Effects of Small-Scale Dead Wood Additions on Beetles in Southeastern U.S. Pine Forests
Abstract
:1. Introduction
2. Materials and Methods
3. Results
| Parameter | 1998 ( n = 12) | 1999 ( n = 9) | 2002 ( n = 9) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| State | Treatment | State*treatment | State | Treatment | State*treatment | State | Treatment | State*treatment | |
| Richness | |||||||||
| All species | F3,16 = 7.5** | F1,3 = 3.4 | F3,16 = 3.3* | F2,12 = 16.4*** | F1,2 = 5.9 | F2,12 = 0.1 | F2,12 = 8.1** | F1,2 = 1.1 | F2,12 = 2.3 |
| Saproxylic species | F3,16 = 0.9 | F1,3 = 6.7 | F3,16 = 4.5* | F2,12 = 18.0*** | F1,2 = 45.1* | F2,12 = 0.1 | F2,12 = 8.9** | F1,2 = 1.2 | F2,12 = 1.3 |
| Ground beetles | F3,16 = 14.3*** | F1,3 = 0.4 | F3,16 = 0.5 | F2,12 = 8.7** | F1,2 = 0.1 | F2,12 = 0.5 | F2,12 = 1.5 | F1,2 = 1.6 | F2,12 = 1.8 |
| Other species | F3,16 = 28.6*** | F1,3 = 1.9 | F3,16 = 1.1 | F2,12 = 10.2** | F1,2 = 5.8 | F2,12 = 0.1 | F2,12 = 3.9 | F1,2 = 0.6 | F2,12 = 1.7 |
| Abundance | |||||||||
| All species | F3,16 = 13.0*** | F1,3 = 8.1 | F3,16 = 2.2 | F2,12 = 18.5*** | F1,2 = 0.4 | F2,12 = 0.4 | F2,12 = 2.2 | F1,2 = 1.1 | F2,12 = 1.9 |
| Saproxylic species | F3,16 = 4.0* | F1,3 = 8.3 | F3,16 = 3.6* | F2,12 = 18.7*** | F1,2 = 1.9 | F2,12 = 0.9 | F2,12 = 6.3* | F1,2 = 1.7 | F2,12 = 1.3 |
| Ground beetles | F3,16 = 18.5*** | F1,3 = 0.4 | F3,16 = 0.7 | F2,12 = 2.7 | F1,2 = 0.7 | F2,12 = 0.8 | F2,12 = 2.0 | F1,2 = 2.4 | F2,12 = 0.9 |
| Other species | F3,16 = 11.1*** | F1,3 = 0.0 | F3,16 = 0.4 | F2,12 = 13.3*** | F1,2 = 0.1 | F2,12 = 0.2 | F2,12 = 1.7 | F1,2 = 0.3 | F2,12 = 2.5 |
| Diversity | |||||||||
| All species | F3,16 = 5.1* | F1,3 = 2.3 | F3,16 = 4.7* | F2,12 = 9.9** | F1,2 = 147.8** | F2,12 = 0.0 | F2,12 = 0.8 | F1,2 = 1.3 | F2,12 = 1.0 |
| Saproxylic species | F3,16 = 1.5 | F1,3 = 4.2 | F3,16 = 4.1* | F2,12 = 1.8 | F1,2 = 62.4* | F2,12 = 0.1 | F2,12 = 1.8 | F1,2 = 0.8 | F2,12 = 0.6 |
| Ground beetles | F3,16 = 7.5** | F1,3 = 0.1 | F3,16 = 0.4 | F2,12 = 16.3*** | F1,2 = 3.0 | F2,12 = 0.2 | F2,12 = 1.0 | F1,2 = 1.3 | F2,12 = 2.0 |
| Other species | F3,16 = 10.2*** | F1,3 = 0.2 | F3,16 = 1.4 | F2,12 = 10.3** | F1,2 = 9.9 | F2,12 = 0.2 | F2,12 = 0.9 | F1,2 = 0.9 | F2,12 = 0.9 |
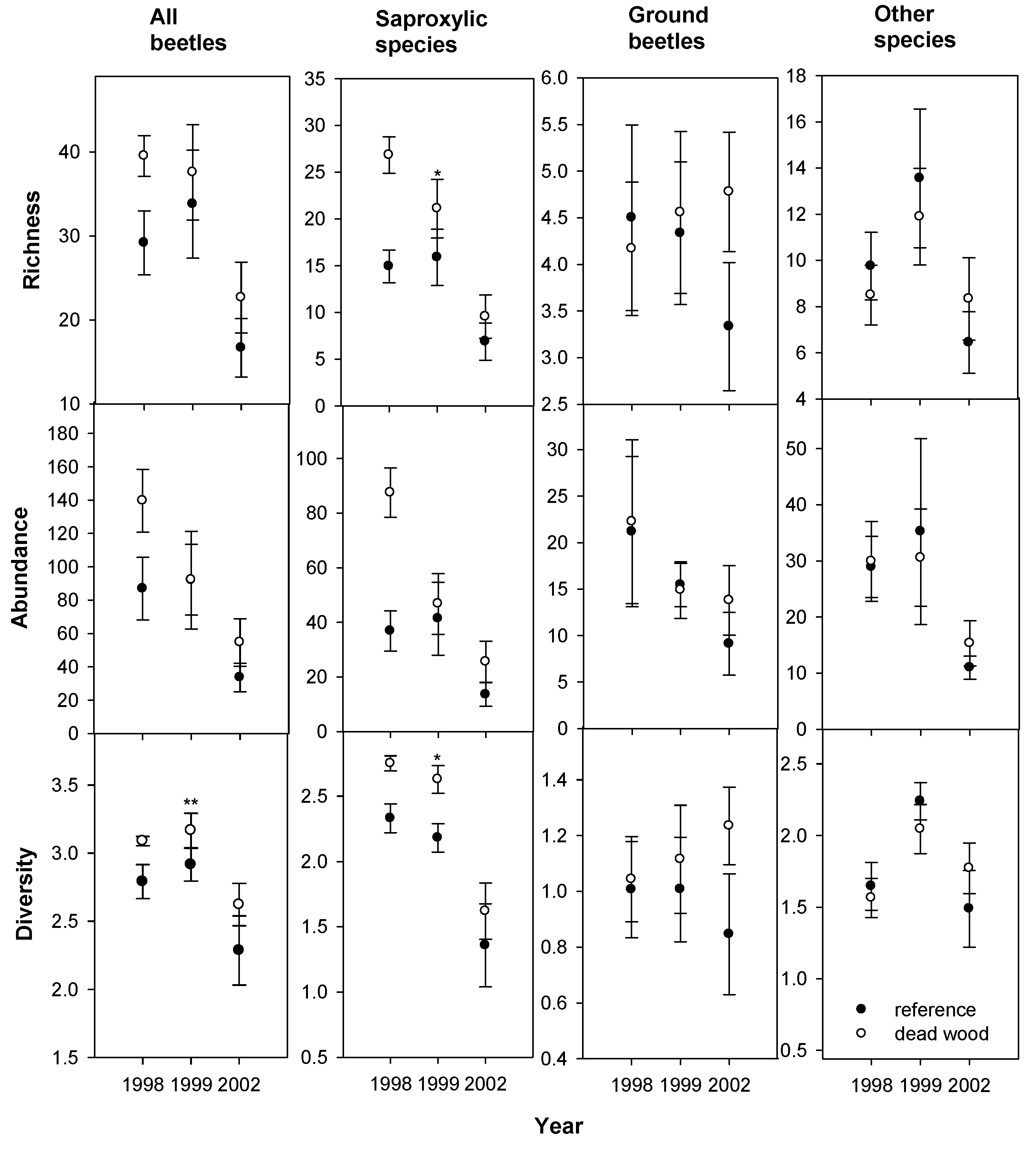
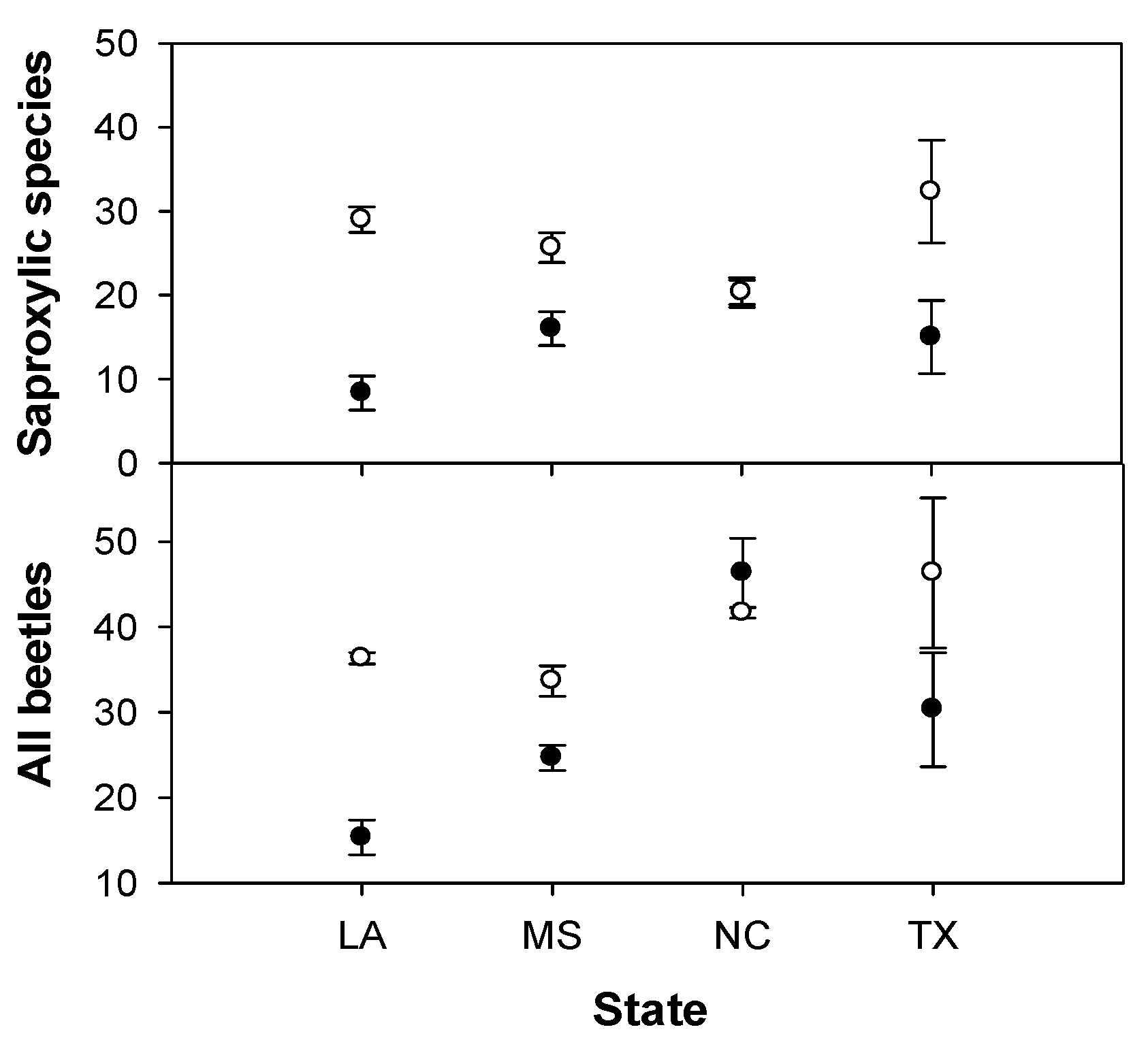
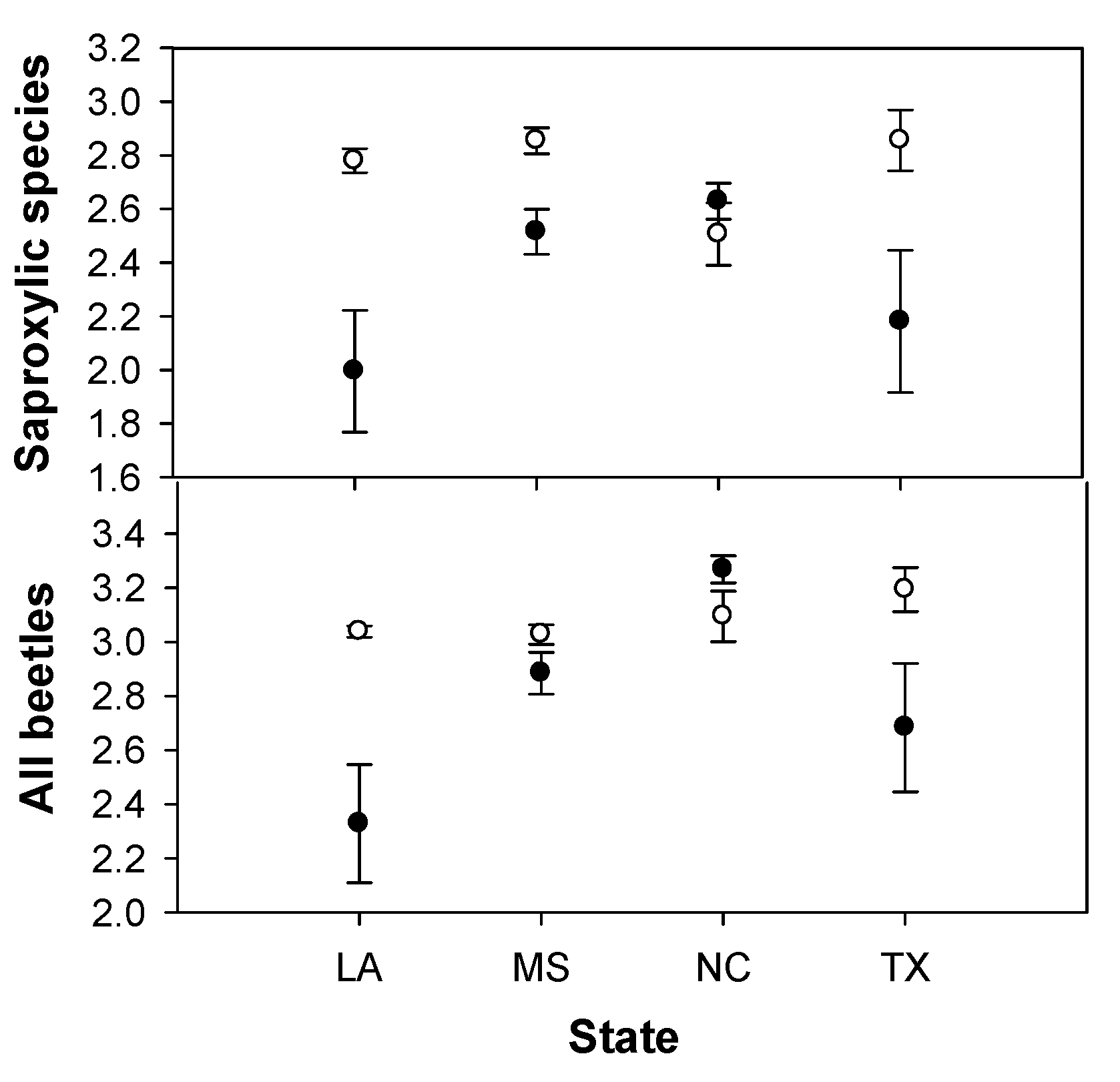
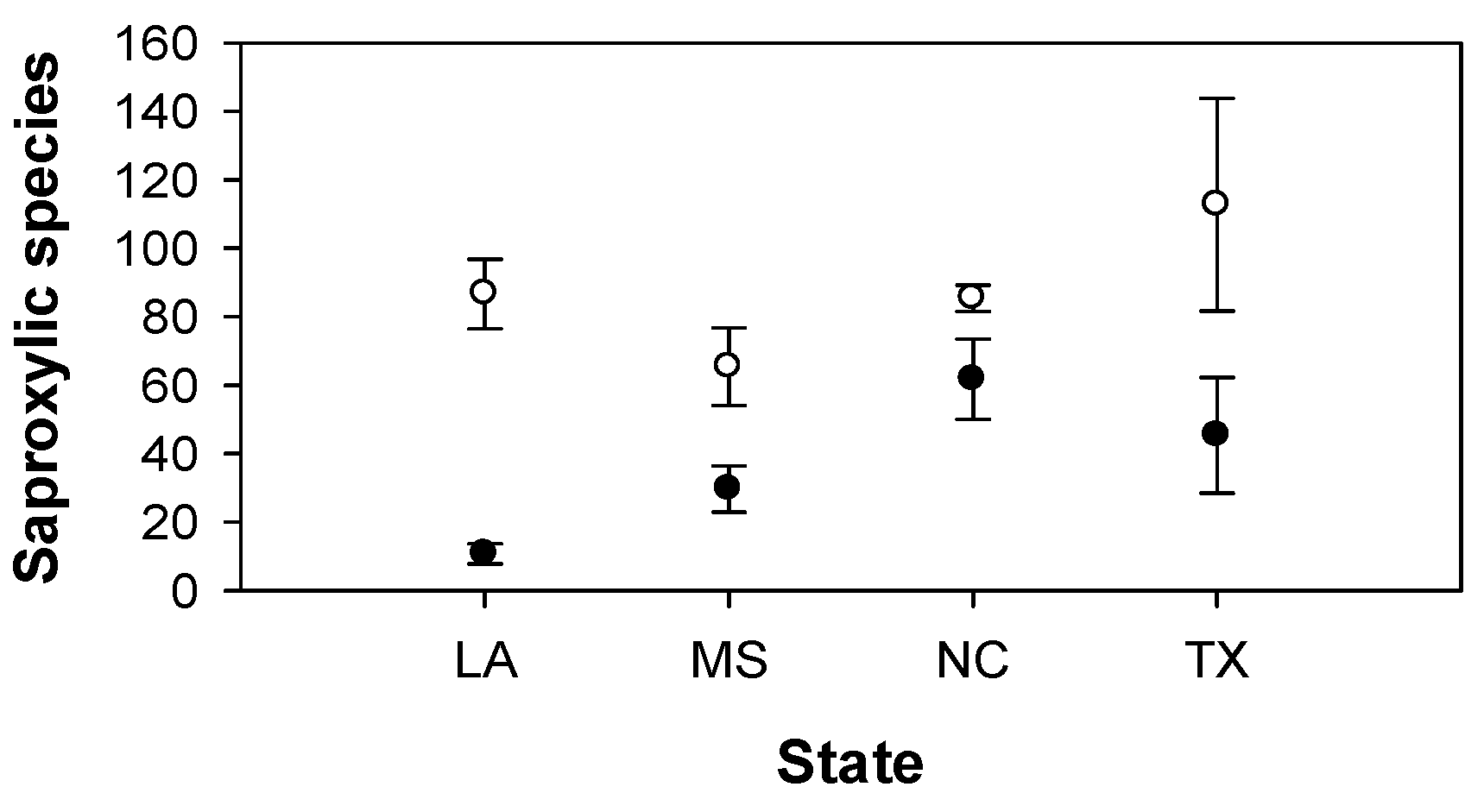
4. Discussion
5. Conclusions
Appendix
| Species | Group | Louisiana | Mississippi | North Carolina | Texas | Total |
|---|---|---|---|---|---|---|
| Aderidae | ||||||
| Zonantes fasciatus (Melsheimer) | S | 0/0 | 0/0 | 1/0 | 0/0 | 1/0 |
| Zonantes signatus (Haldeman) | S | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Zonantes subfasciatus (LeConte) | S | 0/0 | 1/0 | 0/0 | 0/0 | 1/0 |
| Zonantes sp. | S | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Agyrtidae | ||||||
| Necrophilus pettitii Horn | O | 0/0 | 5/2 | 0/0 | 0/0 | 5/2 |
| Anobiidae | ||||||
| Cryptoramorphus sp. | S | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Euvrilletta peltata (Harris) | S | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Ptinus sp. | S | 0/0 | 1/0 | 0/0 | 1/2 | 2/2 |
| Anthicidae | ||||||
| Tomoderus sp. | O | 0/0 | 2/0 | 0/0 | 0/0 | 2/0 |
| Vacusus sp. | O | 0/0 | 1/0 | 0/0 | 0/0 | 1/0 |
| Attelabidae | ||||||
| Pterocolus ovatus (Fabricius) | O | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Biphyllidae | ||||||
| Diplocoelus rudis (LeConte) | S | 8/7 | 12/27 | 8/7 | 10/8 | 38/49 |
| Bostrichidae | ||||||
| Lichenophanes bicornis (Weber) | S | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Melalgus plicatus (LeConte) | S | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Stephanopachys sp. | S | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Xylobiops basilaris (Say) | S | 0/3 | 1/2 | 0/0 | 0/3 | 1/8 |
| Bothrideridae | ||||||
| Bothrideres cryptus Stephan | S | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Brentidae | ||||||
| Sayapion segnipes (Say) | O | 0/0 | 1/0 | 0/0 | 0/0 | 1/0 |
| Buprestidae | ||||||
| Buprestis lineata Fabricius | S | 0/1 | 0/0 | 0/0 | 0/0 | 0/1 |
| Chalcophora virginiensis (Drury) | S | 0/1 | 0/1 | 0/0 | 0/1 | 0/3 |
| Cantharidae | ||||||
| Rhagonycha sp. | O | 3/0 | 0/1 | 0/0 | 0/1 | 3/2 |
| Carabidae | ||||||
| Acupalpus rectangulus Chaudoir | P | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Agonum punctiforme Say | P | 0/0 | 0/0 | 0/0 | 6/15 | 6/15 |
| Amara impuncticollis (Say) | P | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Anisodactylus haplomus Chaudoir | P | 3/0 | 2/0 | 0/0 | 1/0 | 6/0 |
| Apenes lucidulus Chaudoir | P | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Apenes sinuatus (Say) | P | 0/0 | 0/0 | 0/1 | 0/3 | 0/4 |
