Different Adaptive Potential of Norway Spruce Ecotypes in Response to Climate Change in Czech Long-Term Lowland Experiment
Abstract
:1. Introduction
2. Methodology
2.1. Study Area
2.2. Plant Material
2.3. Research Plot Description
2.4. Data Collection
2.5. Data Analysis
2.5.1. Dendrocrhonological Data Processing and Analysis
2.5.2. Tree-Ring Analysis with Precipitation and Temperature
2.5.3. Heritability Data Processing
2.5.4. Stand Structure and Biomass Analysis
3. Results
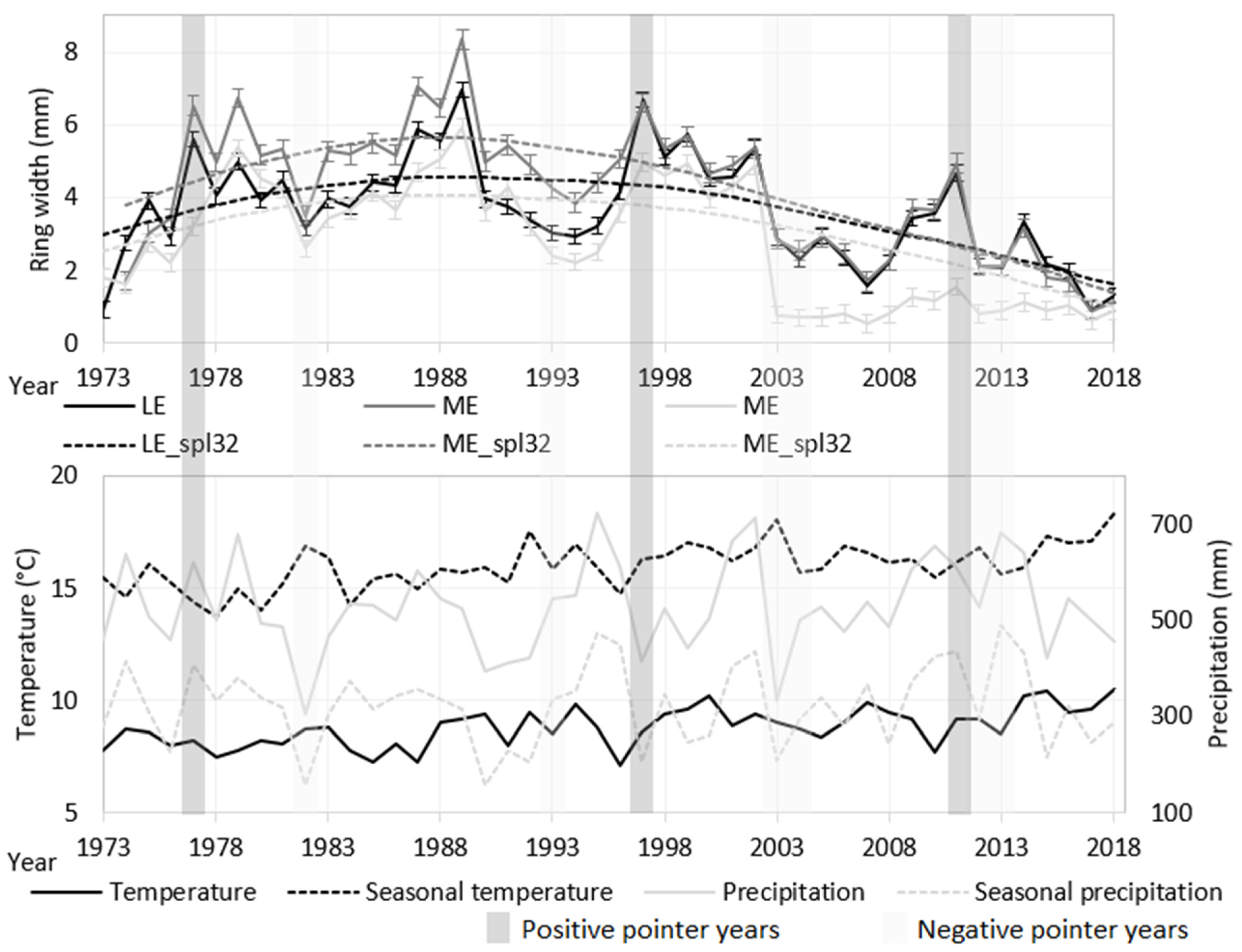
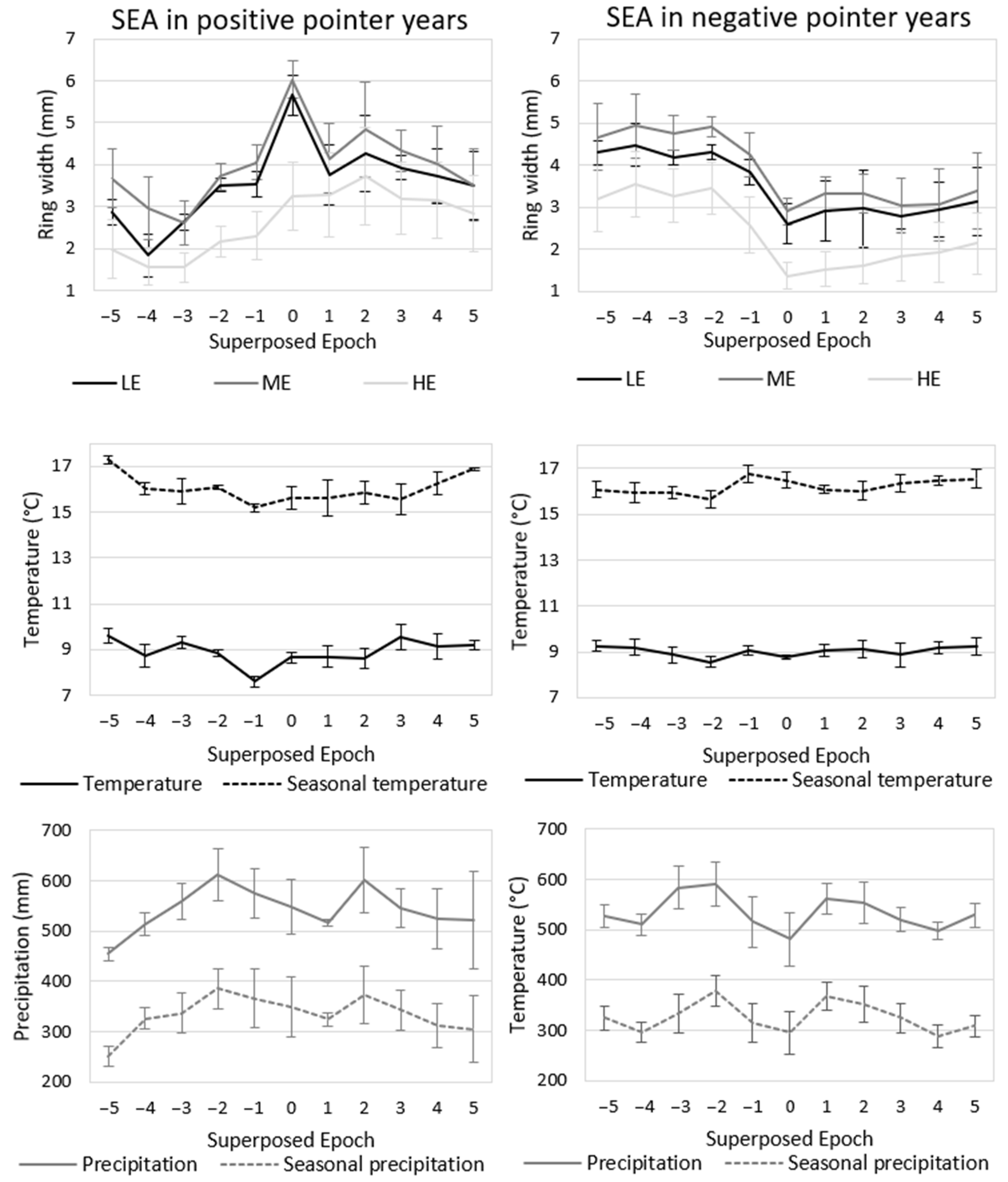
3.1. Tree-Ring Growth of Norway Spruce Ecotypes
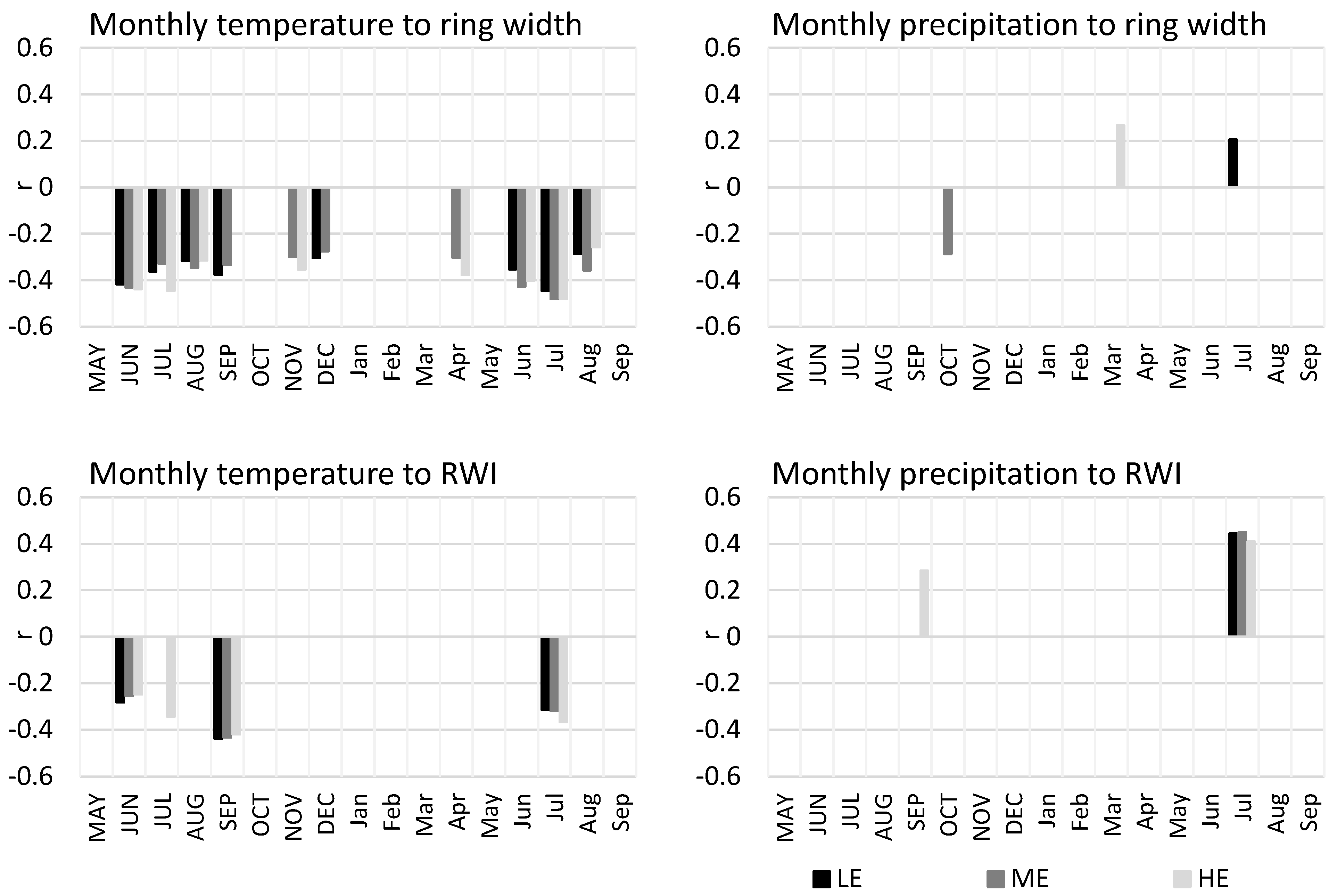
3.2. Temperature and Precipitation in Comparison to Ecotype Tree-Ring Growth
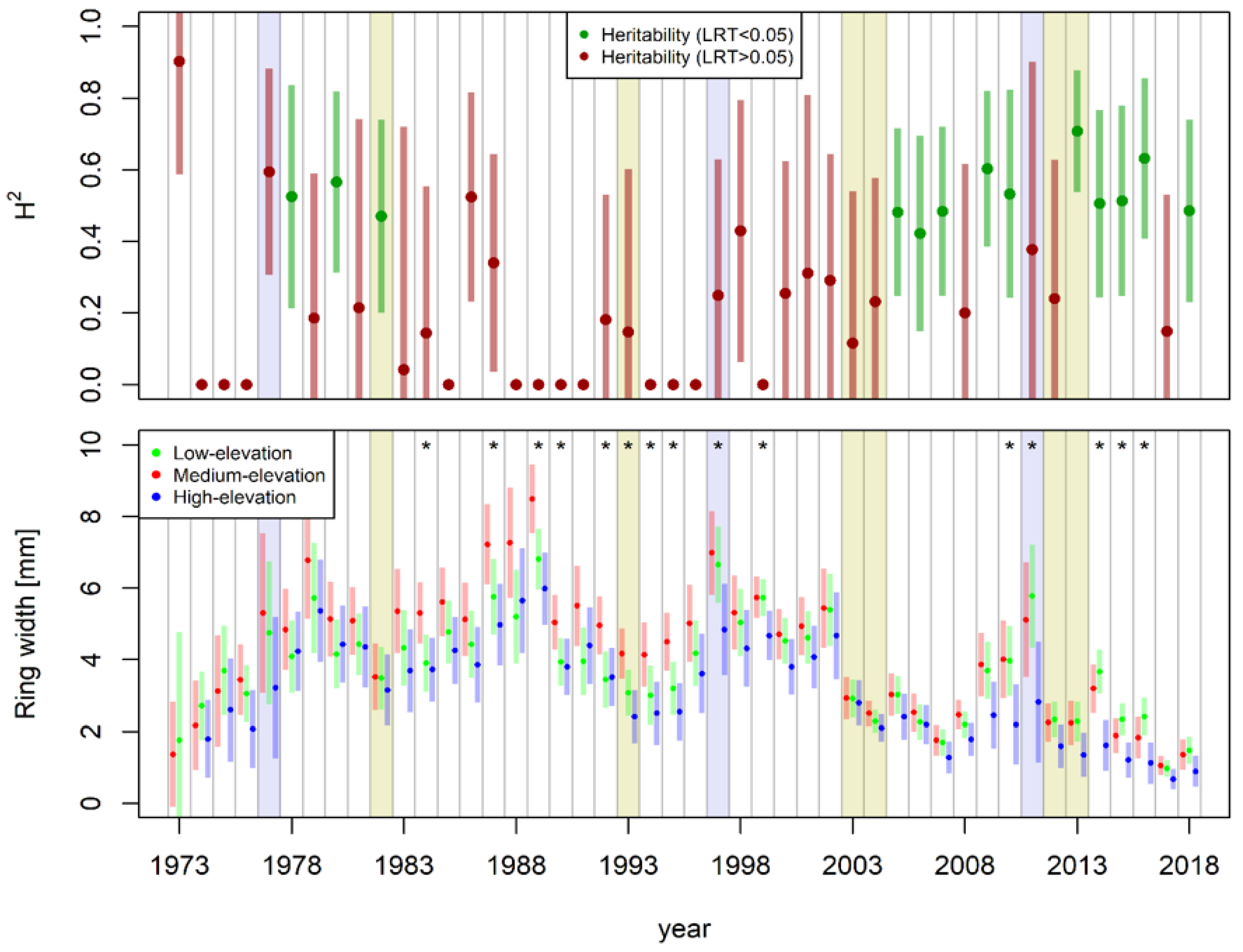
3.3. Clonal Heritability and Ecotypic Variation
3.4. Production Potential
4. Discussion
4.1. Limitations
4.2. Growth Conditions and Ecotype Response of Norway Spruce on the Climate
4.3. Clonal Heritability of Annual Ring Widths
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spiecker, H. Silvicultural management in maintaining biodiversity and resistance of forests in Europe—Temperate zone. J. Environ. Manag. 2003, 67, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture. MAF Zpráva o Stavu Lesa a Lesního Hospdářství v České Republice v Roce 2021; Ministry of Agriculture: Prague, Czech Republic, 2021.
