Consecutive Fertilization-Promoted Soil Nutrient Availability and Altered Rhizosphere Bacterial and Bulk Fungal Community Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Sample Collection
2.2. Soil Chemical Property Analysis
2.3. Soil Enzyme Activity Assays
2.4. Soil Microbiomes DNA Extraction, PCR Amplification, and High-Throughput Sequencing
2.5. Bioinformatics Analysis
2.6. Statistical Analysis
3. Results
3.1. Soil Chemical Property and Enzyme Activity
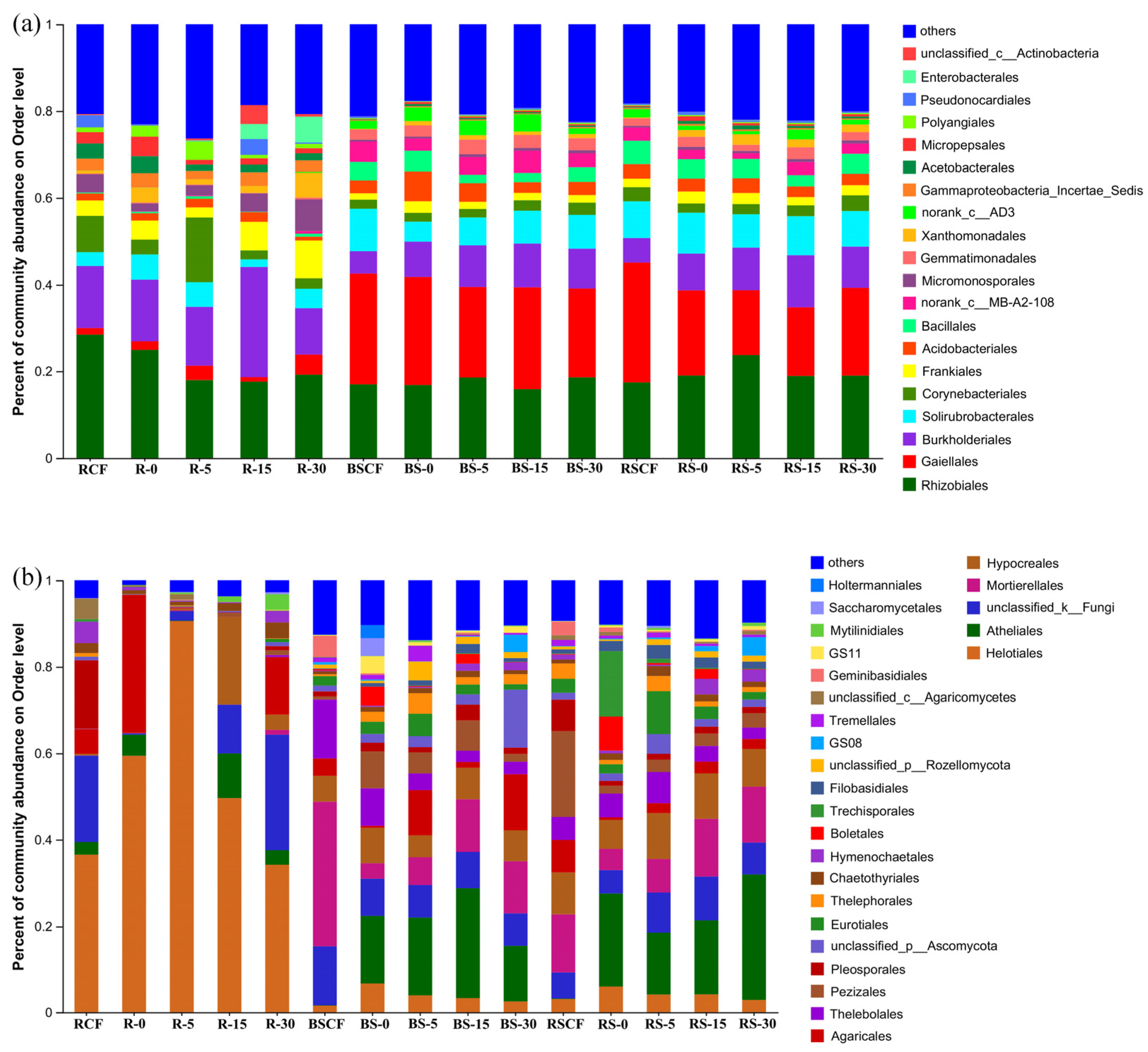
3.2. Composition Characteristics of Bacterial and Fungal Community
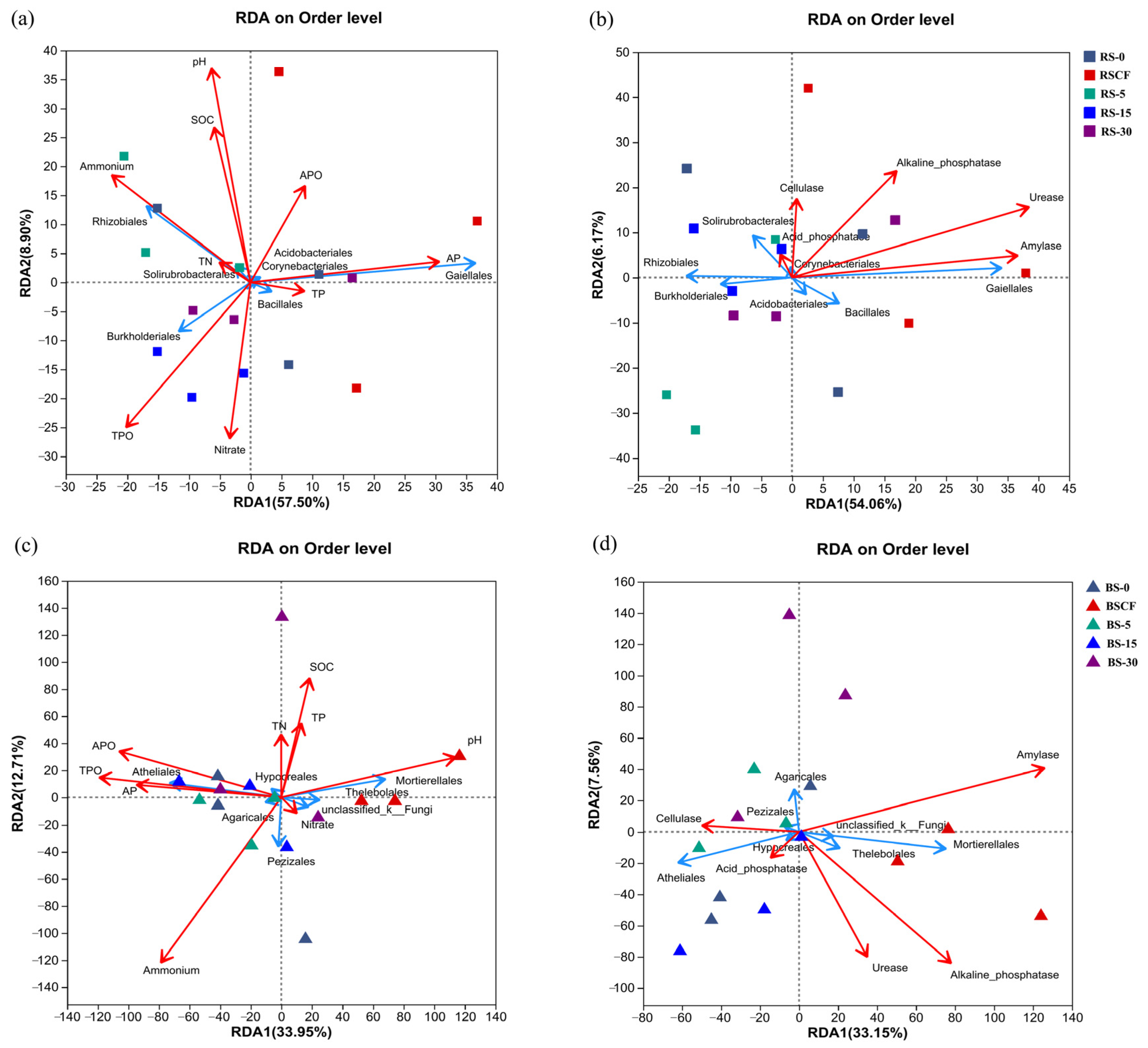
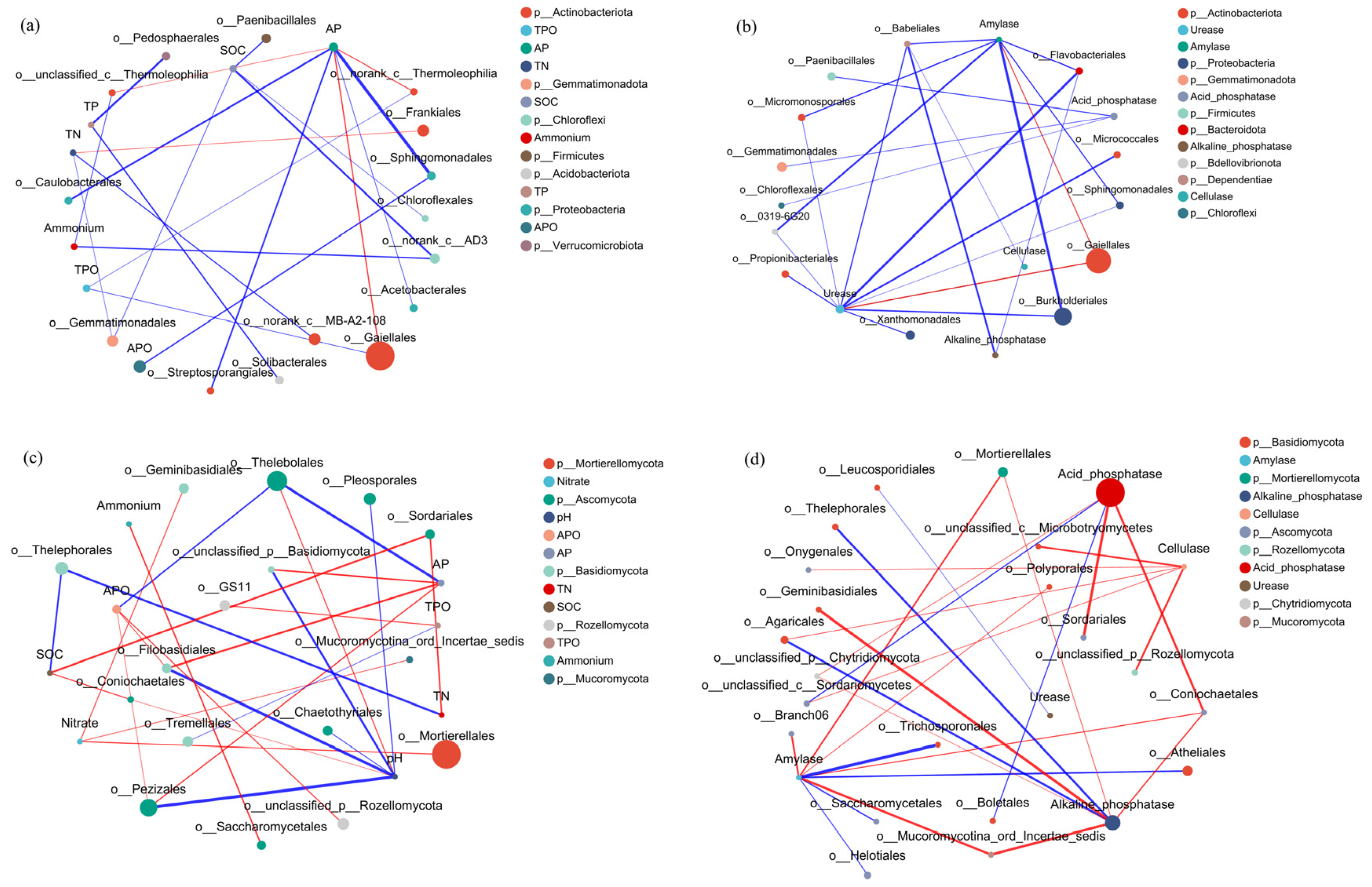
3.3. Correlations between Microbial Composition and Soil Nutrient Content, Enzyme Activity
3.4. Microbial Community Potential for Phosphorus Metabolism Function
4. Discussion
4.1. Consecutive Fertilization Alters Soil Properties, Rhizosphere Soil Bacterial, and Bulk Soil Fungal Community Composition
4.2. Abundance of Soil Nutrients Releasing Relevant Microbes Are Driven by Fertilization Treatment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mead, D.J. Opportunities for improving plantation productivity. How much? How quickly? How realistic? Biomass Bioenergy 2005, 28, 249–266. [Google Scholar] [CrossRef]
- Xue, L.; Ren, H.; Brodribb, T.J.; Wang, J.; Yao, X.; Li, S. Long term effects of management practice intensification on soil microbial community structure and co-occurrence network in a non-timber plantation. For. Ecol. Manag. 2020, 459, 117805. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Komatsu, M.; Tamai, Y.; Harayama, H.; Koike, T. Single and combined effects of fertilization, ectomycorrhizal inoculation, and drought on container-grown Japanese larch seedlings. J. For. Res. 2023, 34, 1077–1094. [Google Scholar] [CrossRef]
- Diez, M.C.; Osorio, N.W.; Moreno, F. Effect of dose and type of fertilizer on flowering and fruiting of vanilla plants. J. Plant Nutr. 2015, 39, 1297–1310. [Google Scholar] [CrossRef]
- Li, B.B.; Roley, S.S.; Duncan, D.S.; Guo, J.; Quensen, J.F.; Yu, H.Q.; Tiedje, J.M. Long-term excess nitrogen fertilizer increases sensitivity of soil microbial community to seasonal change revealed by ecological network and metagenome analyses. Soil Biol. Biochem. 2021, 160, 108349. [Google Scholar] [CrossRef]