| Brachinus alternans Dejean | P | 0/0 | 0/0 | 0/1 | 0/0 | 0/1 |
| Brachinus americanus (LeConte) | P | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Calathus opaculus LeConte | P | 0/0 | 0/0 | 1/0 | 34/43 | 35/43 |
| Calosoma scrutator (Fabricius) | P | 0/0 | 0/0 | 0/0 | 2/2 | 2/2 |
| Carabus goryi Dejean | P | 0/0 | 0/0 | 1/0 | 0/0 | 1/0 |
| Chlaenius amoenus (Dejean) | P | 3/2 | 2/2 | 0/0 | 0/0 | 5/4 |
| Chlaenius emarginatus Say | P | 0/0 | 0/0 | 2/1 | 0/0 | 2/1 |
| Chlaenius erythropus Germar | P | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Clivina ferrea LeConte | P | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Clivina pallida Say | P | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Coptodera aerata Dejean | P | 0/2 | 1/2 | 1/2 | 1/2 | 3/8 |
| Cyclotrachelus alabamae (Van Dyke) | P | 46/39 | 0/2 | 0/0 | 23/24 | 69/65 |
| Cyclotrachelus convivus (LeConte) | P | 0/0 | 23/30 | 0/0 | 0/0 | 23/30 |
| Cyclotrachelus laevipennis (LeConte) | P | 1/0 | 1/1 | 0/0 | 0/0 | 2/1 |
| Cyclotrachelus seximpressus (LeConte) | P | 1/1 | 0/0 | 0/0 | 0/0 | 1/1 |
| Cyclotrachelus sigillatus (Say) | P | 0/0 | 0/0 | 30/31 | 0/0 | 30/31 |
| Cyclotrachelus spoliatus (Newman) | P | 0/0 | 0/0 | 19/14 | 0/0 | 19/14 |
| Cyclotrachelus texensis (Freitag) | P | 6/5 | 0/0 | 0/0 | 42/59 | 48/64 |
| Dicaelus crenatus LeConte | P | 0/0 | 0/0 | 62/57 | 0/0 | 62/57 |
| Dicaelus elongatus Bonelli | P | 0/1 | 5/9 | 3/6 | 7/5 | 15/21 |
| Dicaelus furvus Dejean | P | 1/0 | 1/4 | 3/1 | 0/0 | 5/5 |
| Dicaelus purpuratus Bonelli | P | 0/0 | 0/0 | 3/1 | 0/0 | 3/1 |
| Elaphropus granarius (Dejean) | P | 0/2 | 0/0 | 0/0 | 0/0 | 0/2 |
| Galerita bicolor Drury | P | 0/0 | 4/10 | 57/82 | 0/0 | 61/92 |
| Harpalus rufipes Degeer | P | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Harpalus sp. | P | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Helluomorphoides nigripennis Dejean | P | 5/6 | 38/27 | 4/2 | 0/0 | 47/35 |
| Helluomorphoides praestus bicolor (Larochelle and Lariviere) | P | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Lebia ornata Say | P | 0/0 | 0/0 | 0/1 | 0/0 | 0/1 |
| Megacephala virginica (Linneaus) | P | 0/1 | 0/0 | 0/0 | 0/0 | 0/1 |
| Mioptachys flavicauda (Say) | S | 1/1 | 0/0 | 0/0 | 0/4 | 1/5 |
| Notiophilus novemstriatus LeConte | P | 0/0 | 6/6 | 0/0 | 2/2 | 8/8 |
| Panagaeus fasciatus Say | P | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Pasimachus sublaevis (Beauvois) | P | 0/0 | 0/0 | 2/1 | 0/0 | 2/1 |
| Pterostichus permundus (Say) | P | 0/0 | 0/0 | 0/0 | 4/2 | 4/2 |
| Rhadine larvalis LeConte | P | 0/0 | 0/2 | 0/0 | 0/0 | 0/2 |
| Scaphinotus cavicollis (LeConte) | P | 0/0 | 0/0 | 0/0 | 4/5 | 4/5 |
| Scaphinotus liebecki Van Dyke | P | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Selenophorus ellipticus Dejean | P | 1/0 | 0/0 | 0/0 | 0/0 | 1/0 |
| Selenophorus opalinus (LeConte) | P | 1/1 | 1/0 | 0/0 | 1/0 | 3/1 |
| Selenophorus sp. | P | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Sphaeroderus stenostomus (Weber) | P | 0/0 | 0/0 | 2/0 | 0/0 | 2/0 |
| Tachyta nana (Gyllenhal) | S | 0/1 | 0/0 | 0/0 | 0/0 | 0/1 |
| Cerambycidae | ||||||
| Acanthocinus obsoletus (Olivier) | S | 0/10 | 0/1 | 0/7 | 0/2 | 0/20 |
| Anelaphus pumilus (Newman) | S | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Arhopalus rusticus nubilus (LeConte) | S | 0/1 | 0/0 | 0/0 | 0/0 | 0/1 |
| Astylopsis perplexa (Haldeman) | S | 0/1 | 0/0 | 0/0 | 0/0 | 0/1 |
| Curius dentatus Newman | S | 0/0 | 1/0 | 0/0 | 0/1 | 1/1 |
| Distenia undata (Fabricius) | S | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Eburia quadrigeminata (Say) | S | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Elaphidion mucronatum (Say) | S | 1/0 | 0/1 | 0/0 | 2/5 | 3/6 |
| Enaphalodes atomarius (Drury) | S | 1/0 | 0/0 | 0/0 | 0/0 | 1/0 |
| Eupogonius tomentosus (Haldeman) | S | 0/0 | 1/0 | 0/0 | 2/0 | 3/0 |
| Graphisurus fasciatus (DeGeer) | S | 0/1 | 0/0 | 1/5 | 3/4 | 4/10 |
| Knulliana cincta (Drury) | S | 0/0 | 0/0 | 0/0 | 1/5 | 1/5 |
| Leptostylus transversus (Gyllenhal) | S | 2/3 | 6/4 | 0/0 | 12/28 | 20/35 |
| Monochamus carolinensis (Olivier) | S | 0/0 | 0/0 | 0/1 | 0/0 | 0/1 |
| Neoclytus acuminatus (Fabricius) | S | 0/1 | 2/1 | 1/0 | 10/0 | 13/2 |
| Obrium maculatum (Olivier) | S | 0/1 | 0/0 | 0/0 | 9/7 | 9/8 |
| Orthosoma brunneum (Forster) | S | 0/0 | 1/0 | 0/0 | 2/0 | 3/0 |
| Prionus pocularis Dalman | S | 0/2 | 1/13 | 0/0 | 2/3 | 3/18 |
| Sternidius alpha (Say) | S | 0/0 | 0/0 | 3/1 | 0/0 | 3/1 |
| Styloleptus biustus (LeConte) | S | 0/0 | 1/2 | 0/1 | 3/6 | 4/9 |
| Typocerus lunulatus (Swederus) | S | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Typocerus velutinus (Olivier) | S | 0/0 | 1/0 | 0/0 | 0/0 | 1/0 |
| Typocerus zebra (Olivier) | S | 0/0 | 2/2 | 1/0 | 0/0 | 3/2 |