- Hlásny, T.; Zimová, S.; Merganičová, K.; Štěpánek, P.; Modlinger, R.; Turčáni, M. Devastating outbreak of bark beetles in the Czech Republic: Drivers, impacts, and management implications. For. Ecol. Manag. 2021, 490, 119075. [Google Scholar] [CrossRef]
- Tumajer, J.; Altman, J.; Štěpánek, P.; Treml, V.; Doležal, J.; Cienciala, E. Increasing moisture limitation of Norway spruce in Central Europe revealed by forward modelling of tree growth in tree-ring network. Agric. For. Meteorol. 2017, 247, 56–64. [Google Scholar] [CrossRef]
- Ashraf, M.I.; Bourque, C.P.A.; MacLean, D.A.; Erdle, T.; Meng, F.R. Estimation of potential impacts of climate change on growth and yield of temperate tree species. Mitig. Adapt. Strateg. Glob. Chang. 2015, 20, 159–178. [Google Scholar] [CrossRef]
- Nováková, M.H.; Edwards-Jonášová, M. Restoration of central-european mountain norway spruce forest 15 years after natural and anthropogenic disturbance. For. Ecol. Manag. 2015, 344, 120–130. [Google Scholar] [CrossRef]
- Čermák, P.; Kolář, T.; Žid, T.; Trnka, M.; Rybníček, M. Norway spruce responses to drought forcing in areas affected by forest decline. For. Syst. 2019, 28, e016. [Google Scholar] [CrossRef]
- Hartl-meier, C.; Zang, C.; Büntgen, U.; Esper, J.; Rothe, A.; Göttlein, A.; Dirnböck, T.; Treydte, K. Uniform climate sensitivity in tree-ring stable isotopes across species and sites in a mid-latitude temperate forest. Tree Physiol. 2018, 35, 4–15. [Google Scholar] [CrossRef]
- Šustek, Z.; Vido, J.; Škvareninová, J.; Škvarenina, J.; Šurda, P. Drought impact on ground beetle assemblages (Coleoptera, Carabidae) in Norway spruce forests with different management after windstorm damage—A case study from Tatra Mts. (Slovakia). J. Hydrol. Hydromech. 2017, 65, 333–342. [Google Scholar] [CrossRef]
- Thiele, J.C.; Nuske, R.S.; Ahrends, B.; Panferov, O.; Albert, M.; Staupendahl, K.; Junghans, U.; Jansen, M.; Saborowski, J. Climate change impact assessment—A simulation experiment with Norway spruce for a forest district in Central Europe. Ecol. Modell. 2017, 346, 30–47. [Google Scholar] [CrossRef]
- Hlásny, T.; Barka, I.; Roessiger, J.; Kulla, L.; Trombik, J.; Sarvašová, Z.; Bucha, T.; Kovalčík, M.; Čihák, T. Conversion of Norway spruce forests in the face of climate change: A case study in Central Europe. Eur. J. For. Res. 2017, 136, 1013–1028. [Google Scholar] [CrossRef]
- Šrámek, V.; Hellebrandová, K.N.; Fadrhonsová, V. Interception and soil water relation in Norway spruce stands of different age during the contrasting vegetation seasons of 2017 and 2018. J. For. Sci. 2019, 65, 51–60. [Google Scholar] [CrossRef]
- Vacek, Z.; Cukor, J.; Linda, R.; Vacek, S.; Šimůnek, V.; Brichta, J.; Gallo, J.; Prokůpková, A. Bark stripping, the crucial factor affecting stem rot development and timber production of Norway spruce forests in Central Europe. For. Ecol. Manag. 2020, 474, 118360. [Google Scholar] [CrossRef]
- Krisans, O.; Saleniece, R.; Rust, S.; Elferts, D.; Kapostins, R.; Jansons, A.; Matisons, R. Effect of Bark-Stripping on Mechanical Stability of Norway Spruce. Forests 2020, 11, 357. [Google Scholar] [CrossRef]
- Kozak, I.; Parpan, T. Forecasting drying up of spruce forests in Transcarpathia (Ukraine) using the FORKOME model. J. For. Sci. 2019, 65, 209–217. [Google Scholar] [CrossRef]
- Vacek, Z.; Vacek, S.; Cukor, J. European forests under global climate change: Review of tree growth processes, crises and management strategies. J. Environ. Manag. 2023, 332, 117353. [Google Scholar] [CrossRef]
- Holuša, J.; Lubojacký, J.; Čurn, V.; Tonka, T.; Lukášová, K.; Horák, J. Combined effects of drought stress and Armillaria infection on tree mortality in Norway spruce plantations. For. Ecol. Manag. 2018, 427, 434–445. [Google Scholar] [CrossRef]
- Marini, L.; Økland, B.; Jönsson, A.M.; Bentz, B.; Carroll, A.; Forster, B.; Grégoire, J.C.; Hurling, R.; Nageleisen, L.M.; Netherer, S.; et al. Climate drivers of bark beetle outbreak dynamics in Norway spruce forests. Ecography 2017, 40, 1426–1435. [Google Scholar] [CrossRef]
- Netherer, S.; Panassiti, B.; Pennerstorfer, J.; Matthews, B. Acute Drought Is an Important Driver of Bark Beetle Infestation in Austrian Norway Spruce Stands. Front. For. Glob. Chang. 2019, 2, 39. [Google Scholar] [CrossRef]
- Morgenstern, E.K. Geographic Variation in Forest Trees: Genetic Basis and Application of Knowledge in Silviculture; UBC Press: Vancouver, BC, Canada, 1996. [Google Scholar]
- Hejtmánek, J.; Stejskal, J.; Čepl, J.; Lhotáková, Z.; Korecký, J.; Krejzková, A.; Dvořák, J.; Gezan, S.A. Revealing the Complex Relationship Among Hyperspectral Reflectance, Photosynthetic Pigments, and Growth in Norway Spruce Ecotypes. Front. Plant Sci. 2022, 13, 721064. [Google Scholar] [CrossRef]
- Čepl, J.; Stejskal, J.; Korecký, J.; Hejtmánek, J.; Faltinová, Z.; Lstibůrek, M.; Gezan, S. The dehydrins gene expression differs across ecotypes in Norway spruce and relates to weather fluctuations. Sci. Rep. 2020, 10, 20789. [Google Scholar] [CrossRef]
- Hynek, V. Breeding programmes of Norway spruce Picea abies (L.) Karst. in the Czech Republic. Dendrobiology 2004, 51, 17–21. [Google Scholar]
- Vacek, S. Morfologická proměnlivost autochtonních smrkových populací v Krkonoších. [Morphological variability of autochthonous Norway spruce populations in the Krkonoše Mts]. Lesnictví 1983, 29, 265–284. [Google Scholar]
- Roudná, M. Morfologická proměnlivost původních populací smrku v různých oblastech Československa. Rozpr. Československé Akad. Věd Řada Mat. přírodních Věd 1972, 82, 98. [Google Scholar]
- Fanta, J. Ekologický a hospodářský význam fenotypové proměnlivosti původních populací smrku ztepilého (Picea excelsa Link) v Krkonoších. Lesnictví 1976, 22, 965–976. [Google Scholar]
- Vacek, S. Morfologická proměnlivost šišek autochtonního smrku v Krkonoších. [Morphological variability of cones of the autochthonous Norway spruce in the Krkonoše Mts]. Lesnictví 1983, 29, 755–772. [Google Scholar]
- Kovářová, M.; Vacek, S. Mountain Norway spruce forests: Needle supply and its nutrient content. J. For. Sci. 2003, 49, 327–332. [Google Scholar] [CrossRef]
- Vacek, S. Symptomy poškození smrku obecného (Picea abies /L./Karst.). [Damage symptoms of Norway spruce (Picea abies /L./ Karst.)]. Opera Corcon. 1992, 29, 183–189. [Google Scholar]
- Šrámek, V.; Buriánek, V.; Čihák, T.; Dušek, D.; Frýdl, J.; Kacálek, D.; Knížek, M.; Kotrla, P.; Lubojacký, J.; Hellebrandová Neudertová, K.; et al. Ekologické Limity a Produkční Efekty Pěstování Smrku Ztepilého v Nižších Polohách—Analýza Rizik a Produkčních Možností Populací Chlumního Smrku; Lesy České republiky, s. p: Hradec Králové, Czech Republic, 2018.