- Du, T.Y.; Hu, Q.F.; Mao, W.J.; Yang, Z.; Chen, H.; Sun, L.N.; Zhai, M.Z. Metagenomics insights into the functional profiles of soil carbon, nitrogen, and phosphorus cycles in a walnut orchard under various regimes of long-term fertilisation. Eur. J. Agron. 2023, 148, 126887. [Google Scholar] [CrossRef]
- Guo, Q.; Yan, L.; Korpelainen, H.; Niinemets, Ü.; Li, C. Plant-plant interactions and N fertilization shape soil bacterial and fungal communities. Soil Biol. Biochem. 2018, 128, 127–138. [Google Scholar] [CrossRef]
- Cui, J.; Sun, Z.; Wang, Z.; Gong, L. Effects of the Application of Nutrients on Soil Bacterial Community Composition and Diversity in a Larix olgensis Plantation, Northeast China. Sustainability 2022, 14, 16759. [Google Scholar] [CrossRef]
- Montesinos, D.; Villar-Salvador, P.; García-Fayos, P. Genders in Juniperus thurifera have different functional responses to variations in nutrient availability. New Phytol. 2012, 193, 705–712. [Google Scholar] [CrossRef]
- Du, B.; Zheng, J.; Ji, H.; Zhu, Y.; Yuan, J.; Wen, J.; Kang, H.; Liu, C. Stable carbon isotope used to estimate water use efficiency can effectively indicate seasonal variation in leaf stoichiometry. Ecol. Indic. 2020, 121, 107250. [Google Scholar] [CrossRef]
- Tian, J.; Ge, F.; Zhang, D.; Deng, S.; Liu, X. Roles of Phosphate Solubilizing Microorganisms from Managing Soil Phosphorus Deficiency to Mediating Biogeochemical P Cycle. Biology 2021, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, M.P.; Chandra Nayaka, S.; Nuthan, B.R. Role of Rhizosphere Microflora in Potassium Solubilization. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V., Maurya, B., Verma, J., Meena, R., Eds.; Springer: New Delhi, India, 2016. [Google Scholar] [CrossRef]
- Cui, Y.; Fang, L.; Guo, X.; Wang, X.; Zhang, Y.; Li, P.; Zhang, X. Ecoenzymatic stoichiometry and microbial nutrient limitation in rhizosphere soil in the arid area of the northern Loess Plateau, China. Soil Biol. Biochem. 2018, 116, 11–21. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Dimkpa, C.O.; Pandey, R. Exploring phosphorus fertilizers and fertilization strategies for improved human and environmental health. Biol. Fertil. Soils 2020, 56, 299–317. [Google Scholar] [CrossRef]
