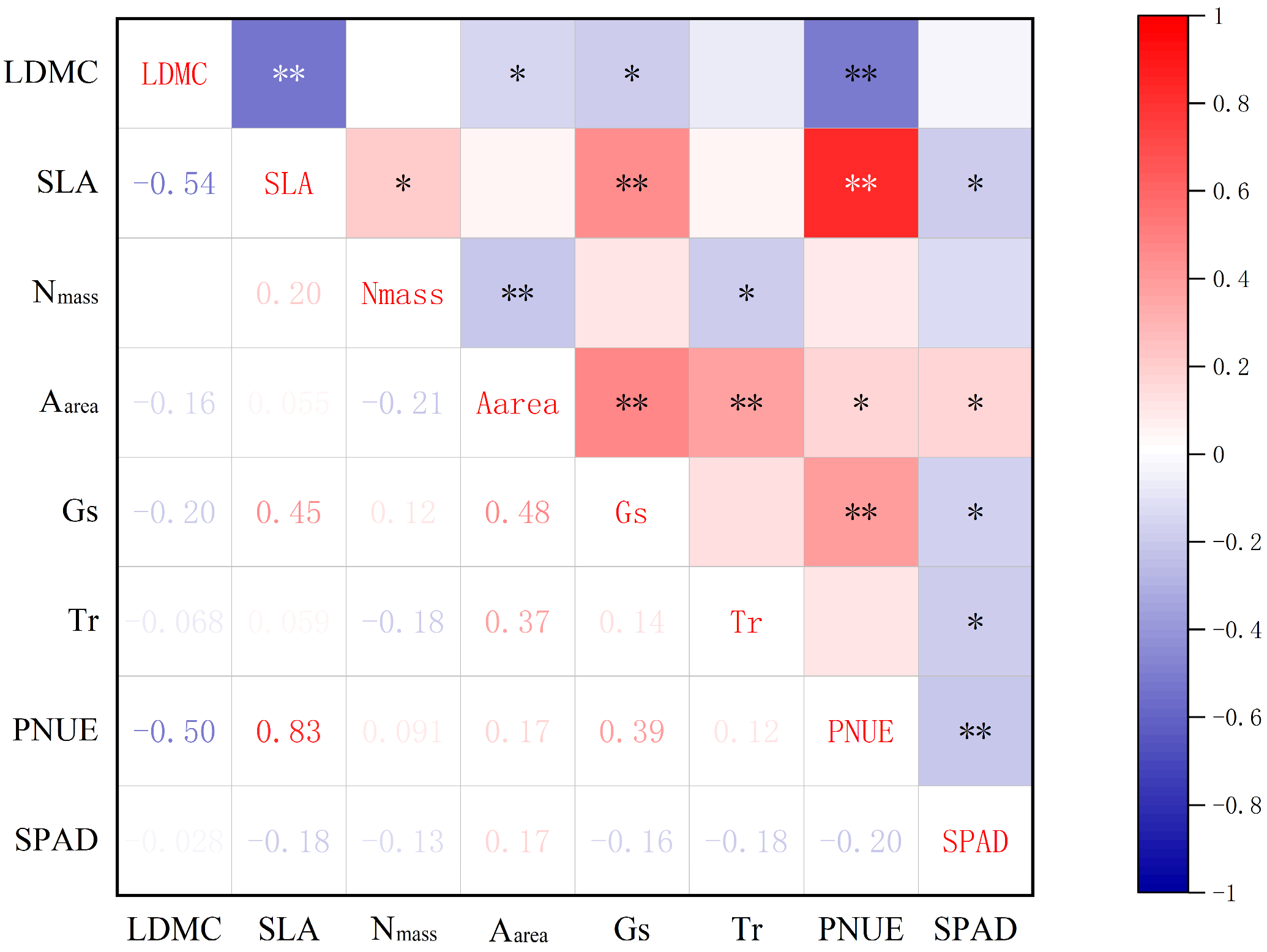
3.5. Correlation Analysis between Leaf Functional Traits
3.5.1. Leaf Traits of Plants
3.5.2. Leaf Traits of Plants of Different Life Forms
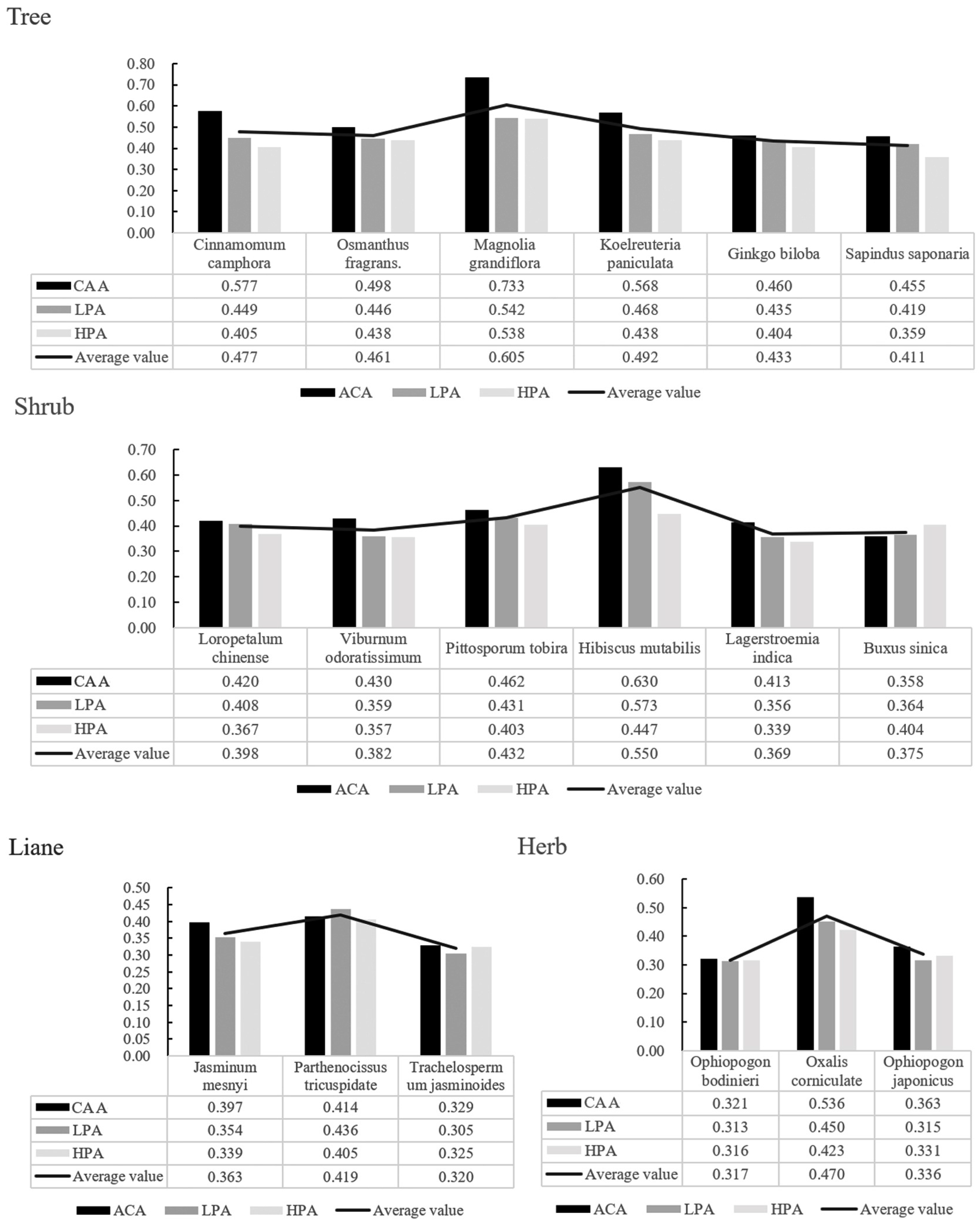
3.6. Fuzzy Affiliation Function Analysis
4. Discussion
4.1. Leaf Functional Traits in Relation to Air Quality
4.2. Relationships between Leaf Traits of Different Life Types
4.3. Analysis of Plants in the Leaf Economic Spectrum
4.4. Research Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, D.; Ramirez, C.D. Air pollution, government pollution regulation, and industrial production in China. J. Syst. Sci. Complex. 2022, 33, 1064–1079. [Google Scholar] [CrossRef]
- Vennemo, H.; Aunan, K.; Lindhjem, H.; Seip, H.M. Environmental pollution in China: Status and trends. Rev. Environ. Econ. Policy 2009, 3, 209–230. [Google Scholar] [CrossRef]
- Zheng, S.; Kahn, M. Understanding China’s urban pollution dynamics. J. Econ. 2013, 51, 731–772. [Google Scholar] [CrossRef]
- Costa, M. Vegetation Dispersion, Interspersion, and Landscape Preference. Front. Psychol. 2022, 13, 771543. [Google Scholar] [CrossRef]
- Temjen, W.; Singh, M.R.; Ajungla, T. Carbon sequestration potential of two Musa cultivars from Mokokchung, Nagaland, North East India along an altitudinal gradient. Curr. Sci. 2022, 123, 925–927. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, E.; Guo, H.; Hu, C.; Zhang, Y.; Yan, D. Comprehensive evaluation of carbon sequestration potential of landscape tree species and its influencing factors analysis: Implications for urban green space management. Carbon Balance Manag. 2023, 18, 17. [Google Scholar] [CrossRef]
- Parr, J.; Sullivan, L.; Chen, B.; Ye, G.; Zheng, W. Carbon bio-sequestration within the phytoliths of economic bamboo species. Glob. Chang. Biol. 2010, 16, 2661–2667. [Google Scholar] [CrossRef]
- Zhao, Y.; Lei, S.; Yang, X.; Gong, C.; Wang, C.; Cheng, W.; Li, H.; She, C. Study on spectral response and estimation of grassland plants dust retention based on hyperspectral data. Remote Sens. 2020, 12, 2019. [Google Scholar] [CrossRef]
- Li, H.; Zhu, X.; Kong, W.; Zheng, M.; Guo, X.; Wang, T. Physiological response of urban greening shrubs to atmospheric particulate matter pollution: An integral view of ecosystem service and plant function. Environ. Exp. Bot. 2023, 213, 105439. [Google Scholar] [CrossRef]