- Putalová, T.; Vacek, Z.; Vacek, S.; Štefančík, I.; Bulušek, D.; Král, J. Tree-ring widths as an indicator of air pollution stress and climate conditions in different Norway spruce forest stands in the Krkonoše Mts. Cent. Eur. For. J. 2019, 65, 21–33. [Google Scholar] [CrossRef]
- Svoboda, P. Lesní Dřeviny a Jejich Porosty. Část I; Státní Zemědělské Nakladatelství: Prague, Czech Republic, 1953.
- Fanta, J. Morphologische Variabilität der Fichte und Grundzüge der genetischen Rekonstruktion der Gebirgsfichtenwälder im Krkonoše-Nationalpark (ČSSR). Archiv. Naturschutz Landsch. Forsch. 1974, 14, 179–200. [Google Scholar]
- Čáp, J.; Novotný, P.; Cvrčková, H.; Machová, P.; Fulín, M.; Frýdl, J.; Dostál, J.; Buriánek, V.; Beran, F.; Lefnar, R.; et al. Genetická charakterizace významných regionálních populací smrku ztepilého v České republice. Specializovaná mapa s odborným obsahem (aktualizované vydání). Lesn. Průvodce 2017, 2. [Google Scholar]
- Fanta, J. Morfologická proměnlivost původních populací smrku v Krkonoších. Lesnictví 1976, 22, 415–434. [Google Scholar]
- Poleno, Z.; Vacek, S.; Podrázský, V.; Remeš, J.; Štefančík, I.; Mikeska, M.; Kobliha, J.; Kupka, I.; Malík, V.; Turčáni, M.; et al. Pěstování Lesů III. Praktické Postupy Pěstování Lesů. [Silviculture III. Practical Methods in Silviculture]; Lesnická Práce, s.r.o.: Kostelec nad Černými Lesy, Czech Republic, 2009. [Google Scholar]
- Vacek, S.; Hejcmanová, P.; Hejcman, M. Vegetative reproduction of Picea abies by artificial layering at the ecotone of the alpine timberline in the Giant (KrkonoŠe) Mountains, Czech Republic. For. Ecol. Manag. 2012, 263, 199–207. [Google Scholar] [CrossRef]
- Vacek, S.; Bílek, L.; Schwarz, O.; Hejcmanová, P.; Mikeska, M. Effect of Air Pollution on the Health Status of Spruce Stands Effect of Air Pollution on the Health Status of Spruce Stands. Mt. Res. Dev. 2013, 33, 40–50. [Google Scholar] [CrossRef]
- Gebauer, R.; Volařík, D.; Urban, J.; Børja, I.; Nagy, N.E.; Eldhuset, T.D.; Krokene, P. Effect of thinning on anatomical adaptations of Norway spruce needles. Tree Physiol. 2011, 31, 1103–1113. [Google Scholar] [CrossRef]
- Kašpar, J.; Hošek, J.; Treml, V. How wind affects growth in treeline Picea abies. Alp. Bot. 2017, 127, 109–120. [Google Scholar] [CrossRef]
- De La Torre, A.R.; Wilhite, B.; Puiu, D.; Clair, J.B.S.; Crepeau, M.W.; Salzberg, S.L.; Langley, C.H.; Allen, B.; Neale, D.B. Dissecting the polygenic basis of cold adaptation using genome-wide association of traits and environmental data in douglas-fir. Genes 2021, 12, 110. [Google Scholar] [CrossRef]
- Popović, V.; Nikolić, B.; Lučić, A.; Rakonjac, L.; Šešlija Jovanović, D.; Miljković, D. Morpho-anatomical trait variability of the Norway spruce (Picea abies (L.) Karst.) needles in natural populations along elevational diversity gradient. Trees 2022, 36, 1131–1147. [Google Scholar] [CrossRef]
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; Wang, T.; Curtis-McLane, S. Adaptation, migration or extirpation: Climate change outcomes for tree populations. Evol. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Webb, J.A.; Stewardson, M.J. Modelling Impacts of Environmental Water on Vegetation of a Semi-Arid Floodplain–Lakes System Using 30-Year Landsat Data. Remote Sens. 2022, 14, 708. [Google Scholar] [CrossRef]
- Da Ros, L.M.; Thomas, B.R.; Mansfield, S.D. Wood quality trait associations with climate: Room for improvement in two northern commercial tree species? For. Ecol. Manag. 2021, 497, 119492. [Google Scholar] [CrossRef]
- Sofron, J. Přirozené smrčiny západních a jihozápadních Čech. Stud. ČSAV 1981, 7, 1–127. [Google Scholar]
- Vacek, S.; Hůnová, I.; Vacek, Z.; Hejcmanová, P.; Podrázský, V.; Král, J.; Putalová, T.; Moser, W.K. Effects of air pollution and climatic factors on Norway spruce forests in the Orlické hory Mts. (Czech Republic), 1979–2014. Eur. J. For. Res. 2015, 134, 1127–1142. [Google Scholar] [CrossRef]
- Lhotáková, Z.; Kopačková-Strnadová, V.; Oulehle, F.; Homolová, L.; Neuwirthová, E.; Švik, M.; Janoutová, R.; Albrechtová, J. Foliage biophysical trait prediction from laboratory spectra in norway spruce is more affected by needle age than by site soil conditions. Remote Sens. 2021, 13, 391. [Google Scholar] [CrossRef]
- Köppen, W. Das Geographische System der Klimate, Handbuch der Klimatologie; Gebrüder Borntraeger: Berlin, Germany, 1936. [Google Scholar]
- ČHMÚ Czech Hydrometeorological Institute. Available online: http://portal.chmi.cz/historicka-data/pocasi/uzemni-srazky (accessed on 1 October 2022).