- Su, J.Q.; Ding, L.J.; Xue, K.; Yao, H.Y.; Quensen, J.; Bai, S.J.; Wei, W.X.; Wu, J.S.; Zhou, J.Z.; Tiedje, J.M.; et al. Long-term balanced fertilization increases the soil microbial functional diversity in a phosphorus-limited paddy soil. Mol. Ecol. 2014, 24, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Du, T.Y.; He, H.Y.; Zhang, Q.; Lu, L.; Mao, W.J.; Zhai, M.Z. Positive effects of organic fertilizers and biofertilizers on soil microbial community composition and walnut yield. Appl. Soil Ecol. 2022, 175, 104457. [Google Scholar] [CrossRef]
- Semenov, M.V.; Krasnov, G.S.; Semenov, V.M.; van Bruggen, A.H.C. Consecutive fertilization rather than plant species shapes rhizosphere and bulk soil prokaryotic communities in agroecosystems. Appl. Soil Ecol. 2020, 154, 103641. [Google Scholar] [CrossRef]
- Cardinale, M.; Ratering, S.; Sadeghi, A.; Pokhrel, S.; Honermeier, B.; Schnell, S. The response of the soil microbiota to long-term mineral and organic nitrogen fertilization is stronger in the bulk soil than in the rhizosphere. Genes 2020, 11, 456. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Meena, V.D. Rhizosphere Effect on Nutrient Availability in Soil and Its Uptake by Plants: A Review. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 1–12. [Google Scholar] [CrossRef]
- Schmidt, J.E.; Kent, A.D.; Brisson, V.L.; Gaudin, A.C. Agricultural management and plant selection interactively affect rhizosphere microbial community structure and nitrogen cycling. Microbiome 2019, 7, 146. [Google Scholar] [CrossRef]
- Soil Survey Staf. Keys to Soil Taxonomy; United States Department of Agriculture-Natural Resources Conservation Service: Washington, DC, USA, 2006.
- Michael, H.; Julian, H.; Stefanie, H.; Janet, R.; Sebastian, P.; Ellen, K.; Bernd, M. Differences in organic matter properties and microbial activity between bulk and rhizosphere soil from the top- and subsoils of three forest stands. Geoderma 2022, 409, 115589. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Wang, Q.; Bermudez, R.S.; Yu, S.; Bu, P.; Wang, Z.; Chen, D.; Feng, J. Effects of plantation type and soil depth on microbial community structure and nutrient cycling function. Front. Microbiol. 2022, 13, 846468. [Google Scholar] [CrossRef]