- Xinru, H.E.; Zhang, Y.; Bing, S.U.N.; Pujie, W.E.I.; Die, H.U. Study on leaf epidermis structure and dust-retention ability of five Machilus species. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 1224–1229. [Google Scholar]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From tropics to tundra: Global convergence in plant functioning. Proc. Natl. Acad. Sci. USA 1997, 94, 13730–13734. [Google Scholar] [CrossRef] [PubMed]
- Shipley, B.; De Bello, F.; Cornelissen, J.H.; Laliberté, E.; Laughlin, D.C.; Reich, P.B. Reinforcing loose foundation stones in trait-based plant ecology. Oecologia 2016, 180, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant ecological strategies: Some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef]
- Anderegg, L.D.; Berner, L.T.; Badgley, G.; Sethi, M.L.; Law, B.E.; HilleRisLambers, J. Within-species patterns challenge our understanding of the leaf economics spectrum. Ecol. Lett. 2018, 21, 734–744. [Google Scholar] [CrossRef]
- Osnas, J.L.; Lichstein, J.W.; Reich, P.B.; Pacala, S.W. Global leaf trait relationships: Mass, area, and the leaf economics spectrum. Science 2013, 340, 741–744. [Google Scholar] [CrossRef]
- Le, Y. Comparative analysis of different software PCA for plant trait integration. J. Cent. South For. Univ. Sci. Technol. 2015, 35, 59–64. [Google Scholar]
- Bucci, S.J.; Goldstein, G.; Meinzer, F.C.; Scholz, F.G.; Franco, A.C.; Bustamante, M. Functional convergence in hydraulic architecture and water relations of tropical savanna trees: From leaf to whole plant. Tree Physiol. 2004, 24, 891–899. [Google Scholar] [CrossRef]
- Chen, W. Characteristics of variation in leaf functional traits of 89 common plant species in Guangdong. J. Ecol. 2016, 35, 2101–2109. [Google Scholar]
- Zhu, J. Spectral characteristics of large-leaved boxwood and its leaf functional traits in response to foliar dustfall. Spectrosc. Spectr. Anal. 2020, 40, 1620–1625. [Google Scholar]
- Evans, L.S.; Albury, K.; Jennings, N. Relationships between anatomical characteristics and ozone sensitivity of leaves of several herbaceous dicotyledonous plant species at Great Smoky Mountains National Park. Environ. Exp. Bot. 1996, 36, 413–420. [Google Scholar] [CrossRef]