| Xylotrechus colonus (Fabricius) | S | 0/0 | 0/0 | 0/0 | 3/4 | 3/4 |
| Xylotrechus s. sagittatus (Germar) | S | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Cerylonidae | ||||||
| Philothermus glabriculus LeConte | S | 0/0 | 0/1 | 0/0 | 0/1 | 0/2 |
| Chrysomelidae | ||||||
| Capraita circumdata (Randall) | O | 0/0 | 0/3 | 0/0 | 0/1 | 0/4 |
| Capraita obsidiana (Fabricius) | O | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Capraita suturalis (Fabricius) | O | 0/0 | 0/2 | 0/0 | 0/0 | 0/2 |
| Capraita thyamoides (Crotch) | O | 0/0 | 0/0 | 0/0 | 3/1 | 3/1 |
| Capraita sp. | O | 0/0 | 0/0 | 2/0 | 0/3 | 2/3 |
| Graphops curtipennis (Melsheimer) | O | 3/1 | 0/0 | 0/0 | 0/0 | 3/1 |
| Graphops floridanus Blake | O | 2/1 | 0/0 | 0/0 | 0/0 | 2/1 |
| Metachroma pellucidum Crotch | O | 0/0 | 0/2 | 0/0 | 0/0 | 0/2 |
| Orthaltica copalina (Fabricius) | O | 0/0 | 1/0 | 0/0 | 0/0 | 1/0 |
| Paria sp. | O | 0/4 | 0/0 | 0/0 | 12/3 | 12/7 |
| Rhabdopterus sp. | O | 0/0 | 3/0 | 2/1 | 0/0 | 5/1 |
| Ciidae | ||||||
| Cis sp. | S | 0/0 | 0/0 | 0/0 | 1/1 | 1/1 |
| Cleridae | ||||||
| Cymatodera wolcotti Barr | S | 1/0 | 0/0 | 0/0 | 0/0 | 1/0 |
| Neorthopleura thoracica (Say) | S | 0/1 | 0/2 | 0/0 | 1/2 | 1/5 |
| Priocera castanea (Newman) | S | 0/5 | 0/1 | 0/0 | 0/0 | 0/6 |
| Coccinellidae | ||||||
| Psyllobora vigintimaculata (Say) | O | 0/0 | 0/0 | 1/0 | 0/0 | 1/0 |
| Corylophidae | ||||||
| Arthrolips fasciata (Erichson) | S | 0/0 | 0/0 | 0/0 | 0/3 | 0/3 |
| Clypastracea sp. | S | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Cryptophagidae | ||||||
| Cryptophagus sp. | S | 1/0 | 0/0 | 0/0 | 0/1 | 1/1 |
| Curculionidae | ||||||
| Acalles clavatus (Say) | S | 0/1 | 3/19 | 3/3 | 1/2 | 7/25 |
| Ambrosiodmus rubricolllis (Eichhoff) | S | 1/1 | 5/8 | 11/13 | 3/5 | 20/27 |
| Apteromechus ferratus (Say) | S | 0/0 | 0/4 | 1/1 | 13/27 | 14/32 |
| Coccotrypes distinctus (Motschulsky) | S | 0/1 | 0/0 | 0/0 | 0/0 | 0/1 |
| Conotrachelus posticatus Boheman | O | 0/0 | 0/0 | 1/0 | 103/68 | 104/68 |
| Cophes fallax (LeConte) | S | 0/0 | 0/0 | 0/0 | 1/3 | 1/3 |
| Corthylus punctatissimus (Zimmermann) | S | 0/0 | 1/0 | 0/1 | 0/0 | 1/1 |
| Cossonus corticola Say | S | 0/13 | 1/6 | 0/0 | 5/41 | 6/60 |
| Cryptorhynchus tristis LeConte | O | 1/2 | 2/3 | 0/0 | 1/0 | 4/5 |
| Cyrtepistomus castaneus (Roelofs) | O | 0/0 | 1/0 | 0/0 | 0/0 | 1/0 |
| Dendroctonus terebrans (Olivier) | S | 0/0 | 0/0 | 0/0 | 0/6 | 0/6 |
| Dryophthorus americanus Bedel | S | 0/2 | 2/3 | 10/0 | 4/3 | 16/8 |
| Dryoxylon onoharaensis (Murayama) | S | 0/0 | 1/4 | 0/0 | 0/0 | 1/4 |
| Euplatypus compositus (Say) | S | 4/7 | 0/2 | 0/0 | 1/1 | 5/10 |
| Euwallacea validus (Eichhoff) | S | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Gnathotrichus materiarius (Fitch) | S | 0/0 | 0/0 | 0/3 | 0/1 | 0/4 |
| Hylastes porculus Erichson | S | 0/2 | 0/0 | 0/1 | 0/1 | 0/4 |
| Hylastes salebrosus Eichhoff | S | 1/2 | 0/0 | 0/0 | 0/2 | 1/4 |
| Hylastes tenuis Eichhoff | S | 1/2 | 1/9 | 6/9 | 1/9 | 9/29 |
| Hylobius pales (Herbst) | S | 2/8 | 8/12 | 11/30 | 3/12 | 24/62 |
| Hypothenemus sp. | S | 0/0 | 0/0 | 1/1 | 0/1 | 1/2 |
| Ips avulsus (Eichhoff) | S | 0/2 | 1/1 | 0/0 | 1/5 | 2/8 |
| Ips calligraphus (Germar) | S | 0/1 | 0/1 | 0/0 | 0/2 | 0/4 |
| Ips grandicollis (Eichhoff) | S | 0/0 | 0/0 | 0/0 | 0/4 | 0/4 |
| Lissorhoptrus oryzophilus Kuschel | O | 0/1 | 0/0 | 0/0 | 0/0 | 0/1 |
| Lissorhoptrus simplex (Say) | O | 1/0 | 0/0 | 0/0 | 0/0 | 1/0 |
| Monarthrum fasciatum (Say) | S | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Monarthrum mali (Fitch) | S | 0/0 | 0/0 | 0/0 | 2/4 | 2/4 |
| Myoplatypus flavicornis (Fabricius) | S | 0/15 | 0/3 | 0/25 | 0/19 | 0/62 |
| Notaris puncticollis (LeConte) | O | 0/0 | 1/0 | 0/0 | 0/0 | 1/0 |
| Orthotomicus caelatus (Eichhoff) | S | 0/38 | 7/10 | 11/52 | 25/59 | 43/159 |
| Pachylobius picivorus (Germar) | S | 5/30 | 10/38 | 4/13 | 25/40 | 44/121 |
| Pityophthorus confusus Blandford | S | 0/1 | 0/0 | 0/0 | 0/0 | 0/1 |
| Sphenophorus sp. | O | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Stethobaris sp. | O | 1/1 | 0/0 | 0/0 | 0/0 | 1/1 |
| Xyleborinus saxeseni (Ratzeburg) | S | 14/10 | 22/12 | 9/2 | 117/98 | 162/122 |
| Xyleborus affinis Eichhoff | S | 4/36 | 28/51 | 27/18 | 13/60 | 72/165 |
| Xyleborus ferrugineus (Fabricius) | S | 3/33 | 6/16 | 23/10 | 39/58 | 71/117 |
| Xyleborus pubescens Zimmermann | S | 1/3 | 0/0 | 1/1 | 1/5 | 3/9 |
| Xyleborus xylographus (Say) | S | 0/0 | 0/0 | 1/0 | 0/1 | 1/1 |
| Xylosandrus compactus (Eichhoff) | S | 0/0 | 1/1 | 22/30 | 1/2 | 24/33 |
| Xylosandrus crassiusculus (Motschulsky) | S | 1/4 | 9/17 | 9/9 | 0/5 | 19/35 |
| Xylosandrus germanus (Blandford) | S | 1/0 | 4/7 | 0/0 | 0/0 | 5/7 |
| Dytiscidae | ||||||
| Copelatus glyphicus (Say) | O | 5/1 | 0/0 | 1/1 | 1/1 | 7/3 |