- Han, W.; He, M. The application of exogenous cellulase to improve soil fertility and plant growth due to acceleration of straw decomposition. Bioresour. Technol. 2010, 101, 3724–3731. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Tang, D.; Feng, H.; Gao, Y.; Zhou, P.; Xu, L.; Wang, L. Determining soil enzyme activities for the assessment of fungi and citric acid-assisted phytoextraction under cadmium and lead contamination. Environ. Sci. Pollut. Res. 2015, 22, 19860–19869. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, Q.; Wang, L.; Liu, W.; Liu, X.; Huang, Y.; Christie, P. Response of soil enzymes and microbial communities to root extracts of the alien Alternanthera philoxeroides. Arch. Agron. Soil Sci. 2017, 64, 708–717. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Averill, C.; Werbin, Z.R.; Atherton, K.F.; Bhatnagar, J.M.; Dietze, M.C. Soil microbiome predictability increases with spatial and taxonomic scale. Nat. Ecol. Evol. 2021, 5, 747–756. [Google Scholar] [CrossRef]
- Wang, W.; Nie, Y.; Tian, H.; Quan, X.; Li, J.; Shan, Q.; Li, H.; Cai, Y.; Ning, S.; Bermudez, R.S.; et al. Microbial community, co-occurrence network relationship and fermentation lignocellulose characteristics of Broussonetia papyrifera ensiled with wheat bran. Microorganisms 2022, 10, 2015. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, D.; Wu, C.; Guo, L.; Sun, X.; Zhang, S. Forest thinning alleviates the negative effects of precipitation reduction on soil microbial diversity and multifunctionality. Biol. Fertil. Soils 2023, 59, 423–440. [Google Scholar] [CrossRef]
- Li, T.; Long, M.; Li, H.; Gatesoupe, F.; Zhang, X.; Zhang, Q.; Feng, D.; Li, A. Multi-omics analysis reveals a correlation between the host phylogeny, gut microbiota and metabolite profiles in cyprinid fishes. Front. Microbiol. 2017, 8, 454. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Q.; Sun, X.; Chen, D.; Insam, H.; Koide, R.; Zhang, S. Effects of mixed-species litter on bacterial and fungal lignocellulose degradation functions during litter decomposition. Soil Biol. Biochem. 2020, 141, 107690. [Google Scholar] [CrossRef]