- Zhang, T.; Shan, L.; Xue, Q. Effect of nitrogen and phosphorus nutrition on water relations in wheat. J. Plant Nutr. Fertil. 2000, 6, 147–151. [Google Scholar]
- Hang, X.; Weng, S.; Yuan, Z. Study on leaf trait characteristics of five garden shrubs in South China and their response to environment. J. Northwest For. Coll. 2014, 29, 243–247. [Google Scholar]
- Zhang, Q. Experimental Study on the Introduction of Raspberries and Blackberries in Ya’an, Sichuan; Sichuan Agricultural University: Ya’an, China, 2004. [Google Scholar]
- Su, X.; Hu, D.; Lin, Z. Effects of atmospheric pollution on chlorophyll fluorescence characteristics of two green plants in Guangzhou city. J. Plant Ecol. 2002, 26, 599–604. [Google Scholar]
- Li, J.; Liu, N.; Ren, H.; Shen, W.J.; Jian, S.G. Ecological adaptations of seven plant species to tropical coral island environments. Ecol. Environ. J. 2016, 25, 790–794. [Google Scholar]
- Gao, C. Research on dust retention effect and physiological characteristics of typical garden plants in Nanning. Res. Soil Water Conserv. 2016, 23, 187–192. [Google Scholar]
- Cai, Y. Study on the Dust Retention Effect of Urban Keystone Tree Species and Their Photosynthetic Characteristics; Fujian University of Agriculture and Forestry: Fuzhou, China, 2010. [Google Scholar]
- Mclaughlin, S.B.; Mcconathy, R.K. Effects of SO2 and O3 on allocation of 14C-labeled photosynthate in Phaseolus vulgaris. Plant Physiol. 1983, 73, 630–635. [Google Scholar] [CrossRef]
- Wu, L.-Y. Effects of sulphur dioxide on photosynthetic and respiratory intensities of crops. Agric. Environ. Prot. 1989, 8, 9–12. [Google Scholar]
- Okano, K.; Totsuka, T.; Fukuzawa, T.; Tazaki, T. Growth responses of plants to various concentrations of nitrogen dioxide. Environ. Pollut. Ser. A Ecol. Biol. 1985, 38, 361–373. [Google Scholar] [CrossRef]
- Sabaratnam, S.; GuPat, G.; Mulehi, C. Effects of nitrogen dioxide on leaf chloroPhyll and Nitrogen content of soybean. Environ. Pollut. 1988, 51, 113–120. [Google Scholar] [CrossRef]
- Sabaratnam, S.; Gupat, G. Effeets of nitrogen dioxide on bioehemieal and Physiologieal Charaeteristies of soybean. Environ. Pollut. 1988, 55, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wang, H.; Zhong, Y. Response of leaf functional traits of nine garden plants to short-term NO2 pollution under artificially controlled conditions. J. Ecol. 2019, 39, 8058–8067. [Google Scholar]