- Šindelář, J. Klonové Archivy Smrku Ztepilého Picea abies Karst. na PLO Zbraslav-Strnady–Polesí Jíloviště; VÚLHM: Jíloviště-Strnady, Czech Republic, 1975. [Google Scholar]
- Rinntech TSAP-WIN: Time Series Analysis and Presentation for Dendrochronology and Related Applications. 2010. Available online: https://rinntech.info/products/tsap-win/ (accessed on 2 February 2023).
- Fabrika, M.; Ďurský, J. Algorithms and software solution of thinning models for SIBYLA growth simulator. J. For. Sci. 2005, 51, 431–445. [Google Scholar] [CrossRef]
- Petráš, R.; Pajtík, J. Sústava česko-slovenských objemových tabuliek drevín. For. J. Lesn. Časopis 1991, 37, 49–56. [Google Scholar]
- Sharma, R.P.; Vacek, Z.; Vacek, S. Modeling individual tree height to diameter ratio for Norway spruce and European beech in Czech Republic. Trees 2016, 30, 1969–1982. [Google Scholar] [CrossRef]
- Bublinec, E. Koncentrácia, Akumulácia a Kolobeh Prvkov v Bukovom a Smrekovom Ekosystéme; Acata Dendrobiologica, Ed.; Ústav Ekológie Lesa Slovenskej Akadémie vied Zvolen: Zvolen, Slovakia, 1994. [Google Scholar]
- Petráš, R.; Košút, M.; Oszlányi, J. Listová biomasa stromov smreka, borovice a buka. Lesn. Časopis 1985, 31, 121–135. [Google Scholar]
- Ledermann, T.; Neumann, M. Ergebnisse vorläufiger Untersuchungen zur Erstellung von Biomassefunktionen aus Daten alter Dauerversuchsflächen. In Mai, Freising; Deutscher Verband Forstlicher Forschungsanstalten: Freiburg, Germany, 2005; pp. 143–152. [Google Scholar]
- Seifert, T.; Schuck, J.; Block, J.; Pretzsch, H. Simulation von Biomasse- und Nährstoffgehalt von Waldbäumen. Dtsch. Verband Forstl. Forschungsanstalten. Sekt Ertragskd 2006, 29, 208–223. [Google Scholar]
- Drexhage, M.; Colin, F. Estimating root system biomass from breast-height diameters. Forestry 2001, 74, 491–497. [Google Scholar] [CrossRef]
- Van der Maaten-Theunissen, M.; van der Maaten, E.; Jetsckhe, G.; Trouillier, M.; van der Maaten-Theunissen, M.M. Package ‘pointRes’, Analyzing Pointer Years and Components of Resilience, Version 2.0.2; CRAN. Available online: https://cran.r-project.org/web/packages/pointRes/pointRes.pdf (accessed on 2 February 2023).
- Team R Core. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Bunn, A.G. Statistical and visual crossdating in R using the dplR library. Dendrochronologia 2010, 28, 251–258. [Google Scholar] [CrossRef]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Bunn, A.; Mikko, K.; Biondi, F.; Campelo, F.; Merian, P.; Qeadan, F.; Zang, C.; Pucha-Cofrep, D.; Wernicke, J. Dendrochronology Program Library in R. R package version 1.6.8. Dendrochronologia 2018, 26, 115–124. [Google Scholar] [CrossRef]
- Cook, E.R.; Shiyatov, S.G.; Mazepa, V.S.; Ecology, A.; Branch, U. Methods of Dendrochronology Applications; Cook, E.R., Kairiukstis, L.A., Eds.; Tree-Ring Laboratory, Lamont-Ooherty Geological Observatory, Columbia University: New York, NY, USA, 1990; ISBN 9789048140602. [Google Scholar]
- Fritts, H.C. Tree Rings and Climate; Academic Press Inc.: Tucson, AZ, USA, 1976. [Google Scholar]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series with applications in dendroclimatology and hydrometeorology. J. Clim. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Bunn, A.; Korpela, M.; Biondi, F.; Campelo, F.; Mérian, P.; Qeadan, F.; Zang, C.; Pucha-Cofrep, D.; Wernicke, J. An Introduction to dplR. 2018. Available online: https://cran.r-project.org/web/packages/dplR/vignettes/chron-dplR.pdf. (accessed on 2 February 2023).