- Mandakovic, D.; Rojas, C.; Maldonado, J.; Latorre, M.; Travisany, D.; Delage, E.; Bihouée, A.; Jean, G.; Díaz, F.P.; Fernández-Gómez, B.; et al. Structure and co-occurrence patterns in microbial communities under acute environmental stress reveal ecological factors fostering resilience. Sci. Rep. 2018, 8, 5875. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Dong, W.; Wang, H.; Feng, Y. Role of acid/alkali-treatment in primary sludge anaerobic fermentation: Insights into microbial community structure, functional shifts and metabolic output by high-throughput sequencing. Bioresour. Technol. 2018, 249, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xia, Y.; Sun, Q.; Liu, K.; Chen, X.; Ge, T.; Zhu, B.; Zhu, Z.; Zhang, Z.; Su, Y. Effects of long-term on phoD-harboring bacterial community in Karst soils. Sci. Total Environ. 2018, 628–629, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.J.; Chen, Z.; Li, X.Y.; Tan, J.; Liu, F.; Wu, J.P. The mechanism of promoting rhizosphere nutrient turnover for arbuscular mycorrhizal fungi attributes to recruited functional bacterial assembly. Mol. Ecol. 2023, 32, 2335–2350. [Google Scholar] [CrossRef]
- Luo, L.; Meng, H.; Gu, J.D. Microbial extracellular enzymes in biogeochemical cycling of ecosystems. J. Environ. Manag. 2017, 197, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. organic amendments: Microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar] [CrossRef] [PubMed]
- Ai, C.; Liang, G.Q.; Sun, J.W.; Wang, X.B.; Zhou, W. Responses of extracellular enzyme activities and microbial community in both the rhizosphere and bulk soil to long-term practices in a fluvo-aquic soil. Geoderma 2012, 173–174, 330–338. [Google Scholar] [CrossRef]
- Li, J.; Xie, T.; Zhu, H.; Zhou, J.; Li, C.; Xiong, W.; Lin, X.; Wu, Y.; He, Z.; Li, X. Alkaline phosphatase activity mediates soil organic phosphorus mineralization in a subalpine forest ecosystem. Geoderma 2021, 404, 115376. [Google Scholar] [CrossRef]
- Cao, N.; Zhi, M.L.; Zhao, W.Q.; Pang, J.Y.; Hu, W.; Zhou, Z.G.; Meng, Y.L. Straw retention combined with phosphorus fertilizer promotes soil phosphorus availability by enhancing soil P-related enzymes and the abundance of phoC and phoD genes. Soil Till. Res. 2022, 220, 105390. [Google Scholar] [CrossRef]