- Liu, M.; Yu, R.; Mu, R. Photosynthetic characteristics of three typical greening tree species at different altitudes in Beishan, Lanzhou. J. Ecol. Environ. 2021, 30, 1943–1951. [Google Scholar]
- Zhu, J.; Yu, Q.; Liu, Y. Plant functional traits and their leaf economic profiles in response to urban thermal environment. J. Beijing For. Univ. 2018, 40, 72–81. [Google Scholar]
- Niinemets, Ü.; Keenan, T.F.; Hallik, L. A worldwide analysis of within-canopy variations in leaf structural, chemical and physiological traits across plant functional types. New Phytol. 2015, 205, 973–993. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, X.; Qu, Y. LES Traits of Garden Plants in Shihu Lake in Response to Water Environment; Northeast Agricultural University: Suzhou, China, 2021. [Google Scholar]
- Zhu, J.; Xu, C.; Qin, G. Response of leaf functional traits of three typical greening plants to air pollution and their leaf economic spectra analyses—A case study in Beijing. J. Cent. South For. Univ. Sci. Technol. 2019, 39, 91–98. [Google Scholar]
- Li, Y.; Zhang, X.; Qu, Y. Response strategies of leaf functional traits of common street trees to air quality in Suzhou. J. Northeast Agric. Univ. 2022, 53, 16–27. [Google Scholar]
- Wang, A.-Y. Particulate Matter Retention Effects and Physiological Responses of Plants under Different Pollution Levels in Zhengzhou City; Henan Agricultural University: Zhengzhou, China, 2023. [Google Scholar]
- Liu, C. Characterisation of volatile organic pollutants in Suqian City during early autumn. Jiangxi Chem. Ind. 2023, 39, 97–100. [Google Scholar]
- Xie, X.; Zhao, Z.; Li, S. Study on pollution characteristics and potential sources of O3 and PM2.5 in Akhdara. J. Environ. Sci. 2023, 1–13. [Google Scholar]
- Yuan, Q. Functional Traits of Plant Leaves and Their Relationship with the Environment in Beishan, Jinhua; Zhejiang Normal University: Jinhua, China, 2020. [Google Scholar]
- He, C.; Li, J.; Guo, M. Changes in photosynthetic properties and water use efficiency of four tree leaves with tree height. J. Ecol. 2008, 28, 3008–3016. [Google Scholar]
- Cheng, J.-F.; Jiang, K.; Shen, Y.-G. Effects of pruning and stemming on the photosynthetic characteristics of leaves of Dicotyledonus pendula. Chin. J. Ecol. Agric. 2009, 17, 469–473. [Google Scholar] [CrossRef]