| Elateridae | ||||||
| Alaus myops (Fabricius) | S | 0/1 | 0/1 | 0/0 | 0/0 | 0/2 |
| Blauta cribraria (Germar) | S | 0/3 | 0/1 | 0/0 | 0/0 | 0/4 |
| Conoderus amplicollis (Gyllenhal) | S | 0/1 | 0/0 | 0/0 | 0/0 | 0/1 |
| Dicrepidius sp. | S | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Glyphonyx bimarginatus Schaeffer | S | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Glyphonyx ferruginosus Schaeffer | S | 0/0 | 0/0 | 0/0 | 1/1 | 1/1 |
| Glyphonyx sp. | S | 0/0 | 0/0 | 0/1 | 0/0 | 0/1 |
| Lacon discoideus (Weber) | S | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Lacon impressicollis (Say) | S | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Limonius quercinus Say | S | 0/0 | 0/0 | 1/0 | 0/0 | 1/0 |
| Megapenthes rufilabris (Germar) | S | 1/0 | 0/1 | 0/0 | 0/1 | 1/2 |
| Megapenthes sp. | S | 0/0 | 0/0 | 0/1 | 0/0 | 0/1 |
| Melanotus corticinus (Say) | S | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Melanotus ignobilis Melsheimer | S | 1/2 | 0/0 | 0/0 | 0/0 | 1/2 |
| Melanotus insipiens (Say) | S | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Melanotus piceatus Blatchley | S | 0/0 | 0/0 | 2/0 | 0/0 | 2/0 |
| Melanotus pilosus Blatchley | S | 1/1 | 0/0 | 0/0 | 0/0 | 1/1 |
| Melanotus similis group | S | 0/0 | 1/1 | 0/1 | 0/0 | 1/2 |
| Melanotus testaceus (Melsheimer) | S | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Melanotus sp. | S | 0/0 | 2/2 | 0/1 | 0/1 | 2/4 |
| Mulsanteus carolinensis (Schaeffer) | S | 0/0 | 0/0 | 0/0 | 13/2 | 13/2 |
| Endomychidae | ||||||
| Aphorista vittata (Fabricius) | S | 1/2 | 2/3 | 0/0 | 4/7 | 7/12 |
| Danae testacea (Ziegler) | S | 0/1 | 2/3 | 7/3 | 4/3 | 13/10 |
| Epipocus punctatus LeConte | S | 1/1 | 0/0 | 0/0 | 0/0 | 1/1 |
| Lycoperdina ferruginea LeConte | S | 0/0 | 4/2 | 1/0 | 8/12 | 13/14 |
| Mycetina perpulchra (Newman) | S | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Erotylidae | ||||||
| Cryptophilus integer (Heer) | S | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Triplax festiva Lacordaire | S | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Tritoma affinis Lacordaire | S | 2/3 | 11/3 | 0/0 | 8/11 | 21/17 |
| Tritoma angulata Say | S | 0/0 | 1/0 | 0/0 | 0/0 | 1/0 |
| Tritoma atriventris LeConte | S | 0/0 | 0/0 | 0/0 | 1/3 | 1/3 |
| Tritoma biguttata affinis Lacordaire | S | 1/2 | 4/4 | 0/0 | 4/3 | 9/9 |
| Tritoma humeralis Fabricius | S | 0/0 | 0/0 | 0/3 | 0/0 | 0/3 |
| Eucinetidae | ||||||
| Eucinetus strigosus LeConte | S | 1/4 | 4/7 | 0/0 | 0/0 | 5/11 |
| Eucnemidae | ||||||
| Dromaeolus cylindricollis (Say) | S | 5/0 | 1/2 | 0/0 | 1/2 | 7/4 |
| Dromaeolus striatus (LeConte) | S | 1/1 | 2/2 | 0/0 | 0/0 | 3/3 |
| Microrhagus triangularis (Say) | S | 0/0 | 0/0 | 0/0 | 2/1 | 2/1 |
| Geotrupidae | ||||||
| Bolboceras thoracicornis (Wallis) | O | 0/0 | 0/0 | 1/0 | 0/0 | 1/0 |
| Bolbocerosoma farctum (Fabricius) | O | 0/0 | 1/1 | 0/0 | 0/0 | 1/1 |
| Geotrupes blackburnii (Fabricius) | O | 1/0 | 0/0 | 0/0 | 1/3 | 2/3 |
| Geotrupes opacus Haldeman | O | 0/0 | 0/0 | 0/1 | 6/0 | 6/1 |
| Odonteus sp. | O | 0/0 | 0/0 | 1/0 | 0/0 | 1/0 |
| Histeridae | ||||||
| Eblisia carolina (Paykull) | S | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Paromalus seminulum Erichson | S | 0/1 | 1/0 | 0/0 | 0/1 | 1/2 |
| Platysoma coarctatum LeConte | S | 0/0 | 0/1 | 0/0 | 0/2 | 0/3 |
| Hydrophilidae | ||||||
| Cercyon occallatus (Say) | O | 0/0 | 1/0 | 9/4 | 0/0 | 10/4 |
| Cercyon pubescens LeConte | O | 1/0 | 0/0 | 0/0 | 3/0 | 4/0 |
| Cymbiodyta chamberlaini Smetana | O | 1/0 | 0/0 | 0/0 | 0/1 | 1/1 |
| Laemophloeidae | ||||||
| Cryptolestes punctatus (LeConte) | S | 0/1 | 0/0 | 0/0 | 0/0 | 0/1 |
| Cryptolestes sp. | S | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Laemophloeus biguttatus Say | S | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Placonotus modestus (Say) | S | 0/0 | 0/2 | 0/0 | 4/2 | 4/4 |
| Lampyridae | ||||||
| Ellychnia corrusca (LeConte) | S | 0/0 | 0/0 | 1/0 | 0/0 | 1/0 |
| Latridiidae | ||||||
| Aridius sp. | S | 0/0 | 0/0 | 0/1 | 0/0 | 0/1 |
| Corticarina sp. | S | 1/2 | 0/0 | 0/0 | 0/0 | 1/2 |
| Leiodidae | ||||||
| Anisotoma basalis (LeConte) | O | 0/0 | 0/0 | 1/0 | 0/0 | 1/0 |
| Anisotoma discolor (Melsheimer) | O | 0/0 | 0/0 | 1/0 | 1/5 | 2/5 |
| Anisotoma sp. | O | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Colenis bifida Peck | O | 0/0 | 0/0 | 2/0 | 0/0 | 2/0 |
| Colenis impunctata LeConte | O | 2/10 | 0/1 | 5/8 | 19/16 | 26/35 |
| Colenis ora Peck | O | 0/1 | 2/1 | 0/0 | 15/20 | 17/22 |
| Colenis stephani Peck | O | 0/0 | 0/0 | 0/0 | 2/1 | 2/1 |
| Colenis sp. | O | 0/1 | 0/0 | 0/1 | 0/0 | 0/2 |
| Dissochaetus oblitus (LeConte) | O | 0/0 | 0/0 | 0/1 | 0/0 | 0/1 |
| Leiodes stephani Baranowski | O | 0/0 | 0/0 | 0/0 | 4/0 | 4/0 |