- Gottel, N.R.; Castro, H.F.; Kerley, M.; Yang, Z.; Pelletier, D.A.; Podar, M.; Karpinets, T.; Uberbacher, E.; Tuskan, G.A.; Vilgalys, R.; et al. Distinct microbial communities within the endosphere and rhizosphere of populus deltoides roots across contrasting soil types. Appl. Environ. Microbiol. 2011, 77, 5934–5944. [Google Scholar] [CrossRef]
- Wang, H.; Guo, S.; Huang, M.; Thorsten, L.H.; Wei, J. Ascomycota has a faster evolutionary rate and higher species diversity than Basidiomycota. Sci. China Life Sci. 2010, 53, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Ma, Y.; Yang, T.; Gao, G.; Wang, D.; Guo, X.; Chu, H.; Gralnick, J. Phosphorus and zinc are strongly associated with belowground fungal communities in wheat field underConsecutive fertilization. Microbiol. Spectr. 2022, 10, e00110-22. [Google Scholar] [PubMed]
- Aira, M.; Gómez-Brandón, M.; Lazcano, C.; Bååth, E.; Domínguez, J. Plant genotype strongly modifies the structure and growth of maize rhizosphere microbial communities. Soil Biol. Biochem. 2010, 42, 2276–2281. [Google Scholar] [CrossRef]
- Neumann, G.; Römheld, V. The release of root exudates as affected by the plant physiological status. In The Rhizosphere: Biochemistry and Organic Substances at the Soil-Plant Interface; Pinton, R., Varanini, Z., Nannipieri, P., Eds.; CRC Press: Boca Ratón, FL, USA, 2007; pp. 23–72. [Google Scholar]
- Wang, Q.; Jiang, X.; Guan, D.; Wei, D.; Zhao, B.; Ma, M.; Chen, S.; Li, L.; Cao, F.; Li, J. Long-term fertilization changes bacterial diversity and bacterial communities in the maize rhizosphere of Chinese Mollisols. Appl. Soil Ecol. 2018, 125, 88–96. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, M.; Jiang, X.; Guan, D.; Wei, D.; Zhao, B.; Chen, S.; Cao, F.; Li, L.; Yang, X.; et al. Impact of 36 years of nitrogen fertilization on microbial community composition and soil carbon cycling-related enzyme activities in rhizospheres and bulk soils in northeast China. Appl. Soil Ecol. 2019, 136, 148–157. [Google Scholar] [CrossRef]
- Roberts, B.A.; Fritschi, F.B.; Horwath, W.R.; Scow, K.M.; Rains, W.D.; Travis, R.L. Comparisons of soil microbial communities influenced by soil texture, nitrogen fertility, and rotations. Soil Sci. 2011, 176, 487–494. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Gtari, M.; Tisa, L.S.; Normand, P. Diversity of frankia strains, actinobacterial symbionts of actinorhizal plants. In Symbiotic Endophytes; Springer: Berlin/Heidelberg, Germany, 2013; pp. 123–148. [Google Scholar] [CrossRef]