- Du, M.; Zhang, N. Effects of atmospheric pollution on chlorophyll content of urban green plants. China Environ. Monit. 2007, 23, 86–88. [Google Scholar]
- Li, J.; He, S.; Jing, Y. Physiological and ecological responses of 10 garden plants to air pollution and ecological responses of 10 garden plant species to atmospheric pollution. J. Ecol. Environ. 2020, 29, 1205–1214. [Google Scholar]
- Li, H.; Lv, G.; Jiang, L.; Wang, J. Scale Change and Correlation of Plant Functional Characteristics in the Desert Community of Ebinur Lake. Sustainability 2021, 13, 4983. [Google Scholar] [CrossRef]
- Tang, Q. Variation of Plant Functional T Raits in Subtropical Evergreen Deciduous Broad-Leaved Mixed Forest; China Academy of Forestry Sciences: Beijing, China, 2016. [Google Scholar]
- Garnier, E.; Shipley, B.; Roumet, C.; Laurent, G. A standardized protocol for the determination of specific leaf area and leaf dry matter content. Funct. Ecol. 2001, 15, 688–695. [Google Scholar] [CrossRef]
- Li, S.; Su, P.; Zhang, H. Characteristics of water and functional traits of desert plant leaves and their interrelationships. J. Plant Physiol. 2013, 49, 153–160. [Google Scholar]
- Wang, R.; Ji, X.; Liu, X. Diversity analysis of leaf and photosynthetic characteristics among millets germplasm in different ecological zones. J. Shanxi Agric. Univ. Nat. Sci. Ed. 2014, 34, 97–102. [Google Scholar]
- Warren, C.R.; Adams, M.A. Evergreen trees do not maximize instantaneous photosynthesis. Trends Plant Sci. 2004, 9, 270–274. [Google Scholar] [CrossRef]
- Poorter, L.; Wright, S.J.; Paz, H.; Ackerly, D.D.; Condit, R.; Ibarra-Manríquez, G.; Harms, K.E.; Licona, J.C.; Martínez-Ramos, M.; Mazer, S.J. Are functional traits good predictors of demographic rates? Evidence from five neotropical forests. Ecology 2008, 89, 1908–1920. [Google Scholar] [CrossRef]
- Takashima, T.; Hikosaka, K.; Hirose, T. Photosynthesis or persistence: Nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ. 2004, 27, 1047–1054. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, J.; Wang, B. Study on summer photosynthetic characteristics and carbon sequestration and oxygen release capacity of major greening tree species in Shanghai. J. Anhui Agric. Univ. 2016, 43, 94–101. [Google Scholar]