| Ptomaphagus consobrinus LeConte | O | 4/5 | 0/0 | 0/0 | 3/5 | 7/10 |
| Ptomaphagus sp. | O | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Lucanidae | ||||||
| Dorcus parallelus (Say) | S | 0/0 | 0/0 | 1/1 | 0/0 | 1/1 |
| Lycidae | ||||||
| Plateros sp. | S | 0/2 | 1/3 | 0/0 | 1/0 | 2/5 |
| Melandryidae | ||||||
| Dircaea liturata (LeConte) | S | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Microtonus sericans LeConte | S | 0/0 | 1/0 | 0/0 | 0/0 | 1/0 |
| Mordellidae | ||||||
| Mordella sp. | O | 0/0 | 1/1 | 0/0 | 0/1 | 1/2 |
| Mordellaria borealis (LeConte) | O | 0/1 | 1/0 | 0/0 | 0/0 | 1/1 |
| Mordellaria serval (Say) | O | 0/0 | 1/0 | 0/0 | 1/3 | 2/3 |
| Mordellistena pubescens (Fabricius) | O | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Mycetophagidae | ||||||
| Typhaea stercorea (Linnaeus) | S | 0/2 | 1/0 | 0/0 | 17/19 | 18/21 |
| Nitidulidae | ||||||
| Amphicrossus ciliatus (Olivier) | O | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Carpophilus antiquus Melsheimer | O | 0/0 | 0/0 | 1/1 | 1/0 | 2/1 |
| Carpophilus sp. | O | 1/1 | 0/2 | 1/4 | 3/2 | 5/9 |
| Colopterus unicolor (Say) | O | 10/16 | 12/39 | 43/55 | 30/66 | 95/176 |
| Epuraea helvola Erichson | O | 0/0 | 1/0 | 3/13 | 0/0 | 4/13 |
| Epuraea planulata Erichson | O | 0/0 | 0/0 | 0/1 | 0/0 | 0/1 |
| Epuraea rufa (Say) | O | 0/0 | 0/0 | 7/4 | 0/0 | 7/4 |
| Glischrochilus sanguinolentus (Olivier) | O | 0/0 | 1/2 | 0/0 | 0/0 | 1/2 |
| Pallodes austrinus Leschen | O | 0/0 | 2/0 | 1/0 | 1/2 | 4/2 |
| Pallodes pallidus Beauvois | O | 0/0 | 1/3 | 1/3 | 0/0 | 2/6 |
| Prometopia sexmaculata (Say) | O | 0/1 | 0/0 | 0/0 | 1/0 | 1/1 |
| Stelidota coenosa Erichson | O | 8/7 | 2/3 | 1/1 | 23/22 | 34/33 |
| Stelidota geminata (Say) | O | 1/3 | 0/1 | 7/12 | 6/3 | 14/19 |
| Stelidota octomaculata (Say) | O | 0/0 | 0/0 | 0/0 | 0/2 | 0/2 |
| Stelidota sexmaculata (Say) | O | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Stelidota strigosa Schoenherr | O | 0/0 | 1/0 | 0/0 | 6/3 | 7/3 |
| Oedemeridae | ||||||
| Oxycopis notoxoides (Fabricius) | O | 1/0 | 0/0 | 0/0 | 0/0 | 1/0 |
| Oxycopis sp. | S | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Oxycopis thoracica (Fabricius) | S | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Passandridae | ||||||
| Catogenus rufus (Fabricius) | S | 0/1 | 0/0 | 0/0 | 0/0 | 0/1 |
| Ptilodactylidae | ||||||
| Ptilodactyla sp. | O | 0/0 | 0/0 | 0/0 | 2/4 | 2/4 |
| Scarabaeidae | ||||||
| Anomala sp. | O | 0/0 | 0/0 | 0/0 | 0/2 | 0/2 |
| Ataenius imbricatus Melsheimer | O | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Ataenius insculptus Horn | O | 0/0 | 2/1 | 0/0 | 0/0 | 2/1 |
| Ataenius sp. | O | 0/0 | 1/0 | 0/0 | 0/0 | 1/0 |
| Ateuchus histeroides Weber | O | 0/0 | 0/0 | 0/0 | 2/1 | 2/1 |
| Canthon ebenus Say | O | 0/0 | 0/0 | 0/0 | 7/7 | 7/7 |
| Canthon viridis (Beauvois) | O | 0/0 | 0/1 | 0/0 | 1/1 | 1/2 |
| Copris minutus (Drury) | O | 0/0 | 1/0 | 0/2 | 0/0 | 1/2 |
| Deltochilum gibbosum (Fabricius) | O | 0/0 | 0/0 | 3/0 | 10/3 | 13/3 |
| Digitonthophagus gazella (Fabricius) | O | 0/0 | 1/0 | 0/0 | 0/0 | 1/0 |
| Diplotaxis sp. | O | 0/0 | 5/1 | 0/0 | 3/6 | 8/7 |
| Euphoria sepulcralis (Fabricius) | O | 0/0 | 0/0 | 0/0 | 2/7 | 2/7 |
| Melanocanthon sp. | O | 0/0 | 0/0 | 0/0 | 2/5 | 2/5 |
| Onthophagus hecate (Panzer) | O | 0/0 | 1/0 | 0/0 | 10/7 | 11/7 |
| Onthophagus medorensis Brown | O | 0/0 | 0/0 | 0/0 | 31/16 | 31/16 |
| Onthophagus pennsylvanicus Harold | O | 0/0 | 0/0 | 0/0 | 2/0 | 2/0 |
| Onthophagus striatulus (Beauvois) | O | 0/1 | 2/0 | 0/0 | 2/2 | 4/3 |
| Onthophagus tuberculifrons Harold | O | 0/0 | 0/0 | 0/0 | 0/2 | 0/2 |
| Onthophagus sp. | O | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Parataenius simulator (Harold) | O | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Phyllophaga forsteri (Burmeister) | O | 0/0 | 1/2 | 0/0 | 0/0 | 1/2 |
| Phyllophaga prunina (LeConte) | O | 2/3 | 0/0 | 0/0 | 0/3 | 2/6 |
| Phyllophaga prununculina (Burmeister) | O | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Phyllophaga scitula (Horn) | O | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Phyllophaga sp. | O | 1/0 | 0/0 | 1/1 | 0/0 | 2/1 |
| Phyllophaga tristis complex | O | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Platytomus longulus (Cartwright) | O | 1/0 | 0/0 | 0/0 | 0/0 | 1/0 |
| Serica sp. | O | 0/0 | 0/0 | 0/0 | 2/0 | 2/0 |
| Scraptiidae | ||||||
| Scraptia sp. | O | 0/0 | 0/0 | 1/0 | 0/0 | 1/0 |
| Silphidae | ||||||
| Necrophila americana (Linnaeus) | O | 0/0 | 0/0 | 0/0 | 0/2 | 0/2 |
| Silvanidae | ||||||
| Ahasverus advena (Waltl) | S | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Ahasverus rectus (LeConte) | S | 1/12 | 1/6 | 1/0 | 7/14 | 10/32 |
| Cathartosilvanus imbellis (LeConte) | S | 0/0 | 0/1 | 0/0 | 0/1 | 0/2 |