- Albuquerque, L.; França, L.; Rainey, F.A.; Schumann, P.; Nobre, M.F.; Costa, M.S. Gaiella occulta gen. nov., sp. nov., a novel representative of a deep branching phylogenetic lineage within the class Actinobacteria and proposal of Gaiellaceae fam. nov. and Gaiellales ord. nov Syst. Appl. Microbiol. 2011, 34, 595–599. [Google Scholar] [CrossRef]
- Novello, G.; Gamalero, E.; Bona, E.; Boatti, L.; Mignone, F.; Massa, N.; Cesaro, P.; Lingua, G.; Berta, G. The rhizosphere bacterial microbiota of vitis vinifera cv. pinot noir in an integrated pest management vineyard. Front. Microbiol. 2017, 8, 1528. [Google Scholar] [CrossRef] [PubMed]
- Severino, R.; Froufe, H.J.C.; Barroso, C.; Albuquerque, L.; Lobo-da-Cunha, A.; da Costa, M.S.; Egas, C. High-quality draft genome sequence of Gaiella occulta isolated from a 150 m deep mineral water borehole and comparison with the genome sequences of other deep-branching lineages of the phylum Actinobacteria. MicrobiologyOpen 2019, 8, e00840. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, P.; Li, F.; Xi, J.; Han, Y. Effects of tillage and biochar on soil physiochemical and microbial properties and its linkage with crop yield. Front. Microbiol. 2022, 13, 929725. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.B.; Dsouza, M.; Gilbert, J.A.; Guo, X.; Wang, D.; Guo, Z.; Ni, Y.; Chu, H. Fungal community composition in soils subjected to long-term chemical fertilization is most influenced by the type of organic matter. Environ. Microbiol. 2016, 18, 5137–5150. [Google Scholar] [CrossRef]
- Osorio, N.W.; Habte, M. Soil phosphate desorption induced by a phosphate-solubilizing fungus. Commun. Soil Sci. Plant Anal. 2014, 45, 451–460. [Google Scholar] [CrossRef]
- Tagawa, M.; Tamaki, H.; Manome, A.; Koyama, O.; Kamagata, Y. Isolation and characterization of antagonistic fungi against potato scab pathogens from potato field soils. FEMS Microbiol. Lett. 2010, 305, 136–142. [Google Scholar] [CrossRef]
- Edgington, S.; Thompson, E.; Moore, D.; Hughes, K.A.; Bridge, P. Investigating the insecticidal potential of Geomyces (Myxotrichaceae: Helotiales) and Mortierella (Mortierellacea: Mortierellales) isolated from Antarctica. SpringerPlus 2014, 3, 289. [Google Scholar] [CrossRef] [PubMed]