- Song, H.; Yu, H.; Chen, Y. Leaf economic spectrum of different functional plants in Beijing Botanical Garden. J. Appl. Ecol. 2016, 27, 1861–1869. [Google Scholar]
- Chen, Y.; Xu, Z. Progress in the study of economic spectrum of plant leaves. J. Plant Ecol. 2014, 38, 1135–1153. [Google Scholar]
- Pérez-Ramos, I.M.; Roumet, C.; Cruz, P.; Blanchard, A.; Autran, P.; Garnier, E. Evidence for a ‘plant community economics spectrum’ driven by nutrient and water limitations in a Mediterranean rangeland of southern France. J. Ecol. 2012, 100, 1315–1327. [Google Scholar] [CrossRef]
- Onoda, Y.; Westoby, M.; Adler, P.B.; Choong, A.M.F.; Clissold, F.J.; Cornelissen, J.H.C.; Díaz, S.; Dominy, N.J.; Elgart, A.; Enrico, L. Global patterns of leaf mechanical properties. Ecol. Lett. 2011, 14, 301–312. [Google Scholar] [CrossRef]
- Peco, B.; Navarro, E.; Carmona, C.P.; Medina, N.G.; Marques, M.J. Effects of grazing abandonment on soil multifunctionality: The role of plant functional traits. Agric. Ecosyst. Environ. 2017, 249, 215–225. [Google Scholar] [CrossRef]
- Medvigy, D.; Jeong, S.J.; Clark, K.L.; Skowronski, N.S.; Schäfer, K.V. Effects of seasonal variation of photosynthetic capacity on the carbon fluxes of a temperate deciduous forest. J. Geophys. Res. Biogeosci. 2013, 118, 1703–1714. [Google Scholar] [CrossRef]



| Number | Plant-Life | Species | Familia | Genus |
|---|---|---|---|---|
| 1 | Tree | Cinnamomum camphora (L.) J.Pres | Lauraceae | Camphora |
| 2 | Osmanthus fragrans (Thunb.) Lour | Oleaceae | Osmanthus | |
| 3 | Magnolia grandiflora L. | Magnoliaceae | Magnolia | |
| 4 | Koelreuteria paniculata Laxm. | Sapindaceae | Koelreuteria | |
| 5 | Ginkgo biloba L. | Ginkgoaceae | Ginkgo | |
| 6 | Sapindus saponaria L. | Sapindaceae | Sapindus | |
| 7 | Shrub | Loropetalum chinense (R. Br.) Oliver | Hamamelidaceae | Loropetalum |
| 8 | Viburnum odoratissimum Ker Gawl. | Viburnaceae | Viburnum | |
| 9 | Pittosporum tobira (Thunb.) W. T. Aiton | Pittosporaceae | Pittosporum | |
| 10 | Hibiscus mutabilis L. | Malvaceae | Hibiscus | |
| 11 | Buxus sinica (Rehder & E. H. Wilson) M. Cheng | Buxaceae | Buxus | |
| 12 | Lagerstroemia indica L. | Lythraceae | Lagerstroemia | |
| 13 | Herb | Ophiopogon bodinieri H. Lév. | Asparagaceae | Ophiopogon |
| 14 | Oxalis corniculata L. | Oxalidaceae | Oxalis | |
| 15 | Ophiopogon japonicus (L. f.) Ker Gawl. | Asparagaceae | Ophiopogon | |