- Speer, J.H. Fundamentals of Tree-Ring Research; University of Arizona Press: Tuscon, AZ, USA, 2010; Volume 26, ISBN 0816526850. [Google Scholar]
- Schweingruber, F.H.; Eckstein, D.; Serre-Bachet, F.; Braker, O.U. Identification, Presentation and Interpretation of Event Years and Pointer Years in Dendrochronology. Dendrochronologia 1990, 8, 9–38. [Google Scholar]
- Desplanque, C.; Rolland, C.; Schweingruber, F.H. Influence of species and abiotic factors on extreme tree ring modulation: Picea abies and Abies alba in Tarentaise and Maurienne (French Alps). Trees 1999, 13, 218–227. [Google Scholar] [CrossRef]
- Van der Maaten-Theunissen, M.; van der Maaten, E.; Bouriaud, O. PointRes: An R package to analyze pointer years and components of resilience. Dendrochronologia 2015, 35, 34–38. [Google Scholar] [CrossRef]
- Lloret, F.; Keeling, E.G.; Sala, A. Components of tree resilience: Effects of successive low-growth episodes in old ponderosa pine forests. Oikos 2011, 120, 1909–1920. [Google Scholar] [CrossRef]
- StatSoft Power Solutions, Inc. Statistica Electronic Manual 2013; StatSoft Power Solutions, Inc.: Tulsa, OK, USA, 2013. [Google Scholar]
- Biondi, F.; Waikul, K. DENDROCLIM2002: A C + + program for statistical calibration of climate signals in tree-ring chronologies $. Comput. Geosci. 2004, 30, 303–311. [Google Scholar] [CrossRef]
- Chree, C. Some phenomena of sunspots and of terrestrial magnetism at Kew observatory. Philos. Trans. R. Soc. Lond. Ser. A 1913, 212, 75–116. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Bartlett, M.S. Properties of sufficiency and statistical tests. Proc. R. Soc. London. Ser. A-Math. Phys. Sci. 1937, 160, 268–282. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Honestly Significant Difference (HSD) Test. In Encyclopedia of Research Design; SAGE: Thousand Oaks, CA, USA, 2012; pp. 1–5. [Google Scholar] [CrossRef]
- Siegel, S.; Castellan, N.J. Book Review: Nonparametric Statistics for the Behavioral Sciences (Second Edition): Sidney Siegel and N. John Castellan, Jr. New York: McGraw-Hill, 1988, 399 pp., approx. $47.95. In Applied Psychological Measurement; SAGE Publications Inc.: Tulsa, OK, USA, 1989; Volume 13, pp. 217–219. [Google Scholar]
- Butler, D.G.; Cullis, B.R.; Gilmour, A.R.; Gogel, B.J.; Thompson, R. ASReml-R Reference Manual Version 4; VSN International Ltd.: Hemel Hempstead, UK, 2017; p. 176. [Google Scholar]
- Isik, F.; Holland, J.; Maltecca, C. Genetic Data Analysis for Plant and Animal Breeding; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 9783319551777. [Google Scholar]
- Vančura, K.; Prokůpková, A.; Bulušek, D.; Šimůnek, V.; Hájek, V.; Králíček, I. Dynamics of mixed lowland forests in Central Bohemia over a 20-year period. J. For. Sci. 2020, 66, 49–62. [Google Scholar] [CrossRef]
- Vejpustková, M. Klimatické faktory limitující růst smrku na území České republiky v období 1968–2013. Zpravy Lesn. Vyzk. 2022, 2022, 60–71. [Google Scholar]
- Kathke, S.; Bruelheide, H. Differences in frost hardiness of two Norway spruce morphotypes growing at Mt. Brocken, Germany. Flora 2011, 206, 120–126. [Google Scholar] [CrossRef]
- Král, J.; Vacek, S.; Vacek, Z.; Putalová, T.; Bulušek, D.; Štefančík, I. Structure, development and health status of spruce forests affected by air pollution in the western Krkonoše Mts. in 1979–2014. For. J. 2015, 61, 175–187. [Google Scholar] [CrossRef]
- Jandl, R. Climate-induced challenges of Norway spruce in Northern Austria. Trees For. People 2020, 1, 100008. [Google Scholar] [CrossRef]
- Wallin, E.; Gräns, D.; Jacobs, D.F.; Lindström, A.; Verhoef, N. Short-day photoperiods affect expression of genes related to dormancy and freezing tolerance in Norway spruce seedlings. Ann. For. Sci. 2017, 74, 1–14. [Google Scholar] [CrossRef]
- Geburek, T.; Robitschek, K.; Milasowszky, N. A tree of many faces: Why are there different crown types in Norway spruce (Picea abies [L.] Karst.)? Flora Morphol. Distrib. Funct. Ecol. Plants 2008, 203, 126–133. [Google Scholar] [CrossRef]
- Krejza, J.; Cienciala, E.; Světlík, J.; Bellan, M.; Noyer, E.; Horáček, P.; Štěpánek, P.; Marek, M.V. Evidence of climate-induced stress of Norway spruce along elevation gradient preceding the current dieback in Central Europe. Trees 2021, 35, 103–119. [Google Scholar] [CrossRef]
- Ponocná, T.; Spyt, B.; Kaczka, R.; Büntgen, U.; Treml, V. Growth trends and climate responses of Norway spruce along elevational gradients in East-Central Europe. Trees 2016, 30, 1633–1646. [Google Scholar] [CrossRef]
- Šimůnek, V.; Vacek, S.; Vacek, Z.; Andrea, G.D. Vztahy těžby listnatých a jehličnatých dřevin dle slunečních cyklů [Harvesting fluctuations of deciduous and coniferous tree species according to solar cycles] Harvesting Fluctuations of Deciduous and Coniferous Tree Species. In Proceedings of the Central European Silviculture—21st International Conference, Opočno, Czech Republic, 7 September 2021; pp. 101–108. [Google Scholar]