| Silvanoprus scuticollis (Walker) | S | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Silvanus muticus Sharp | S | 1/2 | 0/0 | 0/0 | 2/6 | 3/8 |
| Sphindidae | ||||||
| Sphindus sp. | S | 2/0 | 1/0 | 3/0 | 1/4 | 7/4 |
| Staphylinidae | ||||||
| Achenomorphus corticinus (Gravenhorst) | O | 1/0 | 12/11 | 0/4 | 0/1 | 13/16 |
| Anotylus sp. | O | 0/1 | 0/0 | 0/0 | 27/39 | 27/40 |
| Arthmius sp. | O | 0/0 | 0/0 | 1/0 | 0/0 | 1/0 |
| Astenus linearis (Erichson) | O | 0/1 | 0/0 | 0/0 | 0/0 | 0/1 |
| Astenus sp. | O | 1/2 | 2/0 | 0/0 | 0/2 | 3/4 |
| Baeocera laevis (Reitter) | O | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Baeocera sp. | O | 0/0 | 5/2 | 0/1 | 1/4 | 6/7 |
| Belonuchus ephippiatus (Say) | O | 1/0 | 0/1 | 0/0 | 1/0 | 2/1 |
| Belonuchus rufipennis (Fabricius) | S | 0/0 | 0/0 | 1/0 | 0/0 | 1/0 |
| Bryoporus rufescens LeConte | O | 0/0 | 3/1 | 0/1 | 0/0 | 3/2 |
| Carpelimus sp. | O | 0/1 | 0/0 | 0/0 | 0/1 | 0/2 |
| Coproporus laevis LeConte | S | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Coproporus ventriculus (Say) | S | 1/0 | 0/0 | 0/0 | 0/0 | 1/0 |
| Ctenisodes sp. | O | 0/0 | 0/0 | 0/0 | 7/0 | 7/0 |
| Decarthron sp. | O | 1/0 | 0/0 | 0/0 | 0/0 | 1/0 |
| Diochus schaumi Kraatz | O | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Echiaster sp. | O | 0/0 | 0/1 | 0/0 | 0/1 | 0/2 |
| Erichsonius sp. | O | 0/1 | 0/0 | 0/0 | 0/0 | 0/1 |
| Euconnus sp. | O | 0/1 | 0/0 | 2/0 | 0/1 | 2/2 |
| Hesperus baltimorensis (Gravenhorst) | O | 0/2 | 0/0 | 2/0 | 0/0 | 2/2 |
| Homaeotarsus sp. | O | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Hoplandria laevicollis (Notman) | O | 0/0 | 0/0 | 5/3 | 0/0 | 5/3 |
| Hoplandria sp. | O | 0/0 | 0/1 | 12/19 | 1/0 | 13/20 |
| Ischnosoma flavicolle (LeConte) | O | 1/0 | 1/1 | 0/0 | 1/2 | 3/3 |
| Myrmecocephalus concinnus (Erichson) | S | 0/2 | 0/0 | 0/0 | 0/0 | 0/2 |
| Oxybleptes davisi (Notman) | O | 37/4 | 28/23 | 0/0 | 0/0 | 65/27 |
| Oxypoda sp. | O | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Palaminus sp. | S | 0/0 | 1/2 | 0/0 | 2/0 | 3/2 |
| Philonthus umbrinus (Gravenhorst) | O | 0/0 | 0/0 | 1/1 | 0/0 | 1/1 |
| Philotermes sp. | S | 0/0 | 9/5 | 0/0 | 0/0 | 9/5 |
| Pinophilus sp. | O | 1/0 | 0/0 | 0/0 | 0/0 | 1/0 |
| Platydracus fossator (Gravenhorst) | O | 0/0 | 0/0 | 1/0 | 2/1 | 3/1 |
| Platydracus sp. | O | 0/0 | 0/0 | 0/0 | 2/1 | 2/1 |
| Quedius capucinus (Gravenhorst) | O | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Quedius verres Smetana | O | 0/0 | 8/11 | 0/0 | 5/2 | 13/13 |
| Rugilus sp. | O | 0/0 | 3/0 | 0/0 | 2/9 | 5/9 |
| Scaphisoma punctulatum LeConte | O | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Scydmaenus sp. | O | 0/0 | 0/0 | 2/0 | 0/0 | 2/0 |
| Sepedophilus basalis (Erichson) | O | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Sepedophilus crassus (Gravenhorst) | O | 0/0 | 0/0 | 0/1 | 0/0 | 0/1 |
| Sepedophilus debilis (Casey) | O | 0/0 | 0/0 | 1/0 | 0/0 | 1/0 |
| Sepedophilus sp. | O | 22/18 | 2/2 | 0/1 | 13/19 | 37/40 |
| Stenichnus sp. | O | 0/0 | 0/0 | 1/0 | 0/0 | 1/0 |
| Tachinus fimbriatus Gravenhorst | O | 0/0 | 0/0 | 2/0 | 0/0 | 2/0 |
| Thoracophorus costalis (Erichson) | S | 0/0 | 0/0 | 0/0 | 30/26 | 30/26 |
| Tmesiphorus costalis LeConte | O | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Tenebrionidae | ||||||
| Alobates morio (Fabricius) | S | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Alphitophagus bifasciatus (Say) | S | 0/2 | 0/1 | 0/0 | 0/0 | 0/3 |
| Anaedus brunneus (Ziegler) | O | 1/0 | 0/1 | 0/0 | 0/0 | 1/1 |
| Corticeus thoracicus (Melsheimer) | S | 0/2 | 0/0 | 0/0 | 0/1 | 0/3 |
| Gondwanocrypticus obsoletus (Say) | S | 1/0 | 0/0 | 0/0 | 0/0 | 1/0 |
| Helops cisteloides (Germar) | S | 0/0 | 0/0 | 0/0 | 1/1 | 1/1 |
| Helops sp. | S | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Hymenorus sp. | S | 3/4 | 1/2 | 0/0 | 3/5 | 7/11 |
| Isomira sp. | S | 0/0 | 0/0 | 0/0 | 0/2 | 0/2 |
| Lobopoda erythrocnemis (Germar) | S | 3/3 | 2/0 | 0/0 | 11/7 | 16/10 |
| Opatrinus minimus (Beauvois) | O | 0/0 | 0/0 | 0/0 | 1/1 | 1/1 |
| Platydema micans Zimmerman | S | 2/1 | 0/0 | 0/0 | 2/2 | 4/3 |
| Platydema ruficolle Laporte and Brullé | S | 0/0 | 0/1 | 0/0 | 2/1 | 2/2 |
| Platydema ruficorne (Sturm) | S | 0/1 | 0/1 | 0/0 | 0/0 | 0/2 |
| Polypleurus perforatus (Germar) | S | 0/1 | 1/0 | 0/0 | 4/2 | 5/3 |
| Statira gagatina (Melsheimer) | O | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Statira sp. | O | 0/0 | 0/0 | 0/0 | 0/2 | 0/2 |
| Uloma imberbis LeConte | S | 0/0 | 0/0 | 2/0 | 0/1 | 2/1 |
| Uloma punctulata LeConte | S | 0/6 | 2/4 | 0/0 | 0/1 | 2/11 |
| Tetratomidae | ||||||
| Eustrophopsis bicolor (Fabricius) | S | 1/1 | 0/0 | 0/0 | 0/1 | 1/2 |
| Eustrophus tomentosus Say | S | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 |