| 16 | Liane | Jasminum mesnyi Hance | Oleaceae | Jasminum |
| 17 | Parthenocissus tricuspidata (Siebold & Zucc.) Planch. | Vitaceae | Parthenocissus | |
| 18 | Trachelospermum jasminoides (Lindl.) Lem. | Apocynaceae | Trachelospermum |
| Area | SO2 (μg/m3) | NO2 (μg/m3) | PM10 (μg/m3) | PM2.5 (μg/m3) | AQI | |
|---|---|---|---|---|---|---|
| CAA | Average value | 4.87 | 27.00 | 54.00 | 11.00 | 46.00 |
| Min | 1.00 | 5.00 | 8.00 | 3.00 | 18.00 | |
| Max | 17.00 | 84.00 | 94.00 | 63.00 | 170.00 | |
| LPA | Average value | 7.93 | 28.90 | 62.20 | 25.00 | 79.00 |
| Min | 3.00 | 4.00 | 10.00 | 4.00 | 18.00 | |
| Max | 15.00 | 105.00 | 99.00 | 66.00 | 169.00 | |
| HPA | Average value | 9.37 | 43.80 | 65.53 | 27.37 | 126.00 |
| Min | 6.00 | 6.00 | 7.00 | 5.00 | 15.00 | |
| Max | 32.00 | 70.00 | 164.00 | 77.00 | 500.00 | |
| Area | Plant-Life | LDMC | SLA | Aarea | Gs | Tr | Nmass | PNUE | SPAD |
|---|---|---|---|---|---|---|---|---|---|
| CAA | Tree | 0.433 ± 0.071 a | 10,013.975 ± 2606.067 b | 16.48 ± 3.705 b | 0.006 ± 0.004 a | 0.62 ± 0.952 a | 2.586 ± 0.395 b | 2.437 ± 1.148 b | 47.023 ± 9.302 a |
| Shrub | 0.375 ± 0.047 a | 6659.786 ± 2683.895 b | 20.669 ± 2.898 a | 0.013 ± 0.008 ab | 0.762 ± 0.86 a | 2.058 ± 0.583 c | 8.012 ± 6.319 b | 51.42 ± 12.868 a | |
| Herb | 0.296 ± 0.092 b | 12,045.93 ± 7313.533 b | 18.767 ± 3.335 ab | 0.008 ± 0.002 bc | 0.27 ± 0.104 a | 2.68 ± 0.449 b | 17.039 ± 3.079 b | 52.994 ± 8.389 a | |
| Vine | 0.248 ± 0.103 b | 41,495.877 ± 45,318.948 a | 17.148 ± 4.436 b | 0.015 ± 0.014 a | 0.443 ± 0.329 a | 3.105 ± 0.251 a | 35.425 ± 41.661 a | 51.068 ± 8.449 a | |
| LPA | Tree | 0.512 ± 0.105 a | 7254.972 ± 1575.539 b | 14.734 ± 2.957 b | 0.005 ± 0.003 b | 0.162 ± 0.056 b | 2.293 ± 0.239 c | 1.918 ± 1.084 c | 45.018 ± 6.056 bc |
| Shrub | 0.385 ± 0.053 b | 5740.056 ± 3100.166 b | 17.884 ± 2.592 a | 0.009 ± 0.005 ab | 0.577 ± 0.614 a | 2.111 ± 0.476 c | 8.562 ± 8.643 b | 48.891 ± 11.567 ab | |
| Herb | 0.381 ± 0.038 b | 8010.565 ± 5156.448 b | 12.901 ± 1.469 b | 0.006 ± 0.003 b | 0.177 ± 0.076 b | 3.361 ± 0.106 a | 6.29 ± 3.896 bc | 53.133 ± 2.204 a | |
| Vine | 0.253 ± 0.084 c | 27,374.194 ± 27,628.919 a | 12.934 ± 1.397 b | 0.011 ± 0.01 a | 0.201 ± 0.089 b | 2.781 ± 0.781 b | 17.138 ± 11.502 a | 40.67 ± 4.988 c | |