- Piedallu, C.; Dallery, D.; Bresson, C.; Legay, M.; Gégout, J.C.; Pierrat, R. Spatial vulnerability assessment of silver fir and Norway spruce dieback driven by climate warming. Landsc. Ecol. 2023, 38, 341–361. [Google Scholar] [CrossRef]
- Tužinský, L.; Bublinec, E.; Tužinský, M. Development of soil water regime under spruce stands. Folia Oecologica 2017, 44, 46–53. [Google Scholar] [CrossRef]
- Tomiczek, C. Nutrient Deficiency of Spruce Needles Caused By Root and Butt Rots—A Factor in Forest Decline. Arboric. Urban For. 1995, 21, 113–117. [Google Scholar] [CrossRef]
- Hentschel, R.; Rosner, S.; Kayler, Z.E.; Andreassen, K.; Børja, I.; Solberg, S.; Tveito, O.E.; Priesack, E.; Gessler, A. Norway spruce physiological and anatomical predisposition to dieback. For. Ecol. Manag. 2014, 322, 27–36. [Google Scholar] [CrossRef]
- Gömöry, D.; Longauer, R.; Hlásny, T.; Pacalaj, M.; Strmeň, S.; Krajmerová, D. Adaptation to common optimum in different populations of Norway spruce (Picea abies Karst.). Eur. J. For. Res. 2012, 131, 401–411. [Google Scholar] [CrossRef]
- Michalec, K.; Wasik, R.; Barszcz, A. Zmienność wybranych cech makrostruktury i gęstości drewna świerkowego (Picea abies (L.) Karst.) pochodzącego z drzewostanów rosnących na różnych siedliskowych typach lasu. Sylwan 2019, 163, 209–215. [Google Scholar]
- Vacek, Z.; Vacek, S.; Podrázský, V.; Bílek, L.; Štefančík, I.; Moser, W.K.; Bulušek, D.; Král, J.; Remeš, J.; Králíček, I. Effect of tree layer and microsite on the variability of natural regeneration in autochthonous beech forests. Polish J. Ecol. 2015, 63, 233–246. [Google Scholar] [CrossRef]
- George, J.P.; Grabner, M.; Karanitsch-Ackerl, S.; Mayer, K.; Weißenbacher, L.; Schueler, S. Genetic variation, phenotypic stability, and repeatability of drought response in European larch throughout 50 years in a common garden experiment. Tree Physiol. 2017, 37, 33–46. [Google Scholar] [CrossRef]
- Dungey, H.S.; Matheson, A.C.; Kain, D.; Evans, R. Genetics of wood stiffness and its component traits in Pinus radiata. Can. J. For. Res. 2006, 36, 1165–1178. [Google Scholar] [CrossRef]
- Lenz, P.; Cloutier, A.; MacKay, J.; Beaulieu, J. Genetic control of wood properties in Picea glauca—An analysis of trends with cambial age. Can. J. For. Res. 2010, 40, 703–715. [Google Scholar] [CrossRef]


| Plot Name | Location | Altitude (m a.s.l.) | Exposure | Slope (%) | Soils Type | Forest Type | Climate Classif. |
|---|---|---|---|---|---|---|---|
| Ecotype research plot | 49°56′22.2″ N 14°20′57.6″ E | 320 | NW | 8 | Cambisols | 2K4 | Cfb |
| Plot | Meteo. Station Name | GPS of Meteo. Station | Station Altitude (m a.s.l.) | Distance to Plot (km) | Seasonal Period | Annual Temp. (°C) | Seasonal Temp. (°C) | Annual Prec. (mm) | Seasonal Prec. (mm) |
|---|---|---|---|---|---|---|---|---|---|
| Ecotypes study site | Neumětely | 49°51′08.3″ N 14°02′10.6″ E | 322 | 24.51 | May to September | 8.7 | 15.9 | 535 | 323 |
| Plot Name | No. Trees | Mean RW (mm) | SD RW | Mean Min–Max (mm) | Age Sample | AR1 | R-bar | EPS | SNR |
|---|---|---|---|---|---|---|---|---|---|
| LE | 31 | 3.75 | 1.79 | 1.94–5.48 | 46 | 0.54 | 0.52 | 0.96 | 26.13 |
| ME | 25 | 4.28 | 2.10 | 2.68–6.04 | 45 | 0.61 | 0.46 | 0.95 | 17.32 |
| HE | 20 | 3.27 | 1.73 | 1.49–4.74 | 46 | 0.60 | 0.33 | 0.88 | 11.51 |
| Prec. Annual | Seasonal Prec. | Prec. P9-C8 | Prec. C6-7 | Temp. Annual | Seasonal Temp. | Temp. P9-C8 | Temp. C6-7 | |
|---|---|---|---|---|---|---|---|---|
| LE ring width | 0.14 | 0.11 | 0.19 | 0.17 | −0.32 | −0.35 | −0.35 | −0.45 |
| ME ring width | 0.11 | 0.09 | 0.15 | 0.15 | −0.43 | −0.43 | −0.43 | −0.48 |
| HE ring width | 0.03 | −0.01 | 0.06 | 0.05 | −0.36 | −0.39 | −0.35 | −0.50 |
| LE RWI | 0.30 | 0.28 | 0.35 | 0.34 | −0.05 | −0.16 | −0.15 | −0.27 |
| ME RWI | 0.37 | 0.34 | 0.39 | 0.39 | −0.06 | −0.18 | −0.15 | −0.23 |
| HE RWI | 0.20 | 0.16 | 0.30 | 0.35 | −0.07 | −0.13 | −0.15 | −0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šimůnek, V.; Stejskal, J.; Čepl, J.; Korecký, J.; Vacek, Z.; Vacek, S.; Bílek, L.; Švanda, M. Different Adaptive Potential of Norway Spruce Ecotypes in Response to Climate Change in Czech Long-Term Lowland Experiment. Forests 2023, 14, 1922. https://doi.org/10.3390/f14091922
Šimůnek V, Stejskal J, Čepl J, Korecký J, Vacek Z, Vacek S, Bílek L, Švanda M. Different Adaptive Potential of Norway Spruce Ecotypes in Response to Climate Change in Czech Long-Term Lowland Experiment. Forests. 2023; 14(9):1922. https://doi.org/10.3390/f14091922
Chicago/Turabian StyleŠimůnek, Václav, Jan Stejskal, Jaroslav Čepl, Jiří Korecký, Zdeněk Vacek, Stanislav Vacek, Lukáš Bílek, and Michal Švanda. 2023. "Different Adaptive Potential of Norway Spruce Ecotypes in Response to Climate Change in Czech Long-Term Lowland Experiment" Forests 14, no. 9: 1922. https://doi.org/10.3390/f14091922