| Throscidae | ||||||
| Aulonothroscus convergens (Horn) | O | 0/0 | 0/4 | 0/0 | 0/0 | 0/4 |
| Trogidae | ||||||
| Omorgus monachus Herbst | O | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Trox spinulosus Robinson | O | 0/0 | 0/0 | 0/0 | 2/0 | 2/0 |
| Trox variolatus Melsheimer | O | 1/1 | 0/0 | 0/0 | 1/1 | 2/2 |
| Trogossitidae | ||||||
| Temnoscheila virescens (Fabricius) | S | 0/2 | 0/0 | 0/0 | 1/0 | 1/2 |
| Zopheridae | ||||||
| Bitoma quadriguttata (Say) | S | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 |
| Colydium lineola Say | S | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Colydium nigripenne LeConte | S | 0/3 | 0/9 | 0/0 | 0/3 | 0/15 |
| Endeitoma dentata (Horn) | S | 0/1 | 1/0 | 0/0 | 0/0 | 1/1 |
| Endeitoma sp. | S | 0/1 | 0/0 | 0/0 | 0/0 | 0/1 |
| Hyporhagus punctulatus Thomson | S | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Lasconotus pusillus LeConte | S | 0/0 | 0/0 | 0/0 | 0/4 | 0/4 |
| Microsicus parvulus (Guerin) | S | 0/0 | 0/0 | 0/0 | 4/1 | 4/1 |
| Pycnomerus haematodes (Fabricius) | S | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Pycnomerus sulcicollis LeConte | S | 0/4 | 2/9 | 0/0 | 0/1 | 2/14 |
| Total Individuals (reference/wood addition) | 753 | 1016 | 1104 | 2299 | 5172 | |
| (269/484) | (406/610) | (502/602) | (997/1302) | (2174/2998) | ||
| Total Species (reference/wood addition) | 140 | 162 | 108 | 227 | 378 | |
| (79/112) | (105/125) | (82/72) | (147/180) | (261/302) | ||
Acknowledgments
Conflict of Interest
References
- Elton, C.S. The Pattern of Animal Communities; Methuen and Co. Ltd.: London, UK, 1966. [Google Scholar]
- Siitonen, J. Forest management, coarse woody debris and saproxylic organisms: Fennoscandian boreal forests as an example. Ecol. Bull. 2001, 49, 11–41. [Google Scholar]
- Grove, S.J. Saproxylic insect ecology and the sustainable management of forests. Ann. Rev. Ecol. Sys. 2002, 33, 1–23. [Google Scholar]
- Speight, M.C.D. Saproxylic Invertebrates and Their Conservation; Council of Europe: Strasbourg, France, 1989. [Google Scholar]
- Andrew, N.; Rodgerson, L.; York, A. Frequent fuel-reduction burning: The role of logs and associated leaf litter in the conservation of ant biodiversity. Austral Ecol. 2000, 25, 99–107. [Google Scholar] [CrossRef]
- Evans, A.M.; Clinton, P.W.; Allen, R.B.; Frampton, C.M. The influence of logs on the spatial distribution of litter-dwelling invertebrates and forest floor processes in New Zealand forests. For. Ecol. Manag. 2003, 184, 251–262. [Google Scholar] [CrossRef]
- Jabin, M.; Mohr, D.; Kappes, H.; Topp, W. Influence of deadwood on density of soil macro-arthropods in a managed oak-beech forest. For. Ecol. Manag. 2004, 194, 61–69. [Google Scholar] [CrossRef]
- Topp, W.; Kappes, H.; Kulfan, J.; Zach, P. Litter-dwelling beetles in primeval forests of Central Europe: Does deadwood matter? J. Insect Conserv. 2006, 10, 229–239. [Google Scholar] [CrossRef]
- Topp, W.; Kappes, H.; Kulfan, J.; Zach, P. Distribution pattern of woodlice (Isopoda) and millipedes (Diplopoda) in four primeval forests of the western Carpathians (Central Slovakia). Soil Biol. Biochem. 2006, 38, 43–50. [Google Scholar] [CrossRef]
- Kappes, H. Influence of coarse woody debris on the gastropod community of a managed calcareous beech forest in western Europe. J. Molluscan Stud. 2005, 71, 85–91. [Google Scholar] [CrossRef]
- Kappes, H. Relations between forest management and slug assemblages (Gastropoda) of deciduous regrowth forests. For. Ecol. Manag. 2006, 237, 450–457. [Google Scholar] [CrossRef]
- Kappes, H.; Topp, W.; Zach, P.; Kulfan, J. Coarse woody debris, soil properties and snails (Mollusca: Gastropoda) in European primeval forests of different environmental conditions. Eur. J. Soil Biol. 2006, 42, 139–146. [Google Scholar] [CrossRef]
- Kappes, H.; Catalano, C.; Topp, W. Coarse woody debris ameliorates chemical and biotic soil parameters of acidified broad-leaved forests. Appl.Soil Ecol. 2007, 36, 190–198. [Google Scholar] [CrossRef]
- Jabin, M.; Topp, W.; Kulfan, J.; Zach, P. The distribution pattern of centipedes in four primeval forests of central Slovakia. Biodivers. Conserv. 2007, 16, 3437–3445. [Google Scholar] [CrossRef]
- Ulyshen, M.D.; Hanula, J.L. Litter-dwelling arthropod abundance peaks near coarse woody debris in loblolly pine forests of the southeastern United States. Fla. Entomol. 2009, 92, 163–164. [Google Scholar] [CrossRef]
- Castro, A.; Wise, D.H. Influence of fallen coarse woody debris on the diversity and community structure of forest-floor spiders (Arachnida: Araneae). For. Ecol. Manag. 2010, 260, 2088–2101. [Google Scholar] [CrossRef]