| HPA | Tree | 0.491 ± 0.151 a | 6822.454 ± 2559.228 b | 12.926 ± 3.033 b | 0.006 ± 0.003 b | 0.113 ± 0.067 b | 2.466 ± 0.227 c | 1.896 ± 1.17 c | 42.611 ± 9.565 ab |
| Shrub | 0.381 ± 0.136 b | 7111.725 ± 8816.375 b | 18.764 ± 5.07 a | 0.013 ± 0.013 a | 0.449 ± 0.425 a | 2.697 ± 1.01 bc | 9.002 ± 12.428 a | 47.066 ± 12.162 a | |
| Herb | 0.495 ± 0.092 a | 7959.507 ± 4706.202 b | 13.042 ± 2.754 b | 0.008 ± 0.005 ab | 0.123 ± 0.047 b | 3.868 ± 0.749 a | 4.121 ± 3.28 ab | 49.276 ± 1.217 a | |
| Vine | 0.279 ± 0.109 b | 20,180.6 ± 14,521.167 a | 12.046 ± 1.263 b | 0.008 ± 0.005 ab | 0.179 ± 0.135 b | 3.222 ± 0.825 b | 10.265 ± 7.399 a | 35.589 ± 6.299 b |
| Area | Plant-Life | LDMC | SLA | Aarea | Gs | Tr | Nmass | PNUE | SPAD |
|---|---|---|---|---|---|---|---|---|---|
| CAA | Tree | 0.164 | 0.260 | 0.225 | 0.667 | 1.535 | 0.153 | 0.471 | 0.198 |
| Shrub | 0.125 | 0.403 | 0.140 | 0.615 | 1.129 | 0.283 | 0.789 | 0.250 | |
| Herb | 0.311 | 0.607 | 0.178 | 0.250 | 0.385 | 0.168 | 0.181 | 0.158 | |
| Liane | 0.415 | 1.092 | 0.259 | 0.933 | 0.743 | 0.081 | 1.176 | 0.165 | |
| LPA | Tree | 0.206 | 0.217 | 0.201 | 0.620 | 0.344 | 0.104 | 0.565 | 0.135 |
| Shrub | 0.137 | 0.540 | 0.145 | 0.604 | 1.065 | 0.225 | 1.009 | 0.237 | |
| Herb | 0.099 | 0.644 | 0.114 | 0.417 | 0.431 | 0.032 | 0.619 | 0.041 | |
| Liane | 0.332 | 1.009 | 0.108 | 0.853 | 0.444 | 0.281 | 0.671 | 0.123 | |
| HPA | Tree | 0.308 | 0.375 | 0.235 | 0.500 | 0.593 | 0.092 | 0.617 | 0.224 |
| Shrub | 0.357 | 1.240 | 0.270 | 1.000 | 0.947 | 0.374 | 1.381 | 0.258 | |
| Herb | 0.186 | 0.591 | 0.211 | 0.625 | 0.382 | 0.194 | 0.796 | 0.025 | |
| Liane | 0.391 | 0.720 | 0.105 | 0.625 | 0.754 | 0.256 | 0.721 | 0.177 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Zhang, X.; Qu, Y.; Gao, F.; Li, Y. Response of Common Garden Plant Leaf Traits to Air Pollution in Urban Parks of Suzhou City (China). Forests 2023, 14, 2253. https://doi.org/10.3390/f14112253
Yang Z, Zhang X, Qu Y, Gao F, Li Y. Response of Common Garden Plant Leaf Traits to Air Pollution in Urban Parks of Suzhou City (China). Forests. 2023; 14(11):2253. https://doi.org/10.3390/f14112253
Chicago/Turabian StyleYang, Zhiyu, Xing Zhang, Yanting Qu, Fei Gao, and Yutong Li. 2023. "Response of Common Garden Plant Leaf Traits to Air Pollution in Urban Parks of Suzhou City (China)" Forests 14, no. 11: 2253. https://doi.org/10.3390/f14112253