- Choma, M.; Rappe-George, M.O.; Bárta, J.; Čapek, P.; Kaštovská, E.; Gärdenäs, A.I.; Šantrůčková, H. Recovery of the ectomycorrhizal community after termination of long-term nitrogen fertilisation of a boreal Norway spruce forest. Fungal Ecol. 2017, 29, 116–122. [Google Scholar] [CrossRef]
- Hackman, J.J.; Rose, B.D.; Frank, H.E.R.; Vilgalys, R.; Cook, R.L.; Garcia, K. NPK fertilizer use in loblolly pine plantations: Who are we really feeding? For. Ecol. Manag. 2022, 520, 120393. [Google Scholar] [CrossRef]
- Hu, X.; Gu, H.; Liu, J.; Zhou, B.; Wei, D.; Chen, X.; Wang, G. High variation of fungal communities and associated potential plant pathogens induced by long-term addition of N fertilizers rather than P, K fertilizers: A case study in a Mollisol field. Soil Ecol. Lett. 2021, 4, 348–361. [Google Scholar] [CrossRef]
- Truong, C.; Gabbarini, L.A.; Corrales, A.; Mujic, A.B.; Escobar, J.M.; Moretto, A.; Smith, M.E. Ectomycorrhizal fungi and soil enzymes exhibit contrasting patterns along elevation gradients in southern Patagonia. New Phytol. 2019, 222, 1936–1950. [Google Scholar] [CrossRef]
- Luo, S.; Phillips, R.P.; Jo IFei, S.; Liang, J.; Schmid, B.; Eisenhauer, N. Higher productivity in forests with mixed mycorrhizal strategies. Nat. Commun. 2023, 14, 1377. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Yang, Y.; Li, J.; Bu, P.; Lu, A.; Wang, H.; He, W.; Santos Bermudez, R.; Feng, J. Consecutive Fertilization-Promoted Soil Nutrient Availability and Altered Rhizosphere Bacterial and Bulk Fungal Community Composition. Forests 2024, 15, 514. https://doi.org/10.3390/f15030514
Wang W, Yang Y, Li J, Bu P, Lu A, Wang H, He W, Santos Bermudez R, Feng J. Consecutive Fertilization-Promoted Soil Nutrient Availability and Altered Rhizosphere Bacterial and Bulk Fungal Community Composition. Forests. 2024; 15(3):514. https://doi.org/10.3390/f15030514
Chicago/Turabian StyleWang, Wenbo, Yuanyuan Yang, Jinge Li, Pengtu Bu, Aijun Lu, Hao Wang, Wenxing He, Ramon Santos Bermudez, and Jian Feng. 2024. "Consecutive Fertilization-Promoted Soil Nutrient Availability and Altered Rhizosphere Bacterial and Bulk Fungal Community Composition" Forests 15, no. 3: 514. https://doi.org/10.3390/f15030514