- Ulyshen, M.D.; Klooster, W.S.; Barrington, W.T.; Herms, D.A. Impacts of emerald ash borer-induced tree mortality on leaf litter arthropods and exotic earthworms. Pedobiologia 2011, 54, 261–265. [Google Scholar] [CrossRef]
- Maser, C.; Trappe, J.M. The Seen and Unseen World of the Fallen Tree; General Technical Report PNW-164; USDA Forest Service, Pacific Northwest Forest and Range Experiment Station: Portland, OR, USA, 1984. [Google Scholar]
- Amaranthus, M.P.; Parrish, D.S.; Perry, D.A. Decaying logs as moisture reservoirs after drought and wildfire in Stewardship of soil, air and water resources. In Proceedings of Watershed 89, Juneau, AK, USA, 21–23 March 1989; pp. 191–194.
- Marra, J.L.; Edmonds, R.L. Effects of coarse woody debris and soil depth on the density and diversity of soil invertebrates on clearcut and forested sites on the Olympic Peninsula, Washington. Environ. Entomol. 1998, 27, 1111–1124. [Google Scholar]
- Tilman, D.; Socolow, R.; Foley, J.A.; Hill, J.; Larson, E.; Lynd, L.; Pacala, S.; Reilly, J.; Searchinger, T.; Somerville, C.; Williams, R. Beneficial biofuels—The food, energy, and environment trilemma. Science 2009, 325, 270–271. [Google Scholar]
- Landis, D.A.; Werling, B.P. Arthropods and biofuel production systems in North America. Insect Sci. 2010, 17, 220–236. [Google Scholar] [CrossRef]
- Bengtsson, J.; Persson, T.; Lundkvist, H. Long-term effects of logging residue addition and removal on macroarthropods and enchytraeids. J. Appl. Ecol. 1997, 34, 1014–1022. [Google Scholar] [CrossRef]
- Barton, P.S.; Manning, A.D.; Gibb, H.; Wood, J.T.; Lindenmayer, D.B.; Cunningham, S.A. Experimental reduction of native vertebrate grazing and addition of logs benefit beetle diversity at multiple scales. J. Appl. Ecol. 2011, 48, 943–951. [Google Scholar] [CrossRef]
- Gunnarsson, B.; Nittérus, K.; Wirdenäs, P. Effects of logging residue removal on ground-active beetles in temperate forests. For. Ecol. Manag. 2004, 201, 229–239. [Google Scholar] [CrossRef]
- Grove, S.J.; Hanula, J.L. Insect Biodiversity and Dead Wood: Proceedings of a Symposium for the 22nd International Congress of Entomology; General Technical Report SRS-93; USDA Forest Service, Southern Research Station: Asheville, NC, USA, 2006; pp. 57–66.
- Ulyshen, M.D.; Hanula, J.L. Responses of arthropods to large-scale manipulations of dead wood in loblolly pine stands of the southeastern United States. Environ. Entomol. 2009, 38, 1005–1012. [Google Scholar] [CrossRef]
- Houseweart, M.W.; Jennings, D.T.; Rea, J.C. Large capacity pitfall trap. Entomol. News 1979, 90, 51–54. [Google Scholar]
- Ulyshen, M.D.; Hanula, J.L. Patterns of saproxylic beetle succession in loblolly pine. Agr. For. Entomol. 2010, 12, 187–194. [Google Scholar] [CrossRef]
- Hamilton, W.D. Diversity of Insect Faunas. Symposia of the Royal Entomological Society of London; Mound, L.A., Waloff, N., Eds.; Blackwell Scientific: Oxford, UK, 1978; pp. 154–175. [Google Scholar]
- Cárcamo, H.A.; Parkinson, D. Distribution of ground beetle assemblages (Coleoptera, Carabidae) around sour gas processing plants in western Canada. Pedobiologia 1999, 43, 160–173. [Google Scholar]
- Pearce, J.L.; Venier, L.A.; McKee, J.; Pedlar, J.; McKenney, D. Influence of habitat and microhabitat on carabid (Coleoptera: Carabidae) assemblages in four stand types. Can. Entomol. 2003, 135, 337–357. [Google Scholar] [CrossRef]
- Latty, E.F.; Werner, S.M.; Mladenoff, D.J.; Raffa, K.F.; Sickley, T.A. Response of ground beetles (Carabidae) assemblages to logging history in northern hardwood-hemlock forests. For. Ecol. Manag. 2006, 222, 335–347. [Google Scholar] [CrossRef]
- Nittérus, K.; Gunnarsson, Å.; Gunnarsson, B. Manipulated structural variability affects the habitat choice of two ground-living beetle species in a laboratory experiment. Entomol. Fenn. 2008, 19, 122–128. [Google Scholar]
- Nittérus, K.; Gunnarsson, B. Effect of microhabitat complexity on the local distribution of arthropods in clear-cuts. Environ. Entomol. 2006, 35, 1324–1333. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Klepzig, K.D.; Ferro, M.L.; Ulyshen, M.D.; Gimmel, M.L.; Mahfouz, J.B.; Tiarks, A.E.; Carlton, C.E. Effects of Small-Scale Dead Wood Additions on Beetles in Southeastern U.S. Pine Forests. Forests 2012, 3, 632-652. https://doi.org/10.3390/f3030632
Klepzig KD, Ferro ML, Ulyshen MD, Gimmel ML, Mahfouz JB, Tiarks AE, Carlton CE. Effects of Small-Scale Dead Wood Additions on Beetles in Southeastern U.S. Pine Forests. Forests. 2012; 3(3):632-652. https://doi.org/10.3390/f3030632
Chicago/Turabian StyleKlepzig, Kier D., Michael L. Ferro, Michael D. Ulyshen, Matthew L. Gimmel, Jolie B. Mahfouz, Allan E. Tiarks, and Chris E. Carlton. 2012. "Effects of Small-Scale Dead Wood Additions on Beetles in Southeastern U.S. Pine Forests" Forests 3, no. 3: 632-652. https://doi.org/10.3390/f3030632




